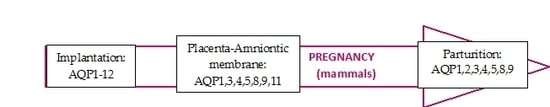
Aquaporins during Pregnancy: Their Function and Significance
Abstract
:1. Introduction
2. Implantation
3. Maternal–Fetal Fluid Flow
3.1. Amniotic Membrane
3.2. Placenta
4. Parturition
4.1. Myometrial Contraction
4.2. Cervical Ripening
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ishibashi, K.; Kondo, S.; Hara, S.; Morishita, Y. The evolutionary aspects of aquaporin family. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R566–R576. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; Moura, T.F.; Soveral, G. Aquaglyceroporins: Implications in adipose biology and obesity. Cell. Mol. Life Sci. 2015, 72, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Verkman, A.S. Physiological roles of glycerol-transporting aquaporins: The aquaglyceroporins. Cell. Mol. Life Sci. 2006, 63, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Bhattacharjee, H.; Rosen, B.P. Aquaglyceroporins: Generalized metalloid channels. Biochim. Biophys. Acta 2014, 1840, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Yakata, K.; Hiroaki, Y.; Ishibashi, K.; Sohara, E.; Sasaki, S.; Mitsuoka, K. Aquaporin-11 containing a divergent NPA motif has normal water channel activity. Biochim. Biophys. Acta Biomembr. 2007, 1768, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Rai, T.; Kuwahara, M.; Ko, S.B.H.; Uchida, S.; Sasaki, S.; Ishibashi, K. Identification of a novel aquaporin, AQP12, expressed in pancreatic acinar cells. Biochem. Biophys. Res. Commun. 2005, 330, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tan, Y.-J.; Qu, F.; Sheng, J.-Z.; Huang, H.-F. Functions of water channels in male and female reproductive systems. Mol. Aspects Med. 2012, 33, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Tornroth-Horsefield, S.; Hedfalk, K.; Fischer, K.; Lindkvist-Petersson, G.; Neutze, R. Structural insights into eukaryotic aquaporin regulation. FEBS Lett. 2010, 584, 2580–2588. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, U.K.; Fischer, G.; Friemann, R.; Enkavi, G.; Tajkhorshid, E.; Neutze, R. Subangstrom resolution X-ray structure details aquaporin-water interactions. Science 2013, 340, 1346–1349. [Google Scholar] [CrossRef] [PubMed]
- Enders, A.; Schlafke, S. A morphological analysis of the early implantation stages in the rat. Am. J. Anat. 1967, 120, 185–225. [Google Scholar] [CrossRef]
- Lindsay, L.A.; Murphy, C.R. Aquaporin-1 increases in the rat myometrium during early pregnancy. J. Mol. Histol. 2004, 35, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, L.A.; Murphy, C.R. Redistribution of aquaporins 1 and 5 in the rat uterus is dependent on progesterone: A study with light and electron microscopy. Reproduction 2006, 131, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Jiang, W.; Zhang, W.; Shen, Q.; Chen, M.; Zhu, X. Expression and significance of aquaporins during pregnancy. Front. Biosci. 2013, 18, 1373–1383. [Google Scholar]
- He, R.H.; Sheng, J.Z.; Luo, Q.; Jin, F.; Wang, B.; Qian, Y.L.; Zhou, C.Y.; Sheng, X.; Huang, H.F. Aquaporin-2 expression in human endometrium correlates with serum ovarian steroid hormones. Life Sci. 2006, 79, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Wray, S.; Marples, D. Distribution of AQP2 and AQP3 water channels in human tissue microarrays. J. Mol. Histol. 2005, 36, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sponchiado, M.; Gomes, N.S.; Fontes, P.K.; Martins, T.; Del Collado, M.; Pastore, A.A.; Pugliesi, G.; Nogueira, M.F.G.; Binelli, M. Pre-hatching embryo-dependent and -independent programming of endometrial function in cattle. PLoS ONE 2017, 12, e0175954. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.L.; Zhang, J.; Fan, Y.; Ding, J.H.; Sha, J.H.; Hu, G. Aquaporin-4 deficiency induces subfertility in female mice. Fertil. Steril. 2009, 92, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, L.A.; Murphy, C.R. Redistribution of aquaporins in uterine epithelial cells at the time of implantation in the rat. Acta Histochem. 2004, 106, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, L.A.; Murphy, C.R. Aquaporins are upregulated in glandular epithelium at the time of implantation in the rat. J. Mol. Histol. 2007, 38, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Gao, J.; Brown, N.; Reese, J. Aquaporin water channel genes are differentially expressed and regulated by ovarian steroids during the periimplantation period in the mouse. Endocrinology 2003, 144, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Qiao, Y.; Yi, F.; Guan, X.; Zhang, D.; Zhang, S.; Hao, F.; Xiao, Y.; Zhang, H.; Guo, L.; et al. Increased female fertility in aquaporin 8-deficient mice. IUBMB Life 2010, 62, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jee, B.C.; Kim, S.K.; Kim, H.; Lee, J.R.; Suh, C.S.; Kim, S.H. Expressions of aquaporin family in human luteinized granulosa cells and their correlations with IVF outcomes. Hum. Reprod. 2016, 31, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.F.; Chen, L.Y.; Xu, K.H.; Yao, J.F.; Shi, Y.F.; Shanguan, X.J. Reduced expression of aquaporin 9 in tubal ectopic pregnancy. J. Mol. Histol. 2013, 44, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Q.; Zhang, H.; Wang, Q.; Li, R.; Jin, Y.; Wang, H.; Ma, T.; Qiao, J.; Duan, E. Aquaporin-dependent excessive intrauterine fluid accumulation is a major contributor in hyper-estrogen induced aberrant embryo implantation. Cell Res. 2015, 25, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, H.; Giribabu, N.; Karim, K.; Kassim, N.; Muniandy, S.; Kumar, K.E.; Salleh, N. Quercetin interferes with the fluid volume and receptivity development of the uterus in rats during the peri-implantation period. Reprod. Toxicol. 2017, 71, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Marunaka, Y. Actions of quercetin, a flavonoid, on ion transporters: Its physiological roles. Ann. N. Y. Acad. Sci. 2017, 1398, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Skowronska, A.; Mlotkowska, P.; Majewski, M.; Nielsen, S.; Skowronski, M.T. Expression of aquaporin 1 and 5 and their regulation by ovarian hormones, arachidonic acid, forskolin and cAMP during implantation in pigs. Physiol. Res. 2016, 8, 637–650. [Google Scholar]
- Brace, R.A.; Vermin, M.L.; Huijssoon, E.I. Regulation of amniotic fluid volume: Intramembranous solute and volume fluxes in late gestation fetal sheep. Am. J. Obstet. Gynecol. 2004, 191, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Yasui, M. Cellular and subcellular localization of aquaporins 1, 3, 8, and 9 in amniotic membranes during pregnancy in mice. Cell Tissue Res. 2010, 342, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Prat, C.; Blanchon, L.; Borel, V.; Gallot, D.; Herbet, A.; Bouvier, D.; Marceau, G.; Sapin, V. Ontogeny of aquaporins in human fetal membranes. Biol. Reprod. 2012, 86, 48. [Google Scholar] [CrossRef] [PubMed]
- Sha, X.Y.; Xiong, Z.F.; Liu, H.S.; Di, X.D.; Ma, T.H. Maternal-fetal fluid balance and aquaporins: From molecule to physiology. Acta Pharmacol. Sin. 2011, 32, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Misaka, T.; Tanaka, Y.; Matsumoto, I.; Ishibashi, K.; Sasaki, S.; Abe, K. Aquaporin-11 knockout mice and polycystic kidney disease animals share a common mechanism of cyst formation. FASEB J. 2008, 22, 3672–3684. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, D.; Rouzaire, M.; Marceau, G.; Prat, C.; Pereira, B.; Lemarié, R.; Deruelle, P.; Fajardy, I.; Gallot, D.; Blanchon, L.; et al. Aquaporins and Fetal Membranes From Diabetic Parturient Women: Expression Abnormalities and Regulation by Insulin. J. Clin. Endocrinol. Metab. 2015, 100, E1270–E1279. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Koukoulas, I.; Ross, M.C.; Wang, S.; Wintour, E.M. Quantitative comparison of placental expression of three aquaporin genes. Placenta 2004, 25, 475–478. [Google Scholar] [CrossRef] [PubMed]
- De Falco, M.; Cobellis, L.; Torella, M.; Acone, G.; Varano, L.; Sellitti, A.; Ragucci, A.; Coppola, G.; Cassandro, R.; Laforgia, V.; et al. Down-regulation of aquaporin 4 in human placenta throughout pregnancy. In Vivo 2007, 21, 813–817. [Google Scholar] [PubMed]
- Escobar, J.; Gormaz, M.; Arduini, A.; Gosens, K.; Martinez, A.; Perales, A.; Escrig, R.; Tormos, E.; Roselló, M.; Orellana, C.; et al. Expression of aquaporins early in human pregnancy. Early Hum. Dev. 2012, 88, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Jiang, S.; Hu, Y.; Zheng, X.; Zou, S.; Wang, Y.; Zhu, X. The expression of aquaporin 8 and aquaporin 9 in fetal membranes and placenta in term pregnancies complicated by idiopathic polyhydramnios. Early Hum. Dev. 2010, 86, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.S.; Zhu, X.J.; Ding, S.D.; Wang, J.J.; Jiang, L.L.; Jiang, W.X.; Zhu, X.Q. Expression and localization of aquaporins 8 and 9 in term placenta with oligohydramnios. Reprod. Sci. 2012, 19, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Belkacemi, L.; Desai, M.; Beall, M.H.; Liu, Q.; Lin, J.T.; Nelson, D.M.; Ross, M.G. Early compensatory adaptations in maternal undernourished pregnancies in rats: Role of the aquaporins. J. Matern. Fetal Neonatal Med. 2011, 24, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Vilariño-García, T.; Pérez-Pérez, A.; Dietrich, V.; Guadix, P.; Dueñas, J.L.; Varone, C.L.; Damiano, A.E.; Sánchez-Margalet, V. Gynecol Endocrinol. Leptin upregulates aquaporin 9 expression in human placenta in vitro. Gynecol. Endocrinol. 2017, 23, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lin, L.; Lai, A. Expression and significance of aquaporin-2 and serum hormones in placenta of patients with preeclampsia. J. Obstet. Gynaecol. 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Szpilbarg, N.; Castro-Parodi, M.; Reppetti, J.; Repetto, M.; Maskin, B.; Martinez, N.; Damiano, A.E. Placental programmed cell death: Insights into the role of aquaporins. Mol. Hum. Reprod. 2016, 22, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Ducza, E.; Seres, A.B.; Hajagos-Tóth, J.; Falkay, G.; Gáspár, R. Oxytocin regulates the expression of aquaporin 5 in the late-pregnant rat uterus. Mol. Reprod. Dev. 2014, 81, 524–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helguera, G.; Eghbali, M.; Sforza, D.; Minosyan, T.Y.; Toro, L.; Stefani, E. Changes in global gene expression in rat myometrium in transition from late pregnancy to parturition. Physiol. Genom. 2009, 8, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Csányi, A.; Bóta, J.; Falkay, G.; Gáspár, R.; Ducza, E. The Effects of Female Sexual Hormones on the Expression of Aquaporin 5 in the Late-Pregnant Rat Uterus. Int. J. Mol. Sci. 2016, 17, 1300. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Brown, N.; Mahendroo, M.S.; Reese, J. Utilization of Different Aquaporin Water Channels in the Mouse Cervix during Pregnancy and Parturition and in Models of Preterm and Delayed Cervical Ripening. Endocrinology 2006, 147, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Soh, Y.M.; Tiwari, A.; Mahendroo, M.; Conrad, K.P.; Parry, L.J. Relaxin regulates hyaluronan synthesis and aquaporins in the cervix of late pregnant mice. Endocrinology 2012, 153, 6054–6064. [Google Scholar] [CrossRef] [PubMed]
| Sites of Expression | AQP1 | AQP2 | AQP3 | AQP4 | AQP5 | AQP6 | AQP7 | AQP8 | AQP9 | AQP11 | AQP12 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oocytes/blastocytes (implantation) | h-gc-R | h-gc-R | h-gc-R | h-gc-R | h-gc-R | h-gc-R | h-gc-R | m-icm-P | h-gc-R m-mt-P | h-gc-R | h-gc-R |
| Uterus (implantation) | r-mm-P m-mm-P+R p-P | h-em-P+R | h-em-P+R | m-mm-P+R c-em-R | m-mm-P+R r-mm-P p-P | - | - | m-mm-P | r-P+R p-P | - | - |
| Amnionic membrane | h-P+R o-P+R m-fc-P | - | h-P+R o-P+R m-ec, fc-P | - | - | - | - | h-P+R o-P+R m-ec-P | h-P+R o-P+R m-aec-P | h-P+R o-P+R | - |
| Placenta | h-pv-R r-bz, lz-P m-vec-P+R o-vec-P+R | - | h-pv-R r-R m-tec-P+R o-tec-P+R | h-pv-R h-st, ec-P | h-pv-R | - | - | h-pv-R r-bz, lz-P m-P+R o-tec-R | h-pv-R r-cp, bz, lz-P m-R | h-pv-R | - |
| Uterus (late pregnant) | r-P+R | r-P+R | r-P+R | r-P+R | - | - | r-P+R | r-P+R | - | - | |
| Cervix (late pregnant) | - | - | m-bcl-P+R | m-acl P+R | m-acl P+R | - | - | m-acl P+R | - | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ducza, E.; Csányi, A.; Gáspár, R. Aquaporins during Pregnancy: Their Function and Significance. Int. J. Mol. Sci. 2017, 18, 2593. https://doi.org/10.3390/ijms18122593
Ducza E, Csányi A, Gáspár R. Aquaporins during Pregnancy: Their Function and Significance. International Journal of Molecular Sciences. 2017; 18(12):2593. https://doi.org/10.3390/ijms18122593
Chicago/Turabian StyleDucza, Eszter, Adrienn Csányi, and Róbert Gáspár. 2017. "Aquaporins during Pregnancy: Their Function and Significance" International Journal of Molecular Sciences 18, no. 12: 2593. https://doi.org/10.3390/ijms18122593
APA StyleDucza, E., Csányi, A., & Gáspár, R. (2017). Aquaporins during Pregnancy: Their Function and Significance. International Journal of Molecular Sciences, 18(12), 2593. https://doi.org/10.3390/ijms18122593