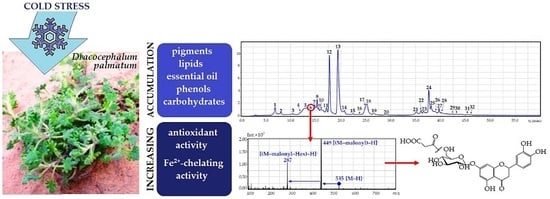
Effect of Low Temperature Cultivation on the Phytochemical Profile and Bioactivity of Arctic Plants: A Case of Dracocephalum palmatum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phenotypic Changes, Electrolyte Leakage, Photosynthetic Pigment Content and Parameters of Photosynthesis of D. palmatum during Low-Temperature (LT) Cultivation
2.2. Changes of the Fatty Acids of D. palmatum during LT-Cultivation
2.3. Changes of the Essential Oil Profile of D. palmatum during LT-Cultivation
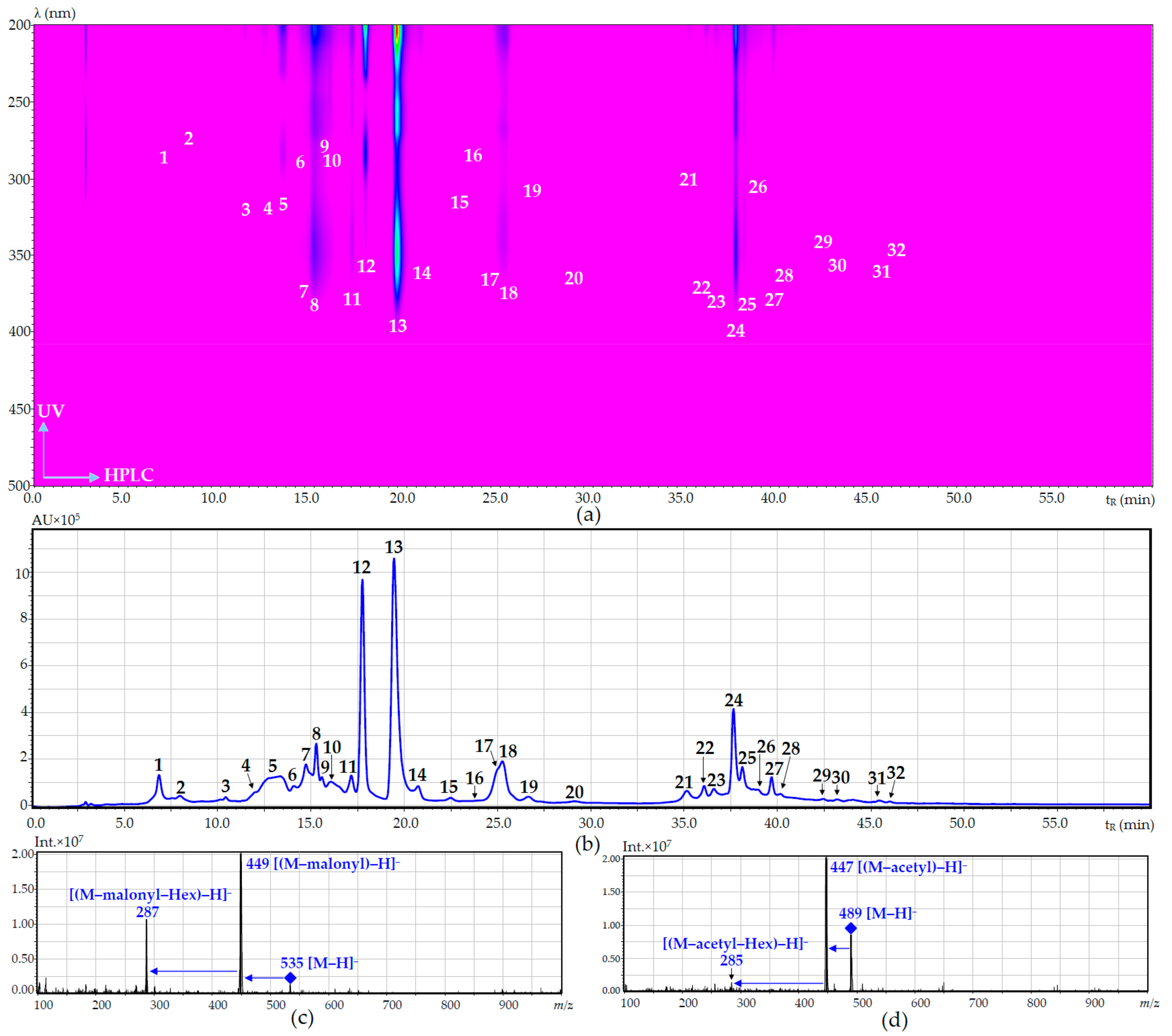
2.4. Changes of Phenolic Compounds of D. palmatum during LT-Cultivation
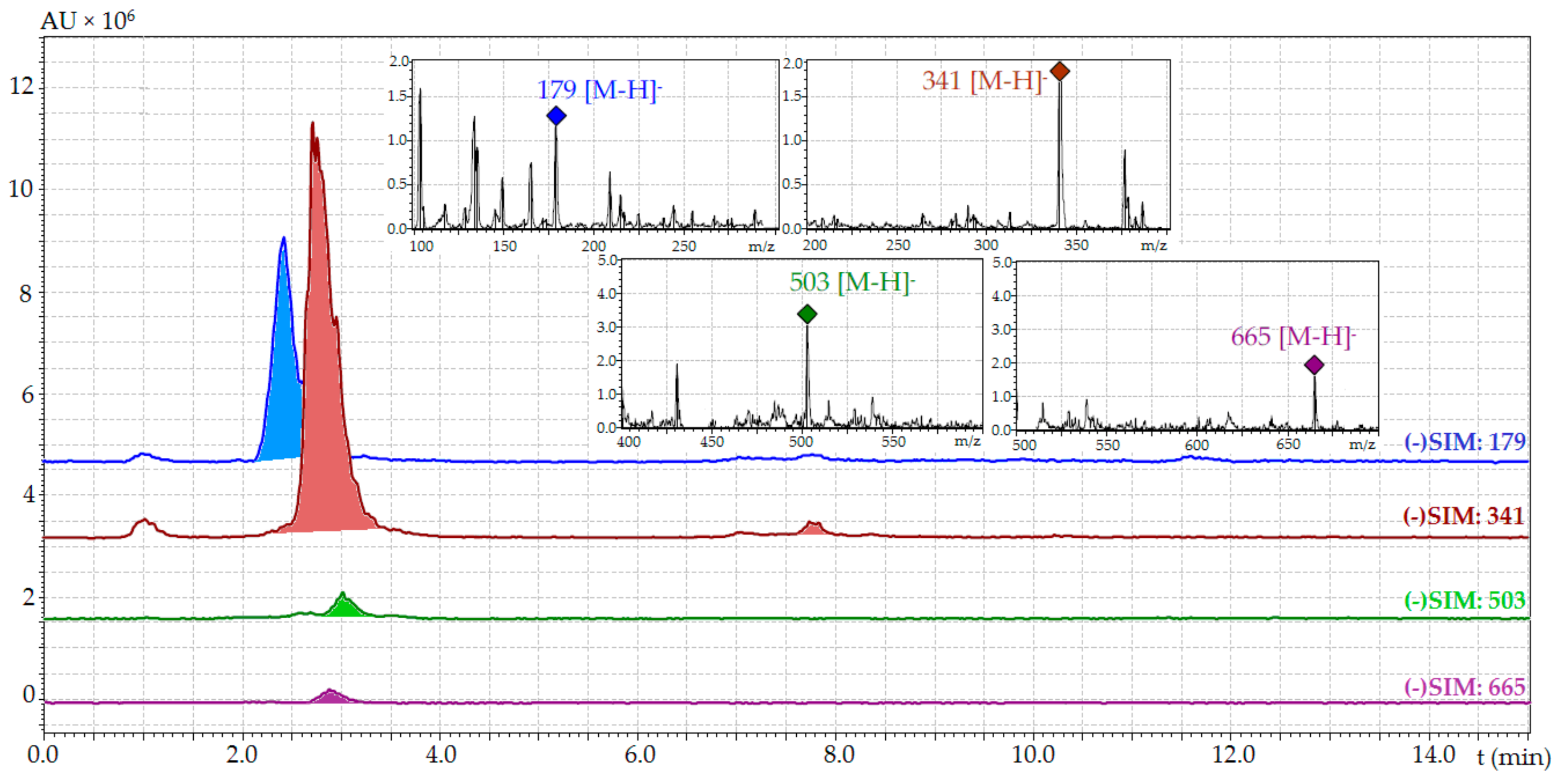
2.5. Changes of Carbohydrates of D. palmatum during LT-Cultivation
2.6. Changes of Malondialdehyde, Antioxidant Enzymes and Antioxidant Potential of D. palmatum during LT-Cultivation
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material, Growth Conditions, and Stress Treatments
3.3. Electrolyte Leakage
3.4. Photosynthetic Pigments Analysis
3.5. Chlorophyll Fluorescence and Carbon Assimilation Rate Measurement
3.6. Fatty Acids Analysis
3.7. Essential Oil Analysis
3.8. Phenolic Compounds Analysis
3.9. Carbohydrates Analysis
3.9.1. RP-HPLC-MS Analysis of Free Sugars
3.9.2. Raw Water Soluble Polysaccharide (RWSP) Analysis
3.10. Antioxidant Activity Assays
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Eremina, M.; Rozhon, W.; Poppenberger, B. Hormonal control of cold stress responses in plants. Cell. Mol. Life Sci. 2016, 73, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Guy, C.; Kaplan, F.; Kopka, J.; Selbig, J.; Hincha, D.K. Metabolomics of temperature stress. Physiol. Plant. 2008, 132, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Safronov, V.M. Climate change and mammals of Yakutia. Biol. Bull. 2016, 43, 1256–1270. [Google Scholar] [CrossRef]
- Desyatkin, R.; Fedorov, A.; Desyatkin, A.; Konstantinov, P. Air temperature changes and their impact on permafrost ecosystems in Eastern Siberia. Therm. Sci. 2015, 19, S351–S360. [Google Scholar] [CrossRef]
- Doron’kin, V.M.; Kovtonyuk, N.K.; Zuev, V.V. Flora of Siberia, 11; Nauka: Novosibirsk, Russia, 1997; pp. 170–185. [Google Scholar]
- Efremova, M.I.; Chirikova, N.K. Medicinal and Food Plants of Yakutia as Renewable Resources of Arctic; SVFU: Yakutsk, Russia, 2015; pp. 195–197. [Google Scholar]
- Olennikov, D.N.; Chirikova, N.K.; Okhlopkova, Z.M.; Zulfugarov, I.S. Chemical composition and antioxidant activity of Tánara Ótó (Dracocephalum palmatum Stephan), a medicinal plant used by the North-Yakutian Nomads. Molecules 2013, 18, 14105–14121. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Chirikova, N.K. Dracopalmaside, a new flavonoid from Dracocephalum palmatum. Chem. Nat. Compd. 2015, 51, 1067–1069. [Google Scholar] [CrossRef]
- Hussein, M.S.; El-Sherbeny, S.E.; Khalil, M.Y.; Naguib, N.Y.; Aly, S.M. Growth characters and chemical constituents of Dracocephalum moldavica L. plants in relation to compost fertilizer and planting distance. Sci. Hortic. 2006, 108, 322–331. [Google Scholar] [CrossRef]
- Zeng, Q.; Jin, H.-Z.; Fu, J.-J.; Qin, J.-J.; Hu, X.-J.; Liu, J.-H.; Yan, L.; Chen, M.; Zhang, W.-D. Chemical constituents of plants from the genus Dracocephalum. Chem. Biodivers. 2010, 7, 1911–1929. [Google Scholar] [CrossRef] [PubMed]
- Alaei, S.; Khosh-Khui, M.; Kobraee, S.; Zaji, B. Effect of different salinity levels on essential oil content and composition of Dracocephalum moldavica. Agric. Commun. 2014, 2, 42–46. [Google Scholar]
- Said-Al Ahl, H.A.H.; Abdou, M.A.A. Impact of water stress and phosphorus fertilizer on fresh herb and essential oil content of dragonhead. Int. Agrophys. 2009, 23, 403–407. [Google Scholar]
- Zhang, C.J.; Li, H.Y.; Yun, T.; Fu, Y.H.; Liu, C.M.; Gong, B.; Neng, B.J. Chemical composition, antimicrobial and antioxidant activities of the essential oil of Tibetan herbal medicine Dracocephalum heterophyllum Benth. Nat. Prod. Res. 2008, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alaei, S.; Mahna, N. Comparison of essential oil composition in Dracocephalum moldavica in greenhouse and field. J. Essent. Oil Bear. Plants 2013, 16, 346–351. [Google Scholar] [CrossRef]
- Monsef-Esfahani, H.R.; Karamkhani, F.; Nickavar, B.; Abdi, K.; Faramarzi, M.A. The volatile constituents of Dracocephalum kotschyi oils. Chem. Nat. Compd. 2007, 43, 40–43. [Google Scholar] [CrossRef]
- Murillo-Amador, B.; Morales-Prado, L.E.; Troyo-Diéguez, E.; Córdoba-Matson, M.V.; Hernández-Montiel, L.G.; Rueda-Puente, E.O.; Nieto-Garibay, A. Changing environmental conditions and applying organic fertilizers in Origanum vulgare L. Front. Plant Sci. 2015, 6, 549. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Amador, B.; Nieto-Garibay, A.; López-Aguilar, R.; Troyo-Diéguez, E.; Rueda-Puente, E.O.; Flores-Hernández, A.; Ruiz-Espinosa, F.H. Physiological, morphometric characteristics and yield of Origanum vulgare L. and Thymus vulgaris L. exposed to open-field and shade-enclosure. Ind. Crop. Prod. 2013, 49, 659–667. [Google Scholar] [CrossRef]
- Georgieva, K.; Lichtenthaler, H.K. Photosynthetic response of different pea cultivars to low and high temperature treatments. Photosynthetica 2006, 44, 569–578. [Google Scholar] [CrossRef]
- Glaszmann, J.C.; Kaw, R.N.; Khush, G.S. Genetic divergence among cold tolerant rices (Oryza satva L.). Euphytica 1990, 45, 95–104. [Google Scholar] [CrossRef]
- Krol, M.; Huner, N.P.A.; McIntosh, A. Chloroplast biogenesis at cold hardening temperatures. Development of photosystem I and photosystem II activities in relation to pigment accumulation. Photosynth. Res. 1988, 14, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Goli, S.A.H.; Sahafi, S.M.; Rashidi, B.; Rahimmalek, M. Novel oilseed of Dracocephalum kotschyi with high n-3 to n-6 polyunsaturated fatty acid ratio. Ind. Crop. Prod. 2013, 43, 188–193. [Google Scholar] [CrossRef]
- Domokos, J.; Peredi, J.; Halasz-Zelnik, K. Characterization of seed oils of dragonhead (Dracocephalum moldavica L.) and catnip (Nepeta cataria var. citriodora Balb.). Ind. Crop. Prod. 1994, 3, 91–94. [Google Scholar] [CrossRef]
- Marin, P.D.; Sajdl, V.; Kapor, S.; Tatic, B.; Petkovic, B. Fatty acids of the Saturejoideae, Ajugoideae and Scutellarioideae (Lamiaceae). Phytochemistry 1991, 30, 2979–2982. [Google Scholar] [CrossRef]
- Tulucku, E. Herbal tea fatty acid content of some medicinal plants grown in Konya, Turkey. Asian J. Chem. 2011, 23, 1369–1379. [Google Scholar]
- Ahmad, P. Oilseed Crops: Yield and Adaptations under Environmental Stress; John Wiley & Sons Ltd.: Chichester, UK, 2017; pp. 80–91. [Google Scholar]
- Schulte, L.R.; Ballard, T.; Samarakoon, T.; Yao, L.; Vadlani, P.; Staggenborg, S.; Rezac, M. Increased growing temperature reduces content of polyunsaturated fatty acids in four oilseed crops. Ind. Crop. Prod. 2013, 51, 212–219. [Google Scholar] [CrossRef]
- Tajabadi, F.; Khalighi-Sigaroodi, F.; Rezazadech, S. Improving gas chromatography–mass spectrometry analysis of essential oils by multivariate curve resolution: Full identification of co-eluting compounds of Dracocephalum moldavica L. Chromatographia 2017, 80, 1069–1077. [Google Scholar] [CrossRef]
- Nezhadali, A.; Khazaeifar, A.; Akbarpour, M.; Masrournia, M. Chemical composition of essential oil and antibacterial activity of Dracocephalum subcapitatum. J. Essent. Oil Bear. Plants 2010, 13, 112–117. [Google Scholar] [CrossRef]
- Sonboli, A.; Esmaeili, M.A.; Gholipour, A.; Kanani, M.R. Composition, cytotoxicity and antioxidant activity of the essential oil of Dracocephalum surmandinum from Iran. Nat. Prod. Commun. 2010, 5, 341–344. [Google Scholar] [PubMed]
- Esmaeli, M.A.; Sonboli, A.; Mirjalili, M.H. Oxidative stress protective effect of Dracocephalum multicaule essential oil against human cancer cell line. Nat. Prod. Res. 2014, 28, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Sonboli, A.; Gholipour, A.; Yousefzadi, M. Antibacterial activity of the essential oil and main components of two Dracocephalum species from Iran. Nat. Prod. Res. 2012, 26, 2121–2125. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Cha, K.H.; Kim, S.N.; Altantsetseg, S.; Shatar, S.; Sarangerel, O.; Nho, C.W. The antimicrobial activity of essential oil from Dracocephalum foetidum against pathogenic microorganisms. J. Microbiol. 2007, 45, 53–57. [Google Scholar] [PubMed]
- Mahmood, U.; Kaul, V.K.; Singh, V.; Brij, L.; Negi, H.R.; Ahuja, P.S. Volatile constituents of the cold desert plant Dracocephalum heterophyllum Benth. Flavour Fragr. J. 2005, 20, 173–175. [Google Scholar] [CrossRef]
- Suleimen, E.M.; Myrzagalieva, A.B.; Ibataev, Z.A.; Iskakova, Z.B.; Samarkhanov, T.N.; Medeubaeva, B.Z. Constituent composition and biological activity of essential oil from Dracocephalum peregrinum. Chem. Nat. Compd. 2017, 53, 173–174. [Google Scholar] [CrossRef]
- Sadraei, H.; Asghari, G.; Kasiri, F. Comparison of antispasmodic effects of Dracocephalum kotschyi essential oil, limonene and α-terpineol. Res. Pharm. Sci. 2015, 10, 109–116. [Google Scholar] [PubMed]
- Ahmadi, L.; Mirza, M. Volatile constituents of Dracocephalum aucherry Boiss. J. Essent. Oil Res. 2001, 13, 202–203. [Google Scholar] [CrossRef]
- Agarwal, S.G.; Kapahi, B.K.; Thappa, R.K. Essential oil constituents of Himalayan Dracocephalum speciosum Benth. J. Essent. Oil Res. 2005, 17, 94–95. [Google Scholar] [CrossRef]
- Sangwan, N.S.A.; Farooqi, H.A.; Shabih, F.; Sangwan, R.S. Regulation of essential oil production in plants. Plant Growth Regul. 2001, 34, 3–21. [Google Scholar] [CrossRef]
- Prakasa Rao, E.V.S.; Rao, R.S.G.; Ramesh, S. Seasonal variation in oil content and its composition in two chemotypes of scented geranium (Pelaronium spp.). J. Essent. Oil Res. 1995, 7, 159–163. [Google Scholar] [CrossRef]
- Putievsky, E.; Ravid, U.; Dudai, N. The influence of season and harvest frequency on essential oil and herbal yields from a pure clone of sage grown under cultivated conditions. J. Nat. Prod. 1986, 49, 326–329. [Google Scholar] [CrossRef]
- Burbott, A.J.; Loomis, W.D. Effects of light and temperature on the monoterpenes of peppermint. Plant Physiol. 1967, 42, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.J.; Menary, R.C. Environmental effects on peppermint (M. piperita L.) effect of day length, photon flux density, night and day temperature on yield and composition of peppermint oil. Aust. J. Plant Physiol. 1980, 7, 685–692. [Google Scholar] [CrossRef]
- Qiao, J.Q.; Xu, D.; Lian, H.Z.; Ge, X. Analysis of related substances in synthetical arbutin and its intermediates by HPLC–UV and LC–ESI–MS. Res. Chem. Intermed. 2015, 41, 691–703. [Google Scholar] [CrossRef]
- Martínez-Vázquez, M.; Estrada-Reyes, R.; Martínez-Laurrabaquio, A.; López-Rubalcava, C.; Heinze, G. Neuropharmacological study of Dracocephalum moldavica L. (Lamiaceae) in mice: Sedative effect and chemical analysis of an aqueous extract. J. Ethnopharmacol. 2012, 141, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Lukas, B.; Schmiderer, C.; Mitteregger, U.; Novak, J. Arbutin in marjoram and oregano. Food Chem. 2010, 121, 185–190. [Google Scholar] [CrossRef]
- Valant-Vetschera, K.M.; Roitman, J.N.; Wollenweber, E. Chemodiversity of exudate flavonoids in some members of the Lamiaceae. Biochem. Syst. Ecol. 2003, 31, 1279–1289. [Google Scholar] [CrossRef]
- Selenge, E.; Murata, T.; Tanaka, S.; Sasaki, K.; Batkhuu, J.; Yoshizaki, F. Monoterpene glycosides, phenylpropanoids, and acacetin glycosides from Dracocephalum foetidum. Phytochemistry 2014, 101, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.-M.; Zhao, C.-C.; Jin, H.-Z.; Tang, J.; Shen, Y.-H.; Li, H.-L.; Peng, C.-Y.; Zhang, W.-D. A new ferulic acid ester and other constituents from Dracocephalum peregrinum. Arch. Pharm. Res. 2008, 31, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Zhao, C.-C.; Tang, J.; Shen, Y.-H.; Xu, X.-K.; Zhang, W.-D. New flavonoid glycosides and cyanogenic glycosides from Dracocephalum peregrinum. Chem. Pharm. Bull. 2009, 57, 207–210. [Google Scholar] [CrossRef] [PubMed]
- She, G.; Guo, Z.; Lv, H.; She, D. New flavonoid glycosides from Elsholtzia rugulosa Hemsl. Molecules 2009, 14, 4190–4196. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Chang, E.J.; Kim, H.J.; Park, J.H.; Choi, S.W. Antioxidative flavonoids from leaves of Carthamus tinctorius. Arch. Pharm. Res. 2002, 25, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Abad-García, B.; Garmón-Lobato, S.; Berrueta, L.A.; Gallo, B.; Vicente, F. On line characterization of 58 phenolic compounds in Citrus fruit juices from Spanish cultivars by high-performance liquid chromatography with photodiode-array detection coupled to electrospray ionization triple quadrupole mass spectrometry. Talanta 2012, 99, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Abad-García, B.; Berrueta, L.A.; Garmón-Lobato, S.; Gallo, B.; Vicente, F. A general analytical strategy for the characterization of phenolic compounds in fruit juices by high-performance liquid chromatography with diode array detection coupled to electrospray ionization and triple quadrupole mass spectrometry. J. Chromatogr. A 2009, 1216, 5398–5415. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Akobirshoeva, A.A. Flavonoids and phenylpropanoids of Nepeta glutinosa and Ziziphora pamiroalaica. Chem. Nat. Compd. 2016, 52, 909–912. [Google Scholar] [CrossRef]
- Fattahi, M.; Nazeri, V.; Torras-Claveria, L.; Sefidkon, F.; Cusido, R.M.; Zamani, Z.; Palazon, J. Identification and quantification of leaf surface flavonoids in wild-growing populations of Dracocephalum kotschyi by LC-DAD-ESI-MS. Food Chem. 2013, 141, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Neugart, S.; Kläring, H.-P.; Zietz, M.; Schreiner, M.; Rohn, S. The effect of temperature and radiation on flavonol aglycones and flavonol glycosides of kale (Brassica oleracea var. sabelica). Food Chem. 2012, 133, 1456–1465. [Google Scholar] [CrossRef]
- Klimov, S.V.; Burakhanova, E.A.; Dubinina, I.M.; Alieva, G.P.; Sal’nikova, E.B.; Olenichenko, N.A.; Zagoskina, N.V.; Trunova, T.I. Suppression of the source activity affects carbon distribution and frost hardiness of vegetating winter wheat plants. Russ. J. Plant Physiol. 2008, 55, 308–314. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. A novel HPLC-assisted method for investigation of the Fe2+-chelating activity of flavonoids and plant extracts. Molecules 2014, 19, 18296–18316. [Google Scholar] [CrossRef] [PubMed]
- Moheb, A.; Ibrahim, R.K.; Roy, R.; Sarhan, F. Changes in wheat leaf phenolome in response to cold acclimation. Phytochemistry 2011, 72, 2294–2307. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Husain, S.Z.; Gil, M.I. The distribution of methylated flavones in the Lamiaceae. Biochem. Syst. Ecol. 1988, 16, 43–46. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Wollenweber, E. Flavonoid aglycones of the leaf surfaces of some Labiatae species. Plant Syst. Evol. 1990, 173, 109–118. [Google Scholar] [CrossRef]
- Amid, A.; Lytovchenko, A.; Fernie, A.R.; Warren, G.; Thorlby, G.J. The sensitive to freezing3 mutation of Arabidopsis thaliana is a cold-sensitive allele of homomeric acetyl-CoA carboxylase that results in cold-induced cuticle deficiencies. J. Exp. Bot. 2012, 14, 5289–5299. [Google Scholar] [CrossRef] [PubMed]
- Yeats, T.H.; Rose, J.K.C. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Pfündel, E.E.; Agati, G.; Cerovic, Z.G. Optical properties of plant surfaces. In Biology of the Plant Cuticle; Riederer, M., Müller, C., Eds.; Blackwell: Oxford, UK, 2006. [Google Scholar]
- Theocharis, A.; Clément, C.; Barka, E.A. Physiological and molecular changes in plants grown at low temperatures. Planta 2012, 235, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.; Vergauwen, R.; Pacolet, P.; Lescrinier, E.; van den Ende, W. Manninotriose is a major carbohydrate in red deadnettle (Lamium purpureum, Lamiaceae). Ann. Bot. 2013, 111, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, H.J.; Sheveleva, E. Plant stress adaptations—Making metabolism move. Curr. Opin. Plant Biol. 1998, 1, 267–274. [Google Scholar] [CrossRef]
- Jouve, L.; Hoffmann, L.; Hausman, J.F. Polyamine, carbohydrate, and proline content changes during salt stress exposure of aspen (Populus tremula L.): Involvement of oxidation and osmoregulation metabolism. Plant Biol. 2004, 6, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Van den Ende, W. Multifunctional fructans and raffinose family oligosaccharides. Front. Plant Sci. 2013, 4, 247. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 16, 112–166. [Google Scholar] [CrossRef] [PubMed]
- Pollock, C.J.; Lloyd, E.J. The effect of low temperature upon starch, sucrose and fructan synthesis in leaves. Ann. Bot. 1987, 60, 231–235. [Google Scholar] [CrossRef]
- Yano, R.; Nakamura, M.; Yoneyama, T.; Nishid, I. Starch-related α-glucan/water dikinase is involved in the cold-induced development of freezing tolerance in Arabidopsis. Plant Physiol. 2005, 138, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Solecka, D.; Zebrowski, J.; Kacperska, A. Are involved in cold acclimation and de-acclimation of winter oil-seed rape plants? Ann. Bot. 2008, 101, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, L.; Domon, J.M.; Klimek, J.F.; Fournet, F.; Sellier, H.; Gillet, F.; Pelloux, J.; Lejeune-Hénaut, I.; Carpita, N.C.; Rayon, C. Structural alteration of cell wall pectins accompanies pea development in response to cold. Phytochemistry 2014, 104, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, Q.; Wang, S.; Hong, Y.; Wang, Z. Rice and cold stress: Methods for its evaluation and summary of cold tolerance-related quantitative trait loci. Rice 2014, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Ren, J.; Zhu, W.; Amombo, E.; Fu, J.; Chen, L. Antioxidant responses and gene expression in bermudagrass under cold stress. J. Am. Soc. Hortic. Sci. 2014, 139, 699–705. [Google Scholar]
- Soydam, A.S.; Büyük, I.; Aras, S. Relationships among lipid peroxidation, SOD enzyme activity, and SOD gene expression profile in Lycopersicum esculentum L. exposed to cold stress. Genet. Mol. Res. 2013, 12, 3220–3229. [Google Scholar] [CrossRef] [PubMed]
- Camejo, D.; Martí Mdel, C.; Nicolás, E.; Alarcón, J.J.; Jiménez, A.; Sevilla, F. Response of superoxide dismutase isoenzymes in tomato plants (Lycopersicon esculentum) during thermo-acclimation of the photosynthetic apparatus. Physiol. Plant. 2007, 131, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Esimi, N.; Atici, Ö. Relationships between some endogenous signal compounds and the antioxidant system in response to chilling stress in maize (Zea mays L.) seedlings. Turk. J. Bot. 2016, 40, 37–44. [Google Scholar] [CrossRef]
- Brown, J.E.; Rice-Evans, C.A. Luteolin-rich artichoke extract protects low density lipoprotein from oxidation in vitro. Free Radic. Res. 1998, 29, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.C.; Mihai, C.T.; Vochita, G.; Rotinberg, P.; Trifan, A.; Luca, S.V.; Petreus, T.; Gille, E.; Miron, A. Antigenotoxic and antioxidant activities of a polyphenolic extract from European Dracocephalum moldavica L. Ind. Crop. Prod. 2016, 79, 248–257. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Han, X.-Z.; Li, X.; Ren, D.-M.; Wang, X.-N.; Lou, H.-X. Flavonoids from Dracocephalum tanguticum and their cardioprotective effects against doxorubicin-induced toxicity in H9c2 cells. Bioorg. Med. Chem. Lett. 2010, 20, 6411–6415. [Google Scholar] [CrossRef] [PubMed]
- Raj, X.J.; Chaurasia, O.P.; Vajpayee, P.K.; Murugan, M.P.; Bala, S.S. Antioxidative activity and phytochemical investigation on a high altitude medicinal plant Dracocephalum heterophyllum Benth. Pharmacogn. J. 2010, 2, 112–117. [Google Scholar]
- Sharifi, B.; Goli, S.A.H.; Maghsoudlou, Y. Antioxidant activity and chemical composition of the methanolic extract and related fractions of Dracocephalum kotschyi leaves using liquid chromatography–tandem mass spectrometry. Ind. Crop. Prod. 2017, 104, 111–119. [Google Scholar] [CrossRef]
- Pouraboli, I.; Nazari, S.; Sabet, N.; Sharififar, F.; Jafari, M. Antidiabetic, antioxidant, and antilipid peroxidative activities of Dracocephalum polychaetum shoot extract in streptozotocin-induced diabetic rats: In vivo and in vitro studies. Pharm. Biol. 2016, 54, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.S.; Quartin, V.; Ramalh, J.C.; Nunes, M.A. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J. Plant Physiol. 2003, 160, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. In Current Protocols in Food Analytical Chemistry; Unit F4.3.1–Unit F4.3.8; John Wiley & Sons: Weinheim, Germany, 2010. [Google Scholar]
- Vernon, L.P. Spectrophotometric determination of chlorophylls and pheophytins in plant extracts. Anal. Chem. 1960, 32, 1144–1150. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Agafonova, S.V.; Penzina, T.A.; Borovskii, G.B. Fatty acid composition of fourteen wood-decaying basidiomycete species growing in permafrost conditions. Rec. Nat. Prod. 2014, 8, 184–188. [Google Scholar]
- Pedneault, K.; Angers, P.; Avis, T.J.; Gosselin, A.; Tweddell, R.J. Fatty acids profiles of polar and non-polar lipids of Pleurotus ostreatus and P. cornucopiae var. ‘citrino-pileatus’ grown at different temperatures. Mycol. Res. 2007, 111, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Sevag, M.G.; Lackman, D.B.; Smolens, J. The isolation of the components of Streptococcal nucleoproteins in serologically active form. J. Biol. Chem. 1938, 124, 425–436. [Google Scholar]
- Olennikov, D.N.; Tankhaeva, L.M.; Samuelsen, A.B. Quantitative analysis of polysaccharides from Plantago major using the Dreywood method. Chem. Nat. Compd. 2006, 42, 265–268. [Google Scholar] [CrossRef]
- Usov, A.T.; Bilan, M.I.; Klochkova, N.G. Polysaccharides of algae. 48. Polysaccharide composition of several calcareous red algae: Isolation of alginate from Corallina pilulitara P. et R. (Rhodophyta, Corallinaceae). Bot. Mar. 1995, 35, 43–51. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 76, 248–254. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Stolbikova, A.V.; Rokhin, A.V.; Khobrakova, V.B.; Tankhaeva, L.M. Polysaccharides from Fabaceae. V. α-Glucan from Sophora flavescens roots. Chem. Nat. Compd. 2011, 47, 1–6. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Tankhaeva, L.M. A quantitative assay for total fructans in burdock (Arctium spp.) roots. Russ. J. Bioorg. Chem. 2011, 37, 893–898. [Google Scholar] [CrossRef]
- Togola, A.; Inngjerdingen, M.; Diallo, D.; Barsett, H.; Rolstad, B. Polysaccharides with complement fixing and macrophage stimulation activity from Opilia celtidifolia, isolation and partial characterization. J. Ethnopharmacol. 2007, 115, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Song, C.; Xia, L.; Wang, X.; Suo, Y.; You, J. Comprehensive comparisons between 1-phenyl-3-methyl-5-pyrazolones, 1-(4-methoxyphenyl)-3-methyl-5-pyrazolones and 1-(2-naphthyl)-3-methyl-5-pyrazolones as labeling reagents used in LC-DAD-ESI-MS-MS analysis of neutral aldoses and uronic acids. Chromatographia 2010, 71, 789–797. [Google Scholar] [CrossRef]
- Chen, J.W.; Cao, K.F. Changes in activities of antioxidative system and monoterpene and photochemical efficiency during seasonal leaf senescence in Hevea brasiliensis trees. Acta Physiol. Plant. 2008, 30, 1–9. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Abassi, N.A.; Kushad, M.M.; Endress, A.G. Active oxygen-scavenging enzymes activities in developing apple flowers and fruits. Sci. Hortic. 1998, 74, 183–184. [Google Scholar] [CrossRef]
- Preito, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. Meadowsweet teas as new functional beverages: Comparative analysis of nutrients, phytochemicals and biological effects of four Filipendula species. Molecules 2017, 27, 16. [Google Scholar] [CrossRef] [PubMed]
- Ozen, T.; Demirtas, I.; Aksit, H. Determination of antioxidant activities of various extracts and essential oil compositions of Thymus praecox subsp. skorpilii var. skorpilii. Food Chem. 2011, 124, 58–64. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, D.; Jusha, M.; Saroha, K.; Singif, N.; Vashishta, B. Antioxidant and free radical scavenging potential of Citrullus colocynthis (L.) Schrad. methanolic fruit extract. Acta Pharm. 2008, 58, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Badami, S.; Channabasavaraj, K.P. In vitro antioxidant activity of thirteen medicinal plants of India’s Western Ghats. Pharm. Biol. 2007, 45, 392–396. [Google Scholar] [CrossRef]

| Parameter | Temperature (°C) | |
|---|---|---|
| 20 | 1 | |
| Electrolyte leakage, % of total electrolytes | 18.2 ± 0.9 a | 26.7 ± 1.4 a |
| Chlorophyll a content (Chla), µg/g FW | 273.45 ± 9.29 a | 406.19 ± 13.40 a |
| Chlorophyll b content (Chlb), µg/g FW | 75.85 ± 2.50 a | 141.21 ± 4.79 a |
| Total chlorophylls content (ΣChl), µg/g FW | 349.30 | 547.40 |
| Chla/Chlb | 3.61 | 2.88 |
| Pheophytin a content, µg/g FW | 6.01 ± 0.16 a | 7.06 ± 0.14 a |
| Pheophytin b content, µg/g FW | 4.57 ± 0.10 a | 5.64 ± 0.11 a |
| Total pheophytins content, µg/g FW | 10.58 | 12.70 |
| Carotenoids content (Car), µg/g DW | 36.86 ± 1.07 a | 53.31 ± 1.55 a |
| ΣChl/Car | 9.48 | 10.27 |
| Carbon assimilation rate, µM CO2/m2·s | 8.3 ± 0.8 b | 5.8 ± 0.4 b |
| Fv/Fm | 0.62 ± 0.04 b | 0.54 ± 0.03 b |
| Compound | Temperature (°C) | |
|---|---|---|
| 20 | 1 | |
| Pelargonic acid (9:0) | 0.1 | Tr. |
| Capric acid (10:0) | 0.2 | Tr. |
| Lauric acid (12:0) | 5.7 | 3.2 |
| Tridecylic acid (13:0) | 1.4 | 0.5 |
| Myristic acid (14:0) | 2.7 | 1.7 |
| Pentadecylic acid (15:0) | 0.4 | 0.3 |
| Palmitic acid (16:0) | 27.9 | 11.2 |
| Palmitoleic acid (16:1) | 3.8 | 6.9 |
| Margaric acid (17:0) | 1.8 | 1.4 |
| Stearic acid (18:0) | 8.0 | 5.2 |
| Oleic acid (18:1 ω-9) | 11.3 | 18.3 |
| Linoleic acid (18:2 ω-6) | 14.1 | 19.3 |
| α-Linolenic acid (18:3 ω-3) | 12.5 | 18.2 |
| γ-Linolenic acid (18:3 ω-6) | 2.1 | 7.4 |
| Arachidic acid (20:0) | 4.5 | 0.5 |
| Gondoic acid (20:1 ω-9) | 2.0 | 3.7 |
| Behenic acid (22:0) | 0.5 | Tr. |
| Erucic acid (22:1 ω-9) | 0.7 | 1.2 |
| Lignoceric acid (24:0) | 0.1 | Tr. |
| Total | 99.8 | 99.0 |
| Saturated FA | 53.3 | 24.0 |
| Unsaturated FA | 46.5 | 75.0 |
| Monounsaturated FA | 17.8 | 30.1 |
| Polyunsaturated FA | 28.7 | 44.9 |
| Compound | RI | MI a | Temperature (°C) | |
|---|---|---|---|---|
| 20 | 1 | |||
| Aliphatic compounds | ||||
| Isoamyl acetate | 875 | i, ii, iii | 0.6 | 0.5 |
| Subtotal | 0.6 | 0.5 | ||
| Simple phenols | ||||
| p-Cymene | 1024 | i, ii, iii | 1.7 | 1.8 |
| p-Cymene-8-ol | 1186 | i, ii | 1.9 | 2.0 |
| p-Cumenol | 1222 | i, ii, iii | 0.4 | 0.4 |
| m-Cumenol | 1225 | i, ii | 0.1 | 0.1 |
| Cuminaldehyde | 1241 | i, ii, iii | 1.2 | 1.2 |
| Subtotal | 5.3 | 5.5 | ||
| Monoterpenes | ||||
| α-Thujene | 926 | i, ii | 0.6 | 0.4 |
| α-Pinene | 932 | i, ii, iii | 1.1 | 1.2 |
| Camphene | 947 | i, ii, iii | 0.1 | 0.2 |
| Sabinene | 973 | i, ii, iii | 2.5 | 2.0 |
| β-Pinene | 975 | i, ii, iii | 8.6 | 9.0 |
| β-Myrcene | 991 | i, ii, iii | 0.6 | 0.2 |
| Pseudolimonene | 1003 | i, ii | 0.3 | 0.2 |
| β-Phellandrene | 1027 | i, ii, iii | 4.8 | 5.2 |
| Limonene | 1029 | i, ii, iii | 1.8 | 1.8 |
| 1,8-Cineol | 1031 | i, ii, iii | 5.5 | 5.8 |
| γ-Terpinene | 1058 | i, ii, iii | 0.2 | 0.3 |
| Linalool | 1100 | i, ii, iii | 1.2 | 1.4 |
| β-Pinone | 1105 | i, ii | 0.8 | 1.0 |
| trans-Pinocarveol | 1138 | i, ii, iii | 1.0 | 1.4 |
| trans-Pinocamphone | 1161 | i, ii | 37.9 | 40.7 |
| cis-Pinocamphone | 1175 | i, ii | 8.0 | 8.7 |
| cis-Pinocarveol | 1186 | i, ii | 0.2 | 0.5 |
| Terpinene-4-ol | 1177 | i, ii, iii | 0.5 | 0.4 |
| Myrtenol | 1197 | i, ii | 2.7 | 3.3 |
| Phellandral | 1276 | i, ii | 0.2 | 0.1 |
| Bornyl acetate | 1287 | i, ii, iii | 0.3 | 0.2 |
| trans-Pinocarvyl acetate | 1301 | i, ii | 1.8 | 2.0 |
| cis-Pinocarvyl acetate | 1315 | i, ii | 0.4 | 0.7 |
| Myrtenyl acetate | 1327 | i, ii, iii | 3.5 | 3.7 |
| p-Mentha-1,4-dien-7-ol | 1329 | i, ii | 0.4 | 0.1 |
| Subtotal | 85.0 | 90.8 | ||
| Sesquiterpenes | ||||
| β-Caryophyllene | 1420 | i, ii, iii | 1.0 | 0.4 |
| γ-Cadinene | 1518 | i, ii | 0.5 | Tr. |
| Germacrene B | 1560 | i, ii | 0.7 | 0.1 |
| Caryophyllene oxide | 1587 | i, ii, iii | 1.8 | 0.5 |
| Viridiflorol | 1594 | i, ii | 3.2 | 1.6 |
| α-Cadinol | 1659 | i, ii | 1.2 | 0.3 |
| Germacrone | 1696 | i, ii | 0.6 | 0.2 |
| Subtotal | 9.0 | 3.1 | ||
| Total | 99.9 | 99.9 | ||
| No. | Compound | tR (min) | UV, λmax (nm) | ESI-MS (m/z) | Refs. Comp.a |
|---|---|---|---|---|---|
| 1 | O-Malonyl-arbutin | 6.79 | 280 | 381 [M + Na]+, 352 [M + H]+, 295 [M + Na]+ | iii [45] |
| 2 | Arbutin | 7.89 | 280 | 295 [M + Na]+, 273 [M + H]+ | i |
| 3 | 5-O-Caffeoylquinic acid | 10.43 | 331 | 353 [M − H]−, 183 | i |
| 4 | 3-O-Caffeoylquinic acid | 12.03 | 331 | 353 [M − H]−, 183 | i |
| 5 | Caffeic acid | 13.15 | 323 | 179 [M − H]− | i |
| 6 | Eriodictyol-O-malonyl-hexoside (isomer) | 14.09 | 284 | 535 [M − H]−, 449 [(M − malonyl) − H]−, 287 [(M − malonyl − Hex) − H]− | - |
| 7 | Luteolin-7,4′-di-O-rutinoside (dracopalmaside) | 14.87 | 253, 265, 345 | 905 [M − H]−, 447 [(M − Rha − Glc) − H]−, 285 [(M – 2 × Rha – 2 × Glc) − H]− | ii [9] |
| 8 | Luteolin-7-O-rutinoside-4′-O-glucoside (cynarotriside) | 15.02 | 253, 265, 345 | 755 [M − H]−, 609 [(M − Rha) − H]−, 447 [(M − Rha − Glc) − H]−, 285 [(M − Rha – 2 × Glc) − H]− | ii [9] |
| 9 | Eriodictyol-O-malonyl-hexoside (isomer) | 15.41 | 283 | 535 [M − H]−, 449 [(M − malonyl) − H]−, 287 [(M − malonyl − Hex) − H]− | - |
| 10 | Eriodictyol-7-O-rutinoside (eriocitrin) | 16.21 | 284 | 595 [M − H]−, 449 [(M − Rha) − H]−, 287 [(M − Rha − Glc) − H]− | i |
| 11 | Luteolin-7-O-rutinoside (scolymoside) | 17.02 | 252, 262, 345 | 593 [M − H]−, 447 [(M − Rha) − H]−, 285 [(M − Rha − Glc) − H]− | i |
| 12 | Eriodictyol-7-O-glucoside | 17.58 | 283 | 899 [2M − H]−, 449 [M − H]−, 287 [(M − Glc) − H]− | i |
| 13 | Luteolin-7-O-glucoside (cynaroside) | 19.48 | 254, 267, 348 | 895 [2M − H]−, 447 [M − H]−, 285 [(M − Glc) − H]− | i |
| 14 | Luteolin-4′-O-glucoside | 20.64 | 260, 335 | 447 [M − H]−, 285 [(M − Glc) − H]− | i |
| 15 | Apigenin-7-O-rutinoside (isorhoifolin) | 22.51 | 266, 334 | 577 [M − H]−, 431 [(M − Rha) − H]−, 269 [(M − Rha − Glc) − H]− | i |
| 16 | Naringenin-7-O-glucoside (prunin) | 23.89 | 283 | 433 [M − H]−, 271 [(M − Glc) − H]− | i |
| 17 | Apigenin-7-O-glucoside (cosmosiin) | 24.47 | 267, 336 | 863 [2M − H]−, 431 [M − H]−, 269 [(M − Glc) − H]− | i |
| 18 | Apigenin-O-hexoside | 25.31 | 265, 334 | 431 [M − H]−, 269 [(M − Glc) − H]− | iii [51] |
| 19 | Rosmarinic acid | 27.26 | 327 | 359 [M − H]−, 183 | i |
| 20 | Luteolin-O-acetyl-hexoside | 29.11 | 251, 263, 346 | 489 [M − H]−, 447 [(M − acetyl) − H]−, 285 [(M − acetyl − Hex) − H]− | iii [52] |
| 21 | Eriodictyol | 35.03 | 283 | 287 [M − H]− | i |
| 22 | Acacetin-7-O-rutinoside (linarin) | 36.14 | 267, 330 | 591 [M − H]−, 445 [(M − Rha) − H]−, 283 [(M − Rha − Glc) − H]− | i |
| 23 | Acacetin-7-O-glucoside (tilianin) | 36.72 | 266, 330 | 445 [M − H]−, 283 [(M − Glc) − H]− | i |
| 24 | Luteolin | 37.72 | 253, 266, 347 | 285 [M − H]− | i |
| 25 | Acacetin-O-acetyl-hexoside | 38.10 | 266, 331 | 487 [M − H]−, 445 [(M − acetyl) − H]−, 283 [(M − acetyl − Hex) − H]− | iii [52] |
| 26 | Naringenin | 38.32 | 283 | 271 [M − H]− | i |
| 27 | Apigenin | 39.75 | 267, 336 | 269 [M − H]− | i |
| 28 | Chrysoeriol | 40.21 | 266, 347 | 299 [M − H]− | i |
| 29 | Acacetin | 42.34 | 267, 330 | 283 [M − H]− | i |
| 30 | Isothymusin | 43.06 | 302, 330 | 329 [M − H]− | ii [46] |
| 31 | Salvigenin | 45.81 | 273, 330 | 327 [M − H]− | i |
| 32 | Genkwanin | 46.04 | 267, 335 | 283 [M − H]− | i |
| Compound (No of Compounds) | Temperature (°C) | |
|---|---|---|
| 20 | 1 | |
| Simple phenols | ||
| O-Malonyl-arbutin (1) | 0.39 ± 0.01 a | 1.24 ± 0.02 a |
| Arbutin (2) | 0.17 ± 0.00 | 0.22 ± 0.00 |
| Subtotal | 0.56 | 1.46 |
| Phenylpropanoids | ||
| 5-O-Caffeoylquinic acid (3) | 0.09 ± 0.00 | 0.05 ± 0.00 |
| 3-O-Caffeoylquinic acid (4) | 0.11 ± 0.00 | 0.09 ± 0.00 |
| Caffeic acid (5) | 0.61 ± 0.02 | 0.84 ± 0.02 |
| Rosmarinic acid (19) | 1.26 ± 0.03 | 1.68 ± 0.04 |
| Subtotal | 2.07 | 2.66 |
| Flavone glycosides. Apigenin derivatives | ||
| Apigenin-7-O-rutinoside (isorhoifolin, 15) | 1.11 ± 0.03 | 0.56 ± 0.02 |
| Apigenin-7-O-glucoside (cosmosiin, 17) | 0.53 ± 0.01 | 6.54 ± 0.17 |
| Apigenin-O-hexoside (18) | 0.47 ± 0.01 b | 8.34 ± 0.18 b |
| Subtotal | 2.11 | 15.44 |
| Flavone glycosides. Acacetin derivatives | ||
| Acacetin-7-O-rutinoside (linarin, 22) | 0.04 ± 0.00 | 0.06 ± 0.00 |
| Acacetin-7-O-glucoside (tilianin, 23) | 0.52 ± 0.01 | 1.27 ± 0.04 |
| Acacetin-O-acetyl-hexoside (25) | ND | 0.08 ± 0.00 c |
| Subtotal | 0.56 | 1.41 |
| Flavone glycosides. Luteolin derivatives | ||
| Luteolin-7,4′-di-O-rutinoside (dracopalmaside, 7) | 0.14 ± 0.00 | 0.52 ± 0.01 |
| Luteolin-7-O-rutinoside-4′-O-glucoside (cynarotriside, 8) | 0.82 ± 0.02 | 1.75 ± 0.04 |
| Luteolin-7-O-rutinoside (scolymoside, 11) | 2.27 ± 0.07 | 2.54 ± 0.07 |
| Luteolin-7-O-glucoside (cynaroside, 13) | 2.56 ± 0.07 | 29.56 ± 0.78 |
| Luteolin-4′-O-glucoside (14) | 0.67 ± 0.02 | 9.57 ± 0.19 |
| Luteolin-O-acetyl-hexoside (20) | ND | 0.92 ± 0.02 d |
| Subtotal | 6.46 | 44.86 |
| Flavanone glycosides. Eriodictyol derivatives | ||
| Eriodictyol-O-malonyl-hexoside (sum of 6 and 9) | ND | 1.84 ± 0.04 e |
| Eriodictyol-7-O-rutinoside (eriocitrin, 10) | 0.21 ± 0.00 | 1.35 ± 0.03 |
| Eriodictyol-7-O-glucoside (12) | 1.77 ± 0.03 | 15.82 ± 0.33 |
| Subtotal | 1.98 | 19.01 |
| Flavanone glycosides. Naringenin derivatives | ||
| Naringenin-7-O-glucoside (pruning, 16) | 1.02 ± 0.02 | 1.64 ± 0.03 |
| Subtotal | 1.02 | 1.64 |
| Flavone aglycones | ||
| Luteolin (24) | 1.19 ± 0.03 | 12.94 ± 0.30 |
| Apigenin (27) | 0.46 ± 0.01 | 1.03 ± 0.03 |
| Chrysoeriol (28) | 0.09 ± 0.00 | 0.14 ± 0.00 |
| Acacetin (29) | ND | 0.18 ± 0.00 |
| Salvigenin (31) | ND | 0.09 ± 0.00 |
| Isothymusin (30) | ND | 0.12 ± 0.00 |
| Genkwanin (32) | ND | 0.10 ± 0.00 |
| Subtotal | 1.74 | 14.60 |
| Flavanone aglycones | ||
| Eriodictyol (21) | 0.24 ± 0.00 | 0.69 ± 0.02 |
| Naringenin (26) | ND | 0.54 ± 0.01 |
| Subtotal | 0.24 | 1.23 |
| Total flavone glycosides | 9.13 | 61.71 |
| Total flavanone glycosides | 3.00 | 20.65 |
| Total flavonoids glycosides | 12.13 | 82.36 |
| Total flavonoids aglycones | 1.98 | 15.83 |
| Total flavonoids | 14.11 | 98.19 |
| Total phenolic compounds | 16.74 | 102.31 |
| Compound | tR (min) | ESI-MS (m/z) | Content (mg/g) 1 | |
|---|---|---|---|---|
| Temperature (°C) | ||||
| 20 | 1 | |||
| Glucose | 2.38 | 179 [M − H]− | 16.86 ± 0.32 | 26.39 ± 0.52 |
| Sucrose | 2.73 | 341 [M − H]− | 35.54 ± 0.78 | 169.21 ± 3.72 |
| Stachyose | 2.89 | 665 [M − H]− | 1.72 ± 0.03 | 38.95 ± 0.82 |
| Raffinose | 3.04 | 503 [M − H]− | 0.87 ± 0.02 | 9.36 ± 0.18 |
| Total content | 54.99 | 243.91 | ||
| Parameter | Temperature (°C) | |
|---|---|---|
| 20 | 1 | |
| RWSP yield (%) a | 2.29 ± 0.04 c | 9.86 ± 0.20 c |
| RWSP general characteristics | ||
| Protein content, % b | 2.61 ± 0.07 c | 2.75 ± 0.09 c |
| Uronic acids, % b | 43.57 ± 1.01 c | 46.16 ± 1.14 c |
| Reaction with I2 (starch) | positive | positive |
| Reaction with resorcinol (inulin) | negative | negative |
| Reaction with Yariv’s reagent (AGP-complexes) | positive | positive |
| Reaction with Fehling’s reagent (mannans) | negative | negative |
| RWSP monosaccharide composition (mol %) | ||
| Ara | 10.1 | 10.2 |
| Gal | 26.1 | 27.7 |
| Glc | 14.4 | 10.2 |
| Fuc | 0.1 | 0.1 |
| Man | 4.3 | 4.0 |
| Rha | 1.9 | 1.7 |
| Rib | Tr. d | Tr. d |
| Xyl | Tr. d | Tr. d |
| GalA | 41.2 | 44.6 |
| GlcA | 1.8 | 1.4 |
| Parameter | Luteolin-7-O-Glucoside | Temperature (°C) | |
|---|---|---|---|
| 20 | 1 | ||
| MDA content (nM/g) FW | - | 92.74 ± 7.41 | 197.02 ± 15.76 |
| SOD activity (U/g·min) FW | - | 57.90 ± 5.21 | 264.32 ± 12.35 |
| Catalase activity (U/g·min) FW | - | 0.93 ± 0.06 | 1.53 ± 0.12 |
| Total antioxidant capacity (mg-eq). luteolin-7-O-glucoside/g | 1000 iii | 280.98 ± 8.99 i | 682.26 ± 21.15 ii,iii |
| DPPH•-radical scavenging activity, IC50 (µg/mL) | 16.97 ± 0.34 iv,v | 33.28 ± 0.73 vi | 11.40 ± 0.24 iv |
| ABTS•+-radical scavenging activity, IC50 (µg/mL) | 9.86 ± 0.19 vii,viii | 14.62 ± 0.31 viii | 5.69 ± 0.11 vii |
| O2•−-radical scavenging activity, IC50 (µg/mL) | 14.92 ± 0.43 ix,x | 18.36 ± 0.56 x | 9.21 ± 0.21 ix |
| Br•-radical scavenging activity (mg-eq). luteolin-7-O-glucoside/g | 1000 xii | 150.19 ± 1.95 xi | 799.63 ± 11.19 xii |
| NO inactivating activity, IC50 (µg/mL) | >100 | 37.92 ± 1.59 | 21.37 ± 0.85 xiii |
| H2O2 inactivating activity (mM/g) | 0.53 ± 0.02 xiv | 1.56 ± 0.04 | 2.75 ± 0.06 xv |
| Fe2+-chelating activity (µM) Fe2+/g | 106.12 ± 3.18 xvi | 142.84 ± 4.42 xvi,xvii | 206.11 ± 4.78 xviii |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olennikov, D.N.; Chirikova, N.K.; Kashchenko, N.I.; Gornostai, T.G.; Selyutina, I.Y.; Zilfikarov, I.N. Effect of Low Temperature Cultivation on the Phytochemical Profile and Bioactivity of Arctic Plants: A Case of Dracocephalum palmatum. Int. J. Mol. Sci. 2017, 18, 2579. https://doi.org/10.3390/ijms18122579
Olennikov DN, Chirikova NK, Kashchenko NI, Gornostai TG, Selyutina IY, Zilfikarov IN. Effect of Low Temperature Cultivation on the Phytochemical Profile and Bioactivity of Arctic Plants: A Case of Dracocephalum palmatum. International Journal of Molecular Sciences. 2017; 18(12):2579. https://doi.org/10.3390/ijms18122579
Chicago/Turabian StyleOlennikov, Daniil N., Nadezhda K. Chirikova, Nina I. Kashchenko, Tat’yana G. Gornostai, Inessa Yu. Selyutina, and Ifrat N. Zilfikarov. 2017. "Effect of Low Temperature Cultivation on the Phytochemical Profile and Bioactivity of Arctic Plants: A Case of Dracocephalum palmatum" International Journal of Molecular Sciences 18, no. 12: 2579. https://doi.org/10.3390/ijms18122579
APA StyleOlennikov, D. N., Chirikova, N. K., Kashchenko, N. I., Gornostai, T. G., Selyutina, I. Y., & Zilfikarov, I. N. (2017). Effect of Low Temperature Cultivation on the Phytochemical Profile and Bioactivity of Arctic Plants: A Case of Dracocephalum palmatum. International Journal of Molecular Sciences, 18(12), 2579. https://doi.org/10.3390/ijms18122579