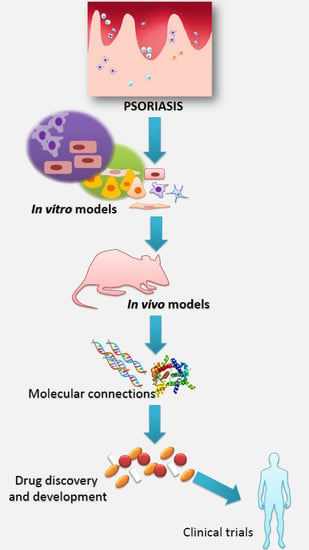
Models in the Research Process of Psoriasis
Abstract
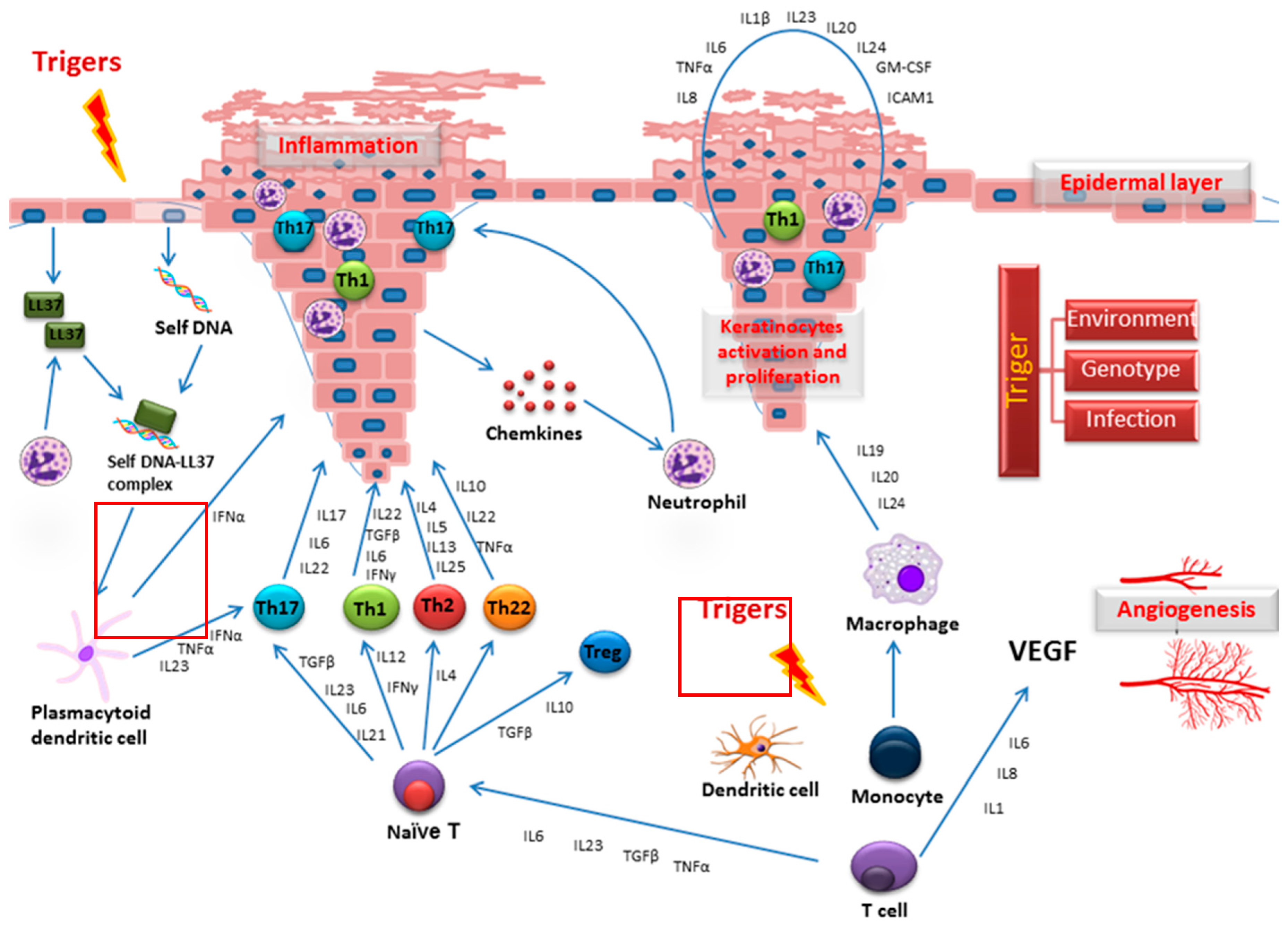
1. Psoriasis and Research of This Disease
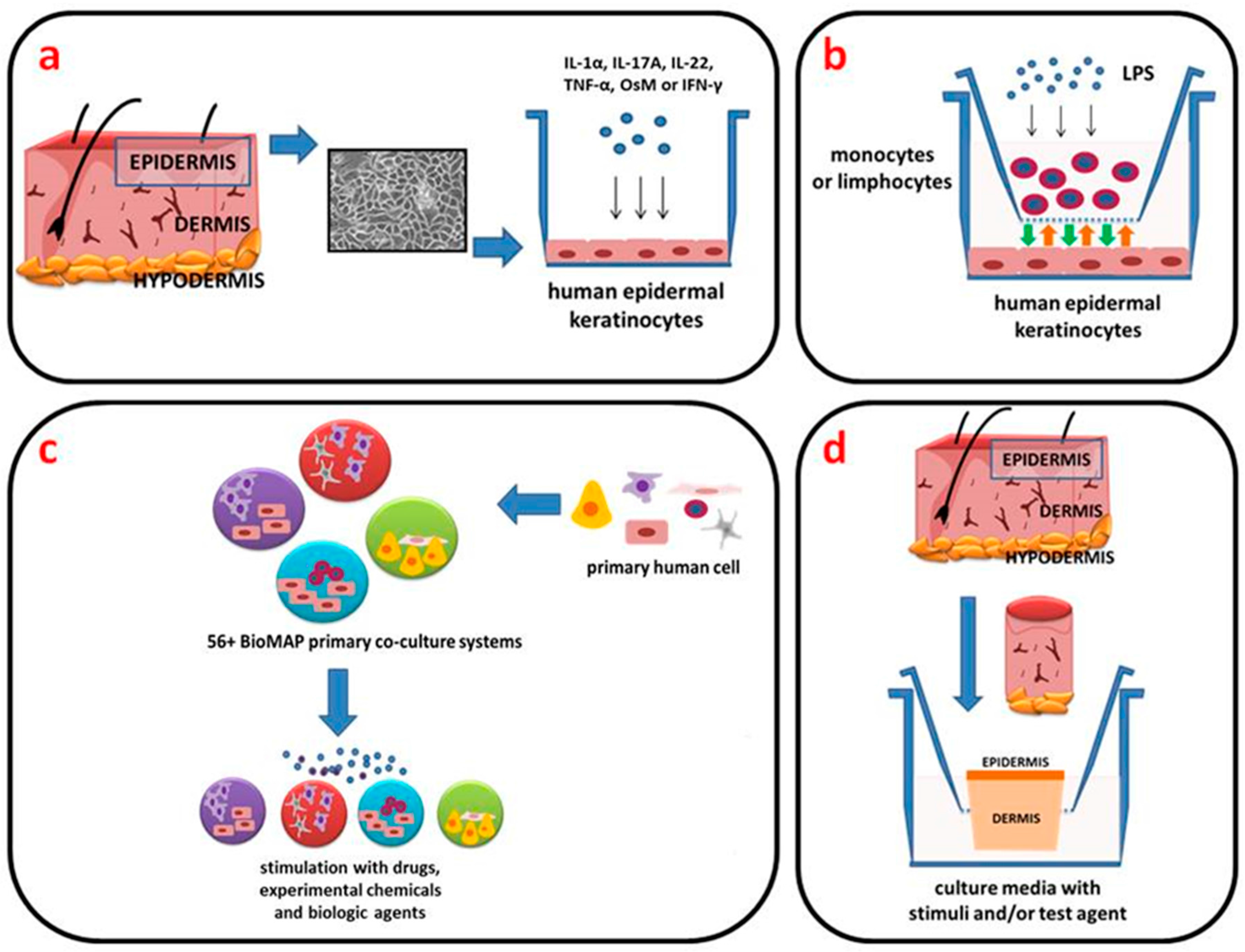
2. In Vitro Models
2.1. Two-Dimensional (2D) Engineered Skin Psoriatic Cell Model
2.2. Reconstituted Human Epidermal Models (3D)
3. BioMAP Model
4. Ex Vivo Models
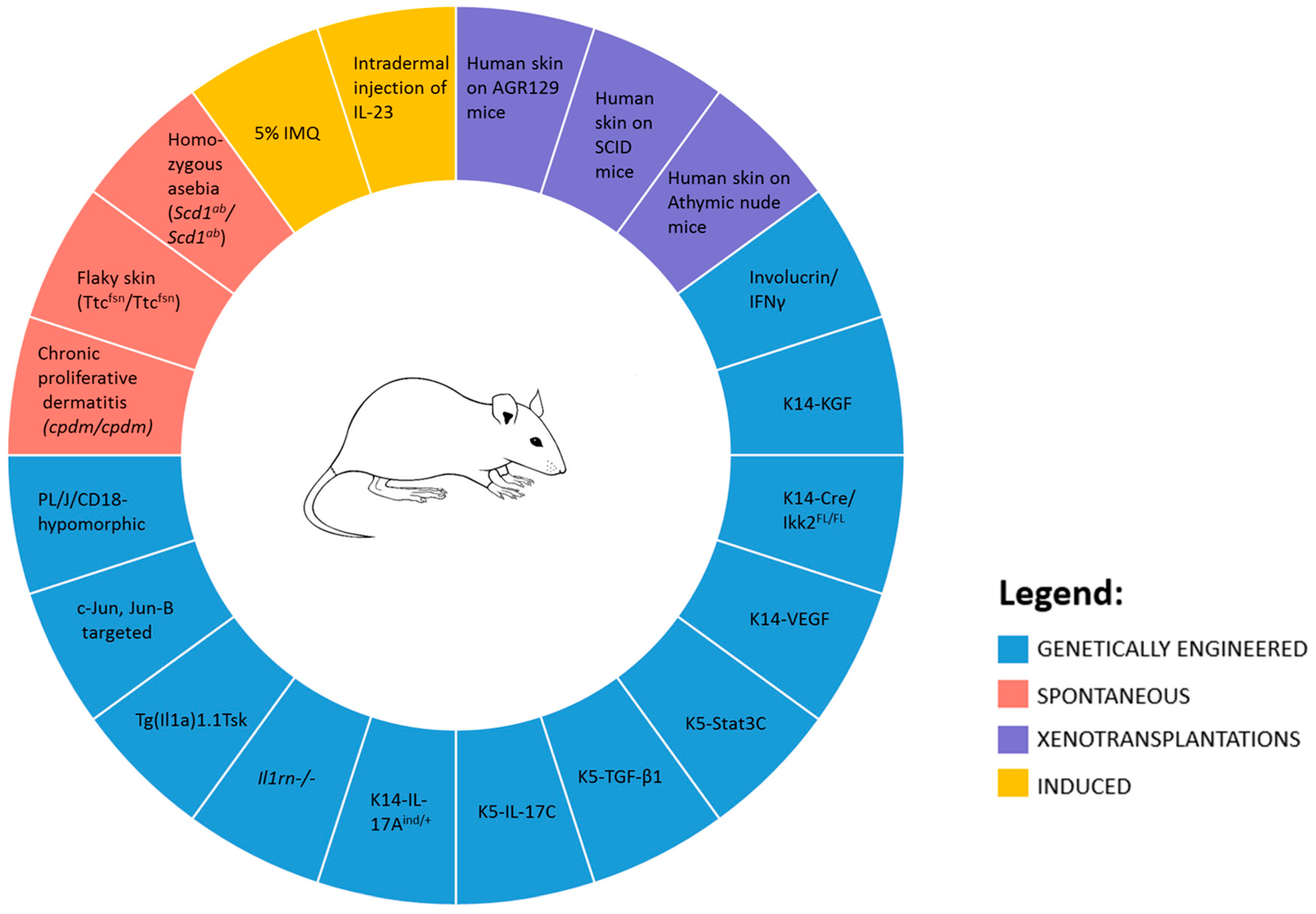
5. In Vivo Models
5.1. Spontaneous Mouse Models
5.2. Genetically Engineered Mouse Models
5.3. Xenotransplantations Models
5.4. Direct Induction
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lowes, M.A.; Suarez-Farinas, M.; Krueger, J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [PubMed]
- Amigo, M.; Schalkwijk, J.; Olthuis, D.; de Rosa, S.; Paya, M.; Terencio, M.C.; Lamme, E. Identification of avarol derivatives as potential antipsoriatic drugs using an in vitro model for keratinocyte growth and differentiation. Life Sci. 2006, 79, 2395–2404. [Google Scholar] [CrossRef] [PubMed]
- Saelee, C.; Thongrakard, V.; Tencomnao, T. Effects of thai medicinal herb extracts with anti-psoriatic activity on the expression on nf-kappab signaling biomarkers in hacat keratinocytes. Molecules 2011, 16, 3908–3932. [Google Scholar] [CrossRef] [PubMed]
- Coda, A.B.; Icen, M.; Smith, J.R.; Sinha, A.A. Global transcriptional analysis of psoriatic skin and blood confirms known disease-associated pathways and highlights novel genomic “hot spots” for differentially expressed genes. Genomics 2012, 100, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Gudjonsson, J.E.; Ding, J.; Li, X.; Nair, R.P.; Tejasvi, T.; Qin, Z.S.; Ghosh, D.; Aphale, A.; Gumucio, D.L.; Voorhees, J.J.; et al. Global gene expression analysis reveals evidence for decreased lipid biosynthesis and increased innate immunity in uninvolved psoriatic skin. J. Investig. Dermatol. 2009, 129, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Gudjonsson, J.E.; Ding, J.; Johnston, A.; Tejasvi, T.; Guzman, A.M.; Nair, R.P.; Voorhees, J.J.; Abecasis, G.R.; Elder, J.T. Assessment of the psoriatic transcriptome in a large sample: Additional regulated genes and comparisons with in vitro models. J. Investig. Dermatol. 2010, 130, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Luo, Y.; Mai, G.; Zhang, M.; Wang, G.; Zhao, M.; Gao, L.; Li, F.; Zhou, F. Gene expression profile based classification models of psoriasis. Genomics 2014, 103, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Swindell, W.R.; Stuart, P.E.; Sarkar, M.K.; Voorhees, J.J.; Elder, J.T.; Johnston, A.; Gudjonsson, J.E. Cellular dissection of psoriasis for transcriptome analyses and the post-gwas era. BMC Med. Genom. 2014, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Krueger, J.G.; Li, K.; Jabbari, A.; Brodmerkel, C.; Lowes, M.A.; Suarez-Farinas, M. Meta-analysis derived (mad) transcriptome of psoriasis defines the “core” pathogenesis of disease. PLoS ONE 2012, 7, e44274. [Google Scholar] [CrossRef] [PubMed]
- Swindell, W.R.; Sarkar, M.K.; Liang, Y.; Xing, X.; Gudjonsson, J.E. Cross-disease transcriptomics: Unique il-17a signaling in psoriasis lesions and an autoimmune pbmc signature. J. Investig. Dermatol. 2016, 136, 1820–1830. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Farinas, M.; Lowes, M.A.; Zaba, L.C.; Krueger, J.G. Evaluation of the psoriasis transcriptome across different studies by gene set enrichment analysis (GSEA). PLoS ONE 2010, 5, e10247. [Google Scholar] [CrossRef] [PubMed]
- Bracke, S.; Desmet, E.; Guerrero-Aspizua, S.; Tjabringa, S.G.; Schalkwijk, J.; van Gele, M.; Carretero, M.; Lambert, J. Identifying targets for topical rnai therapeutics in psoriasis: Assessment of a new in vitro psoriasis model. Arch. Dermatol. Res. 2013, 305, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Dowlatshahi, E.A.; van der Voort, E.A.; Arends, L.R.; Nijsten, T. Markers of systemic inflammation in psoriasis: A systematic review and meta-analysis. Br. J. Dermatol. 2013, 169, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Naves, L.B.; Dhand, C.; Almeida, L.; Rajamani, L.; Ramakrishna, S. In vitro skin models and tissue engineering protocols for skin graft applications. Essays Biochem. 2016, 60, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Greek, R. Pharmacology, toxicology and pharmaceutical science toxicology “new insights into toxicity and drug testing”. In Animal Models in Drug Development; Gowder, S., Ed.; InTech: London, UK, 2013. [Google Scholar]
- McGonigle, P.; Ruggeri, B. Animal models of human disease: Challenges in enabling translation. Biochem. Pharmacol. 2014, 87, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Schofield, P.N.; Sundberg, J.P.; Hoehndorf, R.; Gkoutos, G.V. New approaches to the representation and analysis of phenotype knowledge in human diseases and their animal models. Brief Funct. Genom. 2011, 10, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Jayo, M.J.; Zanolli, M.D.; Jayo, J.M. Psoriatic plaques in macaca fascicularis. Vet. Pathol. 1988, 25, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Gross, T.L.; Halliwell, R.E.; McDougal, B.J.; Rosencrantz, W.S. Psoriasiform lichenoid dermatitis in the springer spaniel. Vet. Pathol. 1986, 23, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Cooper, G.A.; Hoekstra, W.G. The histochemistry of the parakeratotic lesion of swine. J. Investig. Dermatol. 1967, 48, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Avci, P.; Sadasivam, M.; Gupta, A.; de Melo, W.C.; Huang, Y.Y.; Yin, R.; Chandran, R.; Kumar, R.; Otufowora, A.; Nyame, T.; et al. Animal models of skin disease for drug discovery. Expert Opin. Drug Discov. 2013, 8, 331–355. [Google Scholar] [CrossRef] [PubMed]
- Pol, A.; van Ruissen, F.; Schalkwijk, J. Development of a keratinocyte-based screening model for antipsoriatic drugs using green fluorescent protein under the control of an endogenous promoter. J. Biomol. Screen 2002, 7, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Leigh, I.M.; Navsaria, H.; Purkis, P.E.; McKay, I.A.; Bowden, P.E.; Riddle, P.N. Keratins (k16 and k17) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br. J. Dermatol. 1995, 133, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Dallaglio, K.; Marconi, A.; Truzzi, F.; Lotti, R.; Palazzo, E.; Petrachi, T.; Saltari, A.; Coppini, M.; Pincelli, C. E-fabp induces differentiation in normal human keratinocytes and modulates the differentiation process in psoriatic keratinocytes in vitro. Exp. Dermatol. 2013, 22, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Bando, M.; Hiroshima, Y.; Kataoka, M.; Shinohara, Y.; Herzberg, M.C.; Ross, K.F.; Nagata, T.; Kido, J. Interleukin-1alpha regulates antimicrobial peptide expression in human keratinocytes. Immunol. Cell Biol. 2007, 85, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Banno, T.; Gazel, A.; Blumenberg, M. Effects of tumor necrosis factor-alpha (tnf alpha) in epidermal keratinocytes revealed using global transcriptional profiling. J. Biol. Chem. 2004, 279, 32633–32642. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, A.; Guttman-Yassky, E.; Suarez-Farinas, M.; Nograles, K.E.; Tian, S.; Cardinale, I.; Chimenti, S.; Krueger, J.G. Integrative responses to il-17 and tnf-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J. Investig. Dermatol. 2011, 131, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Mee, J.B.; Johnson, C.M.; Morar, N.; Burslem, F.; Groves, R.W. The psoriatic transcriptome closely resembles that induced by interleukin-1 in cultured keratinocytes: Dominance of innate immune responses in psoriasis. Am. J. Pathol. 2007, 171, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Nograles, K.E.; Zaba, L.C.; Guttman-Yassky, E.; Fuentes-Duculan, J.; Suarez-Farinas, M.; Cardinale, I.; Khatcherian, A.; Gonzalez, J.; Pierson, K.C.; White, T.R.; et al. Th17 cytokines interleukin (il)-17 and il-22 modulate distinct inflammatory and keratinocyte-response pathways. Br. J. Dermatol. 2008, 159, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.A.; Towne, J.E.; Kricorian, G.; Klekotka, P.; Gudjonsson, J.E.; Krueger, J.G.; Russell, C.B. The emerging role of il-17 in the pathogenesis of psoriasis: Preclinical and clinical findings. J. Investig. Dermatol. 2013, 133, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Uchi, H.; Terao, H.; Koga, T.; Furue, M. Cytokines and chemokines in the epidermis. J. Dermatol. Sci. 2000, 24 (Suppl. 1), S29–S38. [Google Scholar] [CrossRef]
- Komine, M.; Rao, L.S.; Freedberg, I.M.; Simon, M.; Milisavljevic, V.; Blumenberg, M. Interleukin-1 induces transcription of keratin k6 in human epidermal keratinocytes. J. Investig. Dermatol. 2001, 116, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Kim, H.K. Illicium verum extract suppresses ifn-gamma-induced icam-1 expression via blockade of jak/stat pathway in hacat human keratinocytes. J. Ethnopharmacol. 2013, 149, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Mezentsev, A.V.; Bruskin, S.A.; Soboleva, A.G.; Sobolev, V.V.; Piruzian, E.S. Pharmacological control of receptor of advanced glycation end-products and its biological effects in psoriasis. Int. J. Biomed. Sci. 2013, 9, 112–122. [Google Scholar] [PubMed]
- Soboleva, A.G.; Zolotarenko, A.D.; Sobolev, V.V.; Bruskin, S.A.; Piruzian, E.S.; Mezentsev, A.V. Genetically predetermined limitation in the use of hacat cells that affects their ability to serve as an experimental model of psoriasis. Genetika 2014, 50, 1222–1231. [Google Scholar] [PubMed]
- Wu, Y.; Lu, Z.; Chen, Y.; Xue, F.; Chen, X.; Zheng, J. Replication of association between interleukin-23 receptor (il-23r) and its ligand (il-12b) polymorphisms and psoriasis in the chinese han population. Hum. Immunol. 2010, 71, 1255–1258. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q. New challenges and opportunities for industrial biotechnology. Microb. Cell Fact. 2012, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Goers, L.; Freemont, P.; Polizzi, K.M. Co-culture systems and technologies: Taking synthetic biology to the next level. J. R. Soc. Interface 2014, 11, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Schonefuss, A.; Wendt, W.; Schattling, B.; Schulten, R.; Hoffmann, K.; Stuecker, M.; Tigges, C.; Lubbert, H.; Stichel, C. Upregulation of cathepsin s in psoriatic keratinocytes. Exp. Dermatol. 2010, 19, e80–e88. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Shan, Y.; Fan, Y.; Fan, C.; Chen, S.; Sun, J.; Zhu, L.; Qin, L.; Yu, M.; Lin, Z. Nf-kappab inhibition attenuates lps-induced tlr4 activation in monocyte cells. Mol. Med. Rep. 2016, 14, 4505–4510. [Google Scholar] [CrossRef] [PubMed]
- Shahraz, A.; Kopatz, J.; Mathy, R.; Kappler, J.; Winter, D.; Kapoor, S.; Schutza, V.; Scheper, T.; Gieselmann, V.; Neumann, H. Anti-inflammatory activity of low molecular weight polysialic acid on human macrophages. Sci. Rep. 2015, 5, 16800. [Google Scholar] [CrossRef] [PubMed]
- Krueger, G.G.; Jorgensen, C.M. Experimental models for psoriasis. J. Investig. Dermatol. 1990, 95, 56S–58S. [Google Scholar] [CrossRef] [PubMed]
- Barker, C.L.; McHale, M.T.; Gillies, A.K.; Waller, J.; Pearce, D.M.; Osborne, J.; Hutchinson, P.E.; Smith, G.M.; Pringle, J.H. The development and characterization of an in vitro model of psoriasis. J. Investig. Dermatol. 2004, 123, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, A.; Nograles, K.E.; Johnson-Huang, L.M.; Fuentes-Duculan, J.; Cardinale, I.; Bonifacio, K.M.; Gulati, N.; Mitsui, H.; Guttman-Yassky, E.; Suarez-Farinas, M.; et al. Il-17 induces an expanded range of downstream genes in reconstituted human epidermis model. PLoS ONE 2014, 9, e90284. [Google Scholar] [CrossRef] [PubMed]
- Gazel, A.; Rosdy, M.; Bertino, B.; Tornier, C.; Sahuc, F.; Blumenberg, M. A characteristic subset of psoriasis-associated genes is induced by oncostatin-m in reconstituted epidermis. J. Investig. Dermatol. 2006, 126, 2647–2657. [Google Scholar] [CrossRef] [PubMed]
- Kamsteeg, M.; Bergers, M.; de Boer, R.; Zeeuwen, P.L.; Hato, S.V.; Schalkwijk, J.; Tjabringa, G.S. Type 2 helper t-cell cytokines induce morphologic and molecular characteristics of atopic dermatitis in human skin equivalent. Am. J. Pathol. 2011, 178, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Van den Bogaard, E.H.; Rodijk-Olthuis, D.; Jansen, P.A.; van Vlijmen-Willems, I.M.; van Erp, P.E.; Joosten, I.; Zeeuwen, P.L.; Schalkwijk, J. Rho kinase inhibitor y-27632 prolongs the life span of adult human keratinocytes, enhances skin equivalent development, and facilitates lentiviral transduction. Tissue Eng. Part A 2012, 18, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Tjabringa, G.; Bergers, M.; van Rens, D.; de Boer, R.; Lamme, E.; Schalkwijk, J. Development and validation of human psoriatic skin equivalents. Am. J. Pathol. 2008, 173, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Jean, J.; Lapointe, M.; Soucy, J.; Pouliot, R. Development of an in vitro psoriatic skin model by tissue engineering. J. Dermatol. Sci. 2009, 53, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.H.; Peredo, C.E.; Takeda, Y.; Bui, T.; Neil, J.; Rickard, D.; Millerman, E.; Therrien, J.P.; Nicodeme, E.; Brusq, J.M.; et al. Development of a topical treatment for psoriasis targeting rorgamma: From bench to skin. PLoS ONE 2016, 11, e0147979. [Google Scholar]
- Kunkel, E.J.; Plavec, I.; Nguyen, D.; Melrose, J.; Rosler, E.S.; Kao, L.T.; Wang, Y.; Hytopoulos, E.; Bishop, A.C.; Bateman, R.; et al. Rapid structure-activity and selectivity analysis of kinase inhibitors by biomap analysis in complex human primary cell-based models. Assay Drug Dev. Technol. 2004, 2, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Berg, E.L.; Kunkel, E.J.; Hytopoulos, E. Biological complexity and drug discovery: A practical systems biology approach. Syst. Biol. 2005, 152, 201–206. [Google Scholar] [CrossRef]
- Berg, E.L.; Kunkel, E.J.; Hytopoulos, E.; Plavec, I. Characterization of compound mechanisms and secondary activities by biomap analysis. J. Pharmacol. Toxicol. Methods 2006, 53, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Gillard, G.O.; Collette, B.; Anderson, J.; Chao, J.; Scannevin, R.H.; Huss, D.J.; Fontenot, J.D. Dmf, but not other fumarates, inhibits nf-kappab activity in vitro in an nrf2-independent manner. J. Neuroimmunol. 2015, 283, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Cohn, M. Sourcebook of Models for Biomedical Research; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; pp. 9–33. [Google Scholar]
- Boehncke, W.H. The psoriasis SCID mouse model: A tool for drug discovery? Ernst Scher. Res. Found Workshop 2005, 50, 213–234. [Google Scholar]
- Danilenko, D.M. Review paper: Preclinical models of psoriasis. Vet. Pathol. 2008, 45, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, H.; Richardson, J.A.; Taurog, J.D.; Hammer, R.E. Characterization of psoriasiform and alopecic skin lesions in hla-b27 transgenic rats. Am. J. Pathol. 1995, 147, 955–964. [Google Scholar] [PubMed]
- Schon, M.P. Animal models of psoriasis: A critical appraisal. Exp. Dermatol. 2008, 17, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Zollner, T.M.; Renz, H.; Igney, F.H.; Asadullah, K. Animal models of t-cell-mediated skin diseases. Bioessays 2004, 26, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Gudjonsson, J.E.; Johnston, A.; Dyson, M.; Valdimarsson, H.; Elder, J.T. Mouse models of psoriasis. J. Investig. Dermatol. 2007, 127, 1292–1308. [Google Scholar] [CrossRef] [PubMed]
- Gates, A.H.; Karasek, M. Hereditary absence of sebaceous glands in the mouse. Science 1965, 148, 1471–1473. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, J.P. Handbook of Mouse Mutations with Skin and Hair Abnormalities; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- HogenEsch, H.; Gijbels, M.J.; Offerman, E.; van Hooft, J.; van Bekkum, D.W.; Zurcher, C. A spontaneous mutation characterized by chronic proliferative dermatitis in c57bl mice. Am. J. Pathol. 1993, 143, 972–982. [Google Scholar] [PubMed]
- Wohn, C.T. Mechanisms of Psoriatic Plaque Formation in Mice; Erasmus University Rotterdam: Rotterdam, The Netherlands, 2015; pp. 11–13. [Google Scholar]
- Bullard, D.C.; Scharffetter-Kochanek, K.; McArthur, M.J.; Chosay, J.G.; McBride, M.E.; Montgomery, C.A.; Beaudet, A.L. A polygenic mouse model of psoriasiform skin disease in cd18-deficient mice. Proc. Natl. Acad. Sci. USA 1996, 93, 2116–2121. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peters, T.; Sindrilaru, A.; Scharffetter-Kochanek, K. Key role of macrophages in the pathogenesis of cd18 hypomorphic murine model of psoriasis. J. Investig. Dermatol. 2009, 129, 1100–1114. [Google Scholar] [CrossRef] [PubMed]
- Szabowski, A.; Maas-Szabowski, N.; Andrecht, S.; Kolbus, A.; Schorpp-Kistner, M.; Fusenig, N.E.; Angel, P. C-jun and junb antagonistically control cytokine-regulated mesenchymal-epidermal interaction in skin. Cell 2000, 103, 745–755. [Google Scholar] [CrossRef]
- Zenz, R.; Eferl, R.; Kenner, L.; Florin, L.; Hummerich, L.; Mehic, D.; Scheuch, H.; Angel, P.; Tschachler, E.; Wagner, E.F. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of jun proteins. Nature 2005, 437, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Groves, R.W.; Mizutani, H.; Kieffer, J.D.; Kupper, T.S. Inflammatory skin disease in transgenic mice that express high levels of interleukin 1 alpha in basal epidermis. Proc. Natl. Acad. Sci. USA 1995, 92, 11874–11878. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.; Little, M.C.; Nicklin, M.J. Psoriasis-like cutaneous inflammation in mice lacking interleukin-1 receptor antagonist. J. Investig. Dermatol. 2004, 122, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Croxford, A.L.; Karbach, S.; Kurschus, F.C.; Wortge, S.; Nikolaev, A.; Yogev, N.; Klebow, S.; Schuler, R.; Reissig, S.; Piotrowski, C.; et al. Il-6 regulates neutrophil microabscess formation in il-17a-driven psoriasiform lesions. J. Investig. Dermatol. 2014, 134, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.; Fritz, Y.; Dawes, S.M.; Diaconu, D.; Al-Attar, P.M.; Guzman, A.M.; Chen, C.S.; Fu, W.; Gudjonsson, J.E.; McCormick, T.S.; et al. Keratinocyte overexpression of il-17c promotes psoriasiform skin inflammation. J. Immunol. 2013, 190, 2252–2262. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.M.; Crompton, T.; Seery, J.P.; Watt, F.M. Transgenic mice expressing ifn-gamma in the epidermis have eczema, hair hypopigmentation, and hair loss. J. Investig. Dermatol. 1997, 108, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Marina, M.E.; Roman, I.I.; Constantin, A.M.; Mihu, C.M.; Tataru, A.D. VEGF involvement in psoriasis. Clujul Med. 2015, 88, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.P.; Li, B.; Hylton, D.; Detmar, M.; Yancopoulos, G.D.; Rudge, J.S. Transgenic delivery of vegf to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood 2003, 102, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Swindell, W.R.; Johnston, A.; Carbajal, S.; Han, G.; Wohn, C.; Lu, J.; Xing, X.; Nair, R.P.; Voorhees, J.J.; Elder, J.T.; et al. Genome-wide expression profiling of five mouse models identifies similarities and differences with human psoriasis. PLoS ONE 2011, 6, e18266. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, J.A.; Diaconu, D.; Hatala, D.A.; Rastegar, J.; Knutsen, D.A.; Lowther, A.; Askew, D.; Gilliam, A.C.; McCormick, T.S.; Ward, N.L. Keratinocyte but not endothelial cell-specific overexpression of tie2 leads to the development of psoriasis. Am. J. Pathol. 2009, 174, 1443–1458. [Google Scholar] [CrossRef] [PubMed]
- Sferra, R.; Fargnoli, M.C.; Corbelli, E.; Pellegrini, C.; Peris, K.; Gaudio, E.; Vetuschi, A. Immunopathogenesis of psoriasis: A possible role of TGFβ/smads pathway. Ital. J. Anat. Embryol. 2014, 119, 277–285. [Google Scholar] [PubMed]
- Di Cesare, A.; Di Meglio, P.; Nestle, F.O. The il-23/th17 axis in the immunopathogenesis of psoriasis. J. Investig. Dermatol. 2009, 129, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Williams, C.A.; Salter, K.; Garl, P.J.; Li, A.G.; Wang, X.J. A role for tgfbeta signaling in the pathogenesis of psoriasis. J. Investig. Dermatol. 2010, 130, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Li, A.G.; Wang, D.; Feng, X.H.; Wang, X.J. Latent tgfbeta1 overexpression in keratinocytes results in a severe psoriasis-like skin disorder. EMBO J. 2004, 23, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Fraki, J.E.; Briggaman, R.A.; Lazarus, G.S. Uninvolved skin from psoriatic patients develops signs of involved psoriatic skin after being grafted onto nude mice. Science 1982, 215, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yu, Q.C.; Fuchs, E. Targeting expression of keratinocyte growth factor to keratinocytes elicits striking changes in epithelial differentiation in transgenic mice. EMBO J. 1993, 12, 973–986. [Google Scholar] [PubMed]
- Tak, P.P.; Firestein, G.S. Nf-kappab: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Stratis, A.; Pasparakis, M.; Rupec, R.A.; Markur, D.; Hartmann, K.; Scharffetter-Kochanek, K.; Peters, T.; van Rooijen, N.; Krieg, T.; Haase, I. Pathogenic role for skin macrophages in a mouse model of keratinocyte-induced psoriasis-like skin inflammation. J. Clin. Investig. 2006, 116, 2094–2104. [Google Scholar] [CrossRef] [PubMed]
- Harden, J.L.; Krueger, J.G.; Bowcock, A.M. The immunogenetics of psoriasis: A comprehensive review. J. Autoimmun. 2015, 64, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Krueger, G.G.; Chambers, D.A.; Shelby, J. Involved and uninvolved skin from psoriatic subjects: Are they equally diseased? Assessment by skin transplanted to congenitally athymic (nude) mice. J. Clin. Investig. 1981, 68, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Jean, J.; Pouliot, R. In vivo and in vitro models of psoriasis. In Tissue Engineering; InTech: London, UK, 2010; pp. 359–382. [Google Scholar]
- Gilhar, A.; David, M.; Ullmann, Y.; Berkutski, T.; Kalish, R.S. T-lymphocyte dependence of psoriatic pathology in human psoriatic skin grafted to scid mice. J. Investig. Dermatol. 1997, 109, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.; Mark, N.M.; Machler, B.C.; Levine, V.J. Imiquimod 5% cream induced psoriasis: A case report, summary of the literature and mechanism. Br. J. Dermatol. 2011, 164, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the il-23/il-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Girolomoni, G.; Strohal, R.; Puig, L.; Bachelez, H.; Barker, J.; Boehncke, W.H.; Prinz, J.C. The role of il-23 and the il-23/th 17 immune axis in the pathogenesis and treatment of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Puig, L. The role of il 23 in the treatment of psoriasis. Expert Rev. Clin. Immunol. 2017, 13, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.R.; Blumenschein, W.; Murphy, E.; Diveu, C.; Wiekowski, M.; Abbondanzo, S.; Lucian, L.; Geissler, R.; Brodie, S.; Kimball, A.B.; et al. Il-23 stimulates epidermal hyperplasia via tnf and il-20r2-dependent mechanisms with implications for psoriasis pathogenesis. J. Exp. Med. 2006, 203, 2577–2587. [Google Scholar] [CrossRef] [PubMed]
| Features | In Vitro Models | In Vivo Models |
|---|---|---|
| Pros | Easy access to cells Reduced complexity Utilization of human tissue Avoids the need for animals Individual cell responses can be measured | Similar and complex histopathological image Large amount of interacting factors and signaling pathways for manipulation studies or new drugs testing Varied amount of modifications, depending on the purpose of research |
| Cons | Excludes microenvironment influences and might be misleading Excludes macroenvironmental influences | Differences between human and animal skin and immunity Limitation resulting from the polygene nature of the disease—difficulties in a complete phenotype reconstruction by single mutation Influence of environmental factors |
| Best use of model | Evaluation of cell physiology, viability, phenotype, function, and responses to stimulators and inhibitors | Suitable for research the multifarious interactions between skin cells, vascular endothelium and immune response |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocheńska, K.; Smolińska, E.; Moskot, M.; Jakóbkiewicz-Banecka, J.; Gabig-Cimińska, M. Models in the Research Process of Psoriasis. Int. J. Mol. Sci. 2017, 18, 2514. https://doi.org/10.3390/ijms18122514
Bocheńska K, Smolińska E, Moskot M, Jakóbkiewicz-Banecka J, Gabig-Cimińska M. Models in the Research Process of Psoriasis. International Journal of Molecular Sciences. 2017; 18(12):2514. https://doi.org/10.3390/ijms18122514
Chicago/Turabian StyleBocheńska, Katarzyna, Elwira Smolińska, Marta Moskot, Joanna Jakóbkiewicz-Banecka, and Magdalena Gabig-Cimińska. 2017. "Models in the Research Process of Psoriasis" International Journal of Molecular Sciences 18, no. 12: 2514. https://doi.org/10.3390/ijms18122514
APA StyleBocheńska, K., Smolińska, E., Moskot, M., Jakóbkiewicz-Banecka, J., & Gabig-Cimińska, M. (2017). Models in the Research Process of Psoriasis. International Journal of Molecular Sciences, 18(12), 2514. https://doi.org/10.3390/ijms18122514