Structure and Functional Analysis of Promoters from Two Liver Isoforms of CPT I in Grass Carp Ctenopharyngodon idella
Abstract
:1. Introduction
2. Results
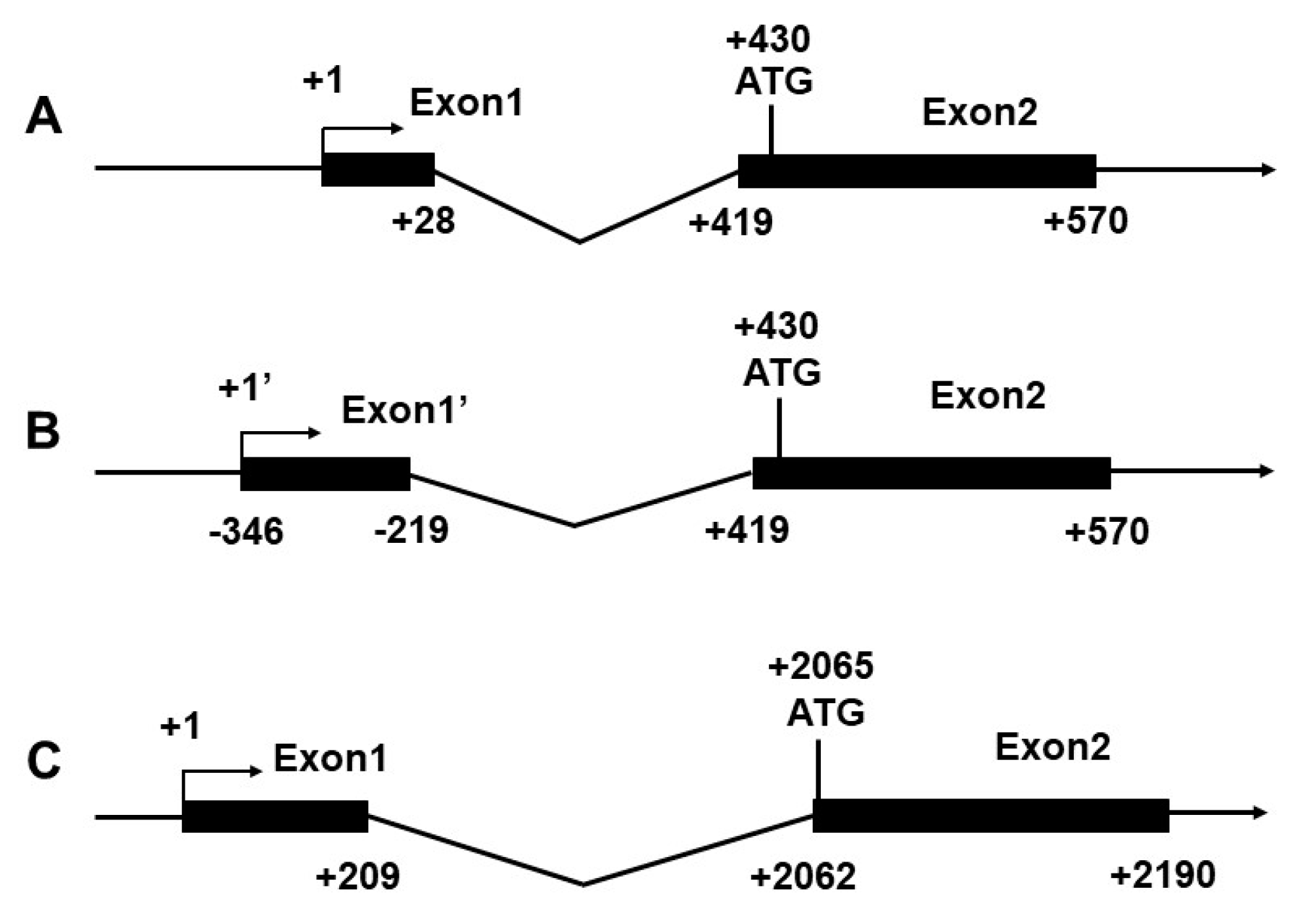
2.1. Identification of Transcription Start Site (TSS)
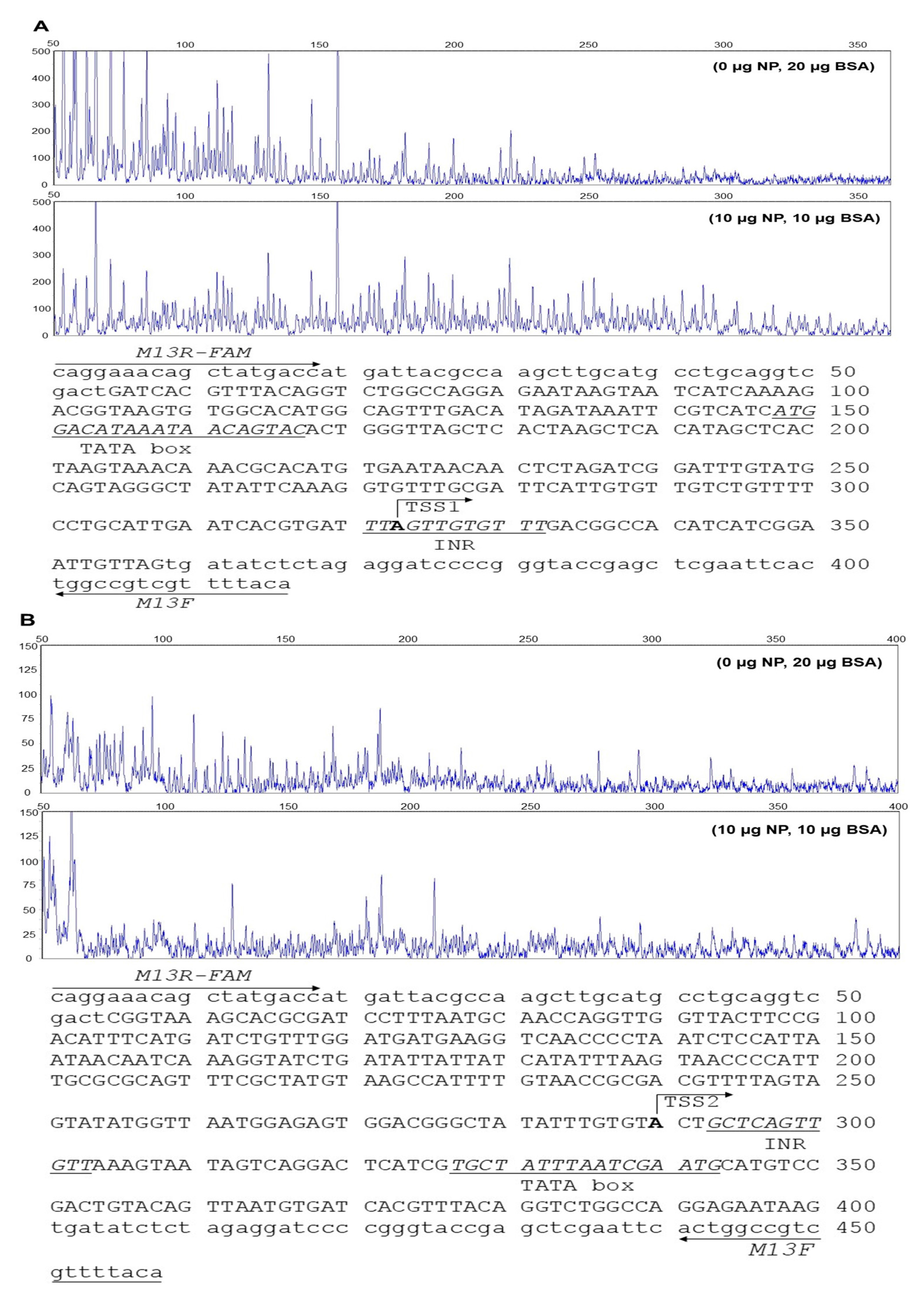
2.2. DNase I Foot Printing Assay of Core Promoter of CPT Iα1b
2.3. Sequence Analysis of the CPT Iα1b and CPT Iα2a Promoters
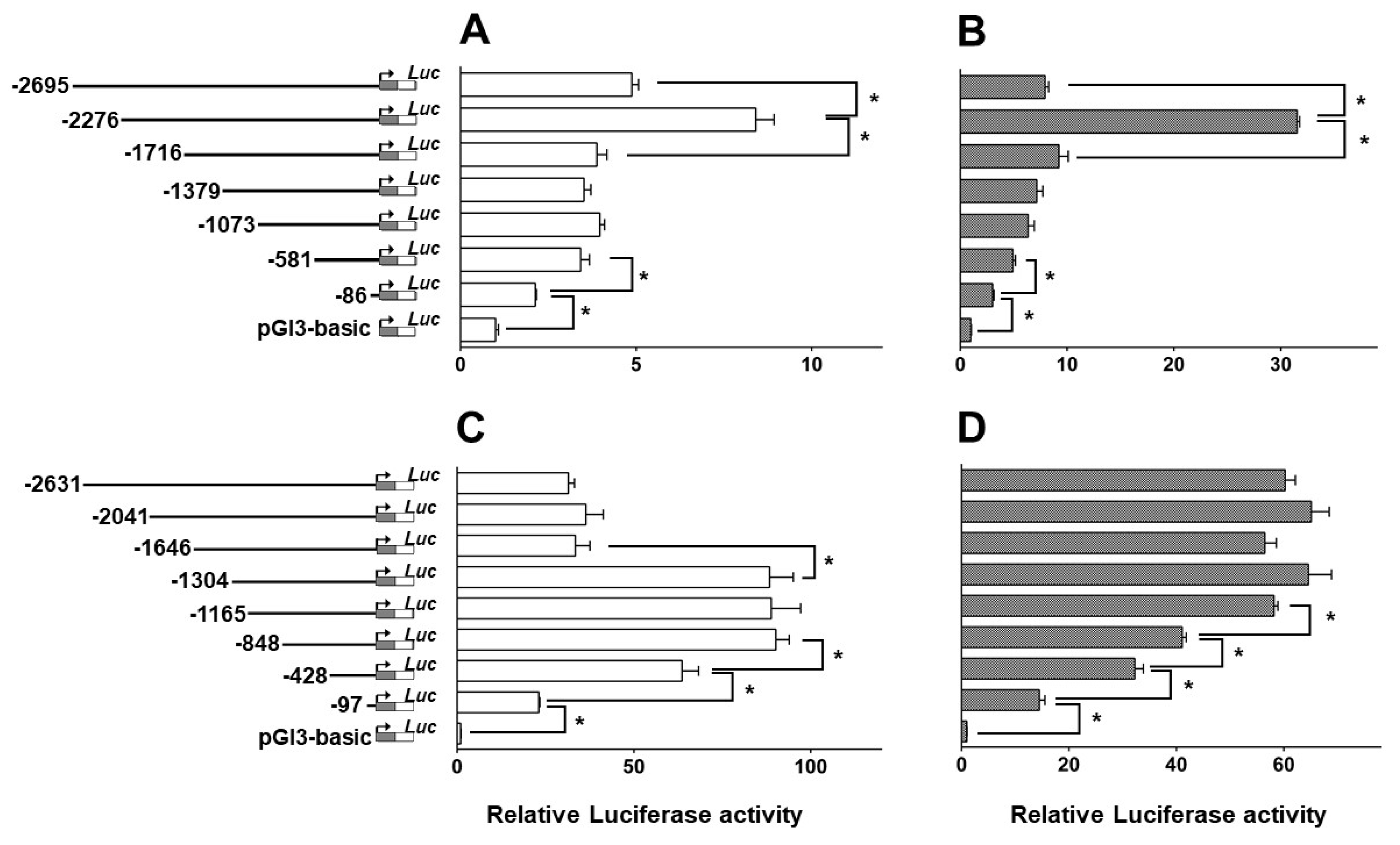
2.4. Deletion Assay of the CPT Iα1b and CPT Iα2a Promoter
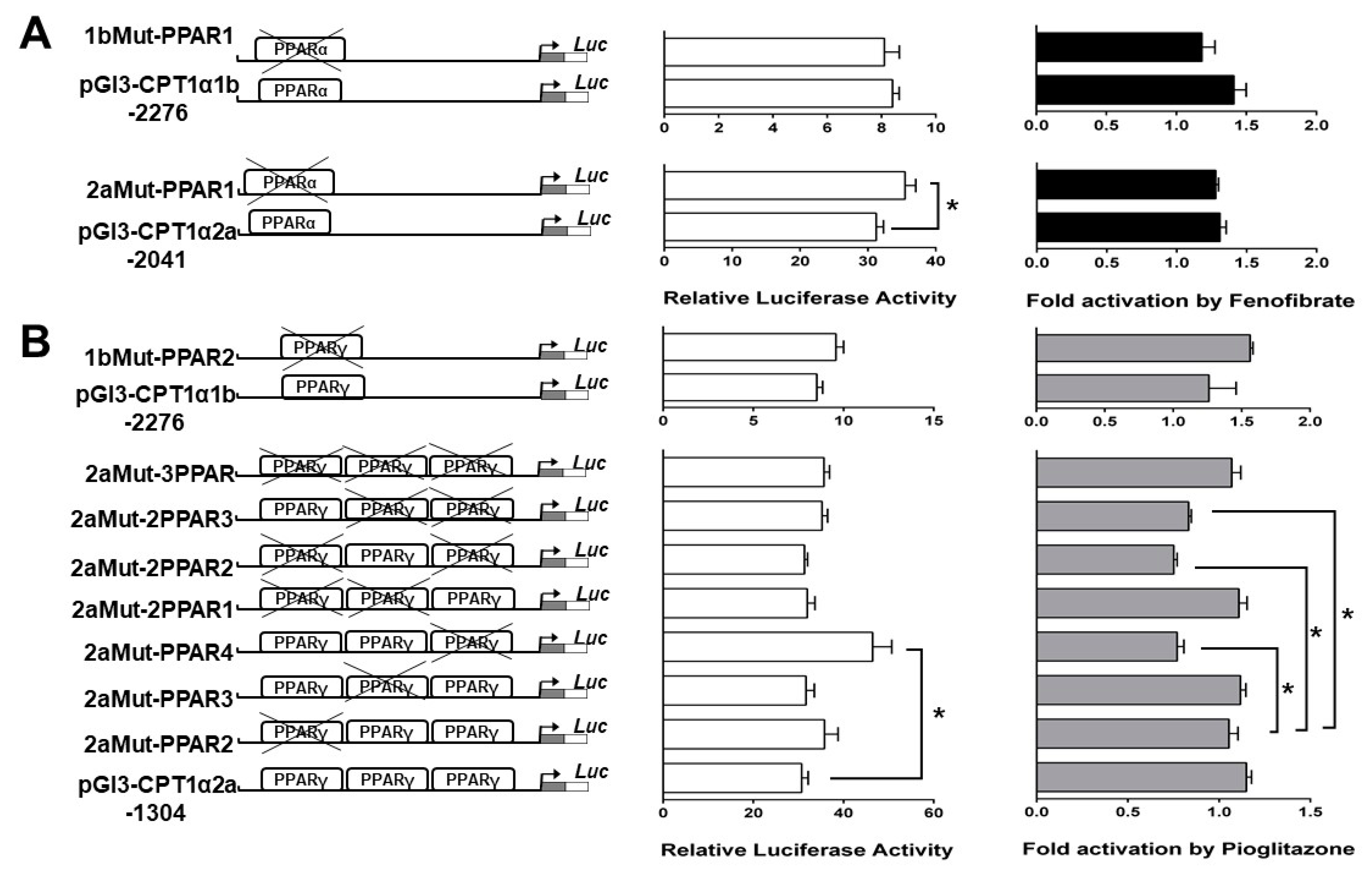
2.5. Site-Mutation Analysis of PPAR Binding Sites
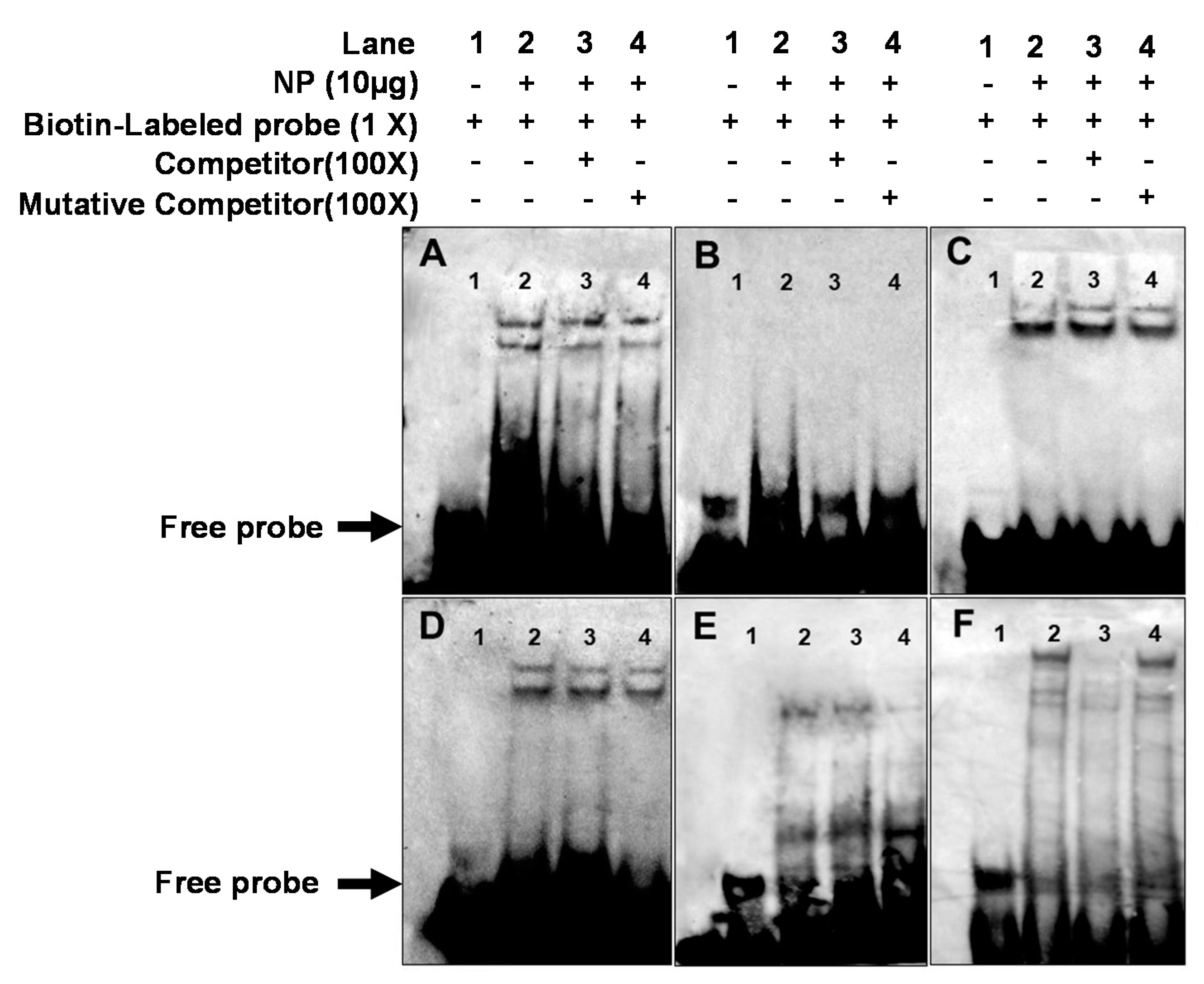
2.6. EMSA of Each PPAR Binding Sequence
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Cells
4.2. Rapid Amplification of 5′ cDNA Ends (5′ RACE)
4.3. Cloning of Promoter and Plasmid Construction
4.4. Sequence Analysis
4.5. DNase I Foot Printing Assay
4.6. Transfections and Luciferase Assays
4.7. Site-Mutation Analysis of PPAR Binding Sites on the Grass Carp CPT Iα1b and CPT Iα2a Promoters
4.8. Electrophoretic Mobility-Shift Assay (EMSA)
4.9. Statistical Analysis
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CPT I | carnitine palmitoyltransferase I |
| FAM | fluorescent 6-carboxy-fluorescein |
| L-CPT | liver carnitine palmitoyltransferase |
| LCFA | long-chain fatty acid |
| M-CPT | muscle carnitine palmitoyltransferase |
| PCR | polymerase chain reaction |
| PPAR | peroxisome proliferator-activated receptor |
| PPRE | peroxisome proliferator-responsive element |
| RLM-5′RACE | RNA ligase-mediated rapid amplification of 5′ cDNA ends |
| RXR | retinoid X receptor |
| TFBS | transcription factor binding site |
| TR | thyroid hormone receptor |
| TRE | thyroid hormone response elements |
References
- Sargent, J.R.; Tocher, D.R.; Bell, J.G. The lipids. In Fish Nutrition, 2nd ed.; Halver, J., Hardy, R., Eds.; Academic Press: New York, NY, USA; San Diego, CA, USA, 2002; pp. 181–257. [Google Scholar]
- Bartlett, K.; Eaton, S. Mitochondrial β-oxidation. FEBS J. 2004, 271, 462–469. [Google Scholar] [CrossRef]
- Skiba-Cassy, S.; Collin, A.; Chartrin, P.; Médale, F.; Simon, J.; Duclos, M.J.; Tesseraud, S. Chicken liver and muscle carnitine palmitoyltransferase 1: Nutritional regulation of messengers. Comp. Biochem. Physiol. B 2007, 147, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Morash, A.J.; Kajimura, M.; McClelland, G.B. Intertissue regulation of carnitine palmitoyltransferase I (CPTI): Mitochondrial membrane properties and gene expression in rainbow trout (Oncorhynchus mykiss). BBA-Biomembr. 2008, 1778, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Britton, C.H.; Schultz, R.A.; Zhang, B.; Esser, V.; Foster, D.W.; McGarry, J.D. Human liver mitochondrial carnitine palmitoyltransferase I: Characterization of its cDNA and chromosomal localization and partial analysis of the gene. Proc. Natl. Acad. Sci. USA 1995, 92, 1984–1988. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, N.; Shinohara, Y.; Shima, A.; Yamanaka, Y.; Terada, H. Isolation and characterization of cDNA and genomic clones encoding human muscle type carnitine palmitoyltransferase I. BBA-Gene Struct. Expr. 1996, 1307, 157–161. [Google Scholar] [CrossRef]
- Price, N.T.; van der Leij, F.R.; Jackson, V.N.; Corstorphine, C.G.; Thomson, R.; Sorensen, A.; Zammit, V.A. A novel brain-expressed protein related to carnitine palmitoyltransferase I. Genomics 2002, 80, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.L.; Luo, Z.; Zhu, Q.L.; Chen, Q.L.; Gong, Y. Molecular characterization, tissue distribution and kinetic analysis of carnitine palmitoyltransferase I in juvenile yellow catfish Pelteobagrus fulvidraco. Genomics 2013, 101, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zheng, J.L.; Luo, Z.; Chen, Q.L.; Zhu, Q.L. Carnitine palmitoyltransferase I gene in Synechogobius hasta: Cloning, mRNA expression and transcriptional regulation by insulin in vitro. Gene 2016, 576, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Hu, W. Isolation, Purification, Cloning, Kinetic Properties of Enzymes Related to Lipid Metabolism and Response to Copper and Zinc in Ctenopharyngodon idella. Ph.D. Dissertation, Huazhong Agricultural University, Wuhan, China, 2015. [Google Scholar]
- Shi, X.C.; Sun, J.; Yang, Z.; Li, X.X.; Ji, H.; Li, Y.; Chang, Z.G.; Du, Z.Y.; Chen, L.Q. Molecular characterization and nutritional regulation of carnitine palmitoyltransferase (CPT) family in grass carp (Ctenopharyngodon idellus). Comp. Biochem. Physiol. 2017, 203, 11–19. [Google Scholar] [CrossRef] [PubMed]
- McGarry, J.D.; Brown, N.F. The mitochondrial carnitine palmitoyltransferase system: From concept to molecular analysis. Eur. J. Biochem. 1997, 244, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Boukouvala, E.; Leaver, M.J.; Favre-Krey, L.; Theodoridou, M.; Krey, G. Molecular characterization of a gilthead sea bream (Sparus aurata) muscle tissue cDNA for carnitine palmitoyltransferase 1B (CPT 1B). Comp. Biochem. Physiol. 2010, 157, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.L.; Hu, W.; Luo, Z.; Zhao, Y.H.; Zhu, Q.L.; Li, X.D. Comparative study on the kinetic behaviour of carnitine palmitoyltransferase I between Javelin goby Synechogobius hasta (carnivorous) and grass carp Ctenopharyngodon idella (herbirovous). Aquacult. Nutr. 2013, 19, 665–676. [Google Scholar] [CrossRef]
- Plagnes-Juan, E.; Lansard, M.; Seiliez, I.; Médale, F.; Corraze, G.; Kaushik, S.; Panserat, S.; Skiba-Cassy, S. Insulin regulates the expression of several metabolism-related genes in the liver and primary hepatocytes of rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 2008, 211, 2510–2518. [Google Scholar] [CrossRef] [PubMed]
- Morash, A.J.; Bureau, D.P.; McClelland, G.B. Effects of dietary fatty acid composition on the regulation of carnitine palmitoyltransferase (CPT) I in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. 2009, 152, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Polakof, S.; Médale, F.; Skiba-Cassy, S.; Corraze, G.; Panserat, S. Molecular regulation of lipid metabolism in liver and muscle of rainbow trout subjected to acute and chronic insulin treatments. Domest. Anim. Endocrin. 2010, 39, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.L.; Luo, Z.; Hu, W.; Liu, C.X.; Chen, Q.L. Differential effects of dietary Zn deficiency and excess on carnitine status, kinetics and expression of CPT I in yellow catfish Pelteobagrus fulvidraco. Aquaculture 2014, 420–421, 10–17. [Google Scholar] [CrossRef]
- Chen, Q.L.; Luo, Z.; Liu, C.X.; Zheng, J.L. Differential effects of dietary Cu deficiency and excess on carnitine status, kinetics and expression of CPT I in liver and muscle of yellow catfish Pelteobagrus fulvidraco. Comp. Biochem. Physiol. 2015, 188, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Park, A.E.; Steffen, L.M.; Song, S.; Park, M.V.; Cook, A.G. Cloning and characterization of the promoter for the liver isoform of the rat carnitine palmitoyltransferase I (L-CPT I) gene. Biochem. J. 1998, 330, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Steffen, M.L.; Harrison, W.R.; Elder, F.F. Expression of the rat liver carnitine palmitoyltransferase I (CPT-Iα) gene is regulated by Sp1 and nuclear factor Y: Chromosomal localization and promoter characterization. Biochem. J. 1999, 340, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Mascaró, C.; Acosta, E.; Ortiz, J.A.; Marrero, P.F.; Hegardt, F.G.; Haro, D. Control of human muscle-type carnitine palmitoyltransferase I gene transcription by peroxisome proliferator-activated receptor. J. Biol. Chem. 1998, 273, 8560–8563. [Google Scholar] [CrossRef] [PubMed]
- Leone, T.C.; Weinheimer, C.J.; Kelly, D.P. A critical role for the peroxisome proliferator-activated receptor α (PPARα) in the cellular fasting response: The PPARα-null mouse as a model of fatty acid oxidation disorders. Proc. Natl. Acad. Sci. USA 1999, 96, 7473–7478. [Google Scholar] [CrossRef] [PubMed]
- Ribet, C.; Montastier, E.; Valle, C.; Bezaire, V.; Mazzucotelli, A.; Mairal, A.; Viguerie, N.; Langin, D. Peroxisome proliferator-activated receptor-α control of lipid and glucose metabolism in human white adipocytes. Endocrinology 2010, 151, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.L.; Zhuo, M.Q.; Luo, Z.; Song, Y.F.; Pan, Y.X.; Huang, C.; Hu, W.; Chen, Q.L. Peroxisome proliferator-activated receptor alpha1 in yellow catfish Pelteobagrus fulvidraco: Molecular characterization, mRNA tissue expression and transcriptional regulation by insulin in vivo and in vitro. Comp. Biochem. Physiol. 2015, 183, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Huang, C.J. Changes in liver PPARα mRNA expression in response to two levels of high-safflower-oil diets correlate with changes in adiposity and serum leptin in rats and mice. J. Nutr. Biochem. 2007, 18, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Napal, L.; Marrero, P.F.; Haro, D. An intronic peroxisome proliferator-activated receptor-binding sequence mediates fatty acid induction of the human carnitine palmitoyltransferase 1A. J. Mol. Biol. 2005, 354, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Attia, R.R.; Connaughton, S.; Niesen, M.I.; Ness, G.C.; Elam, M.B.; Hori, R.T.; Cook, G.A.; Park, E.A. Peroxisome proliferator activated receptor α (PPARα) and PPAR gamma coactivator (PGC-1α) induce carnitine palmitoyltransferase IA (CPT-1A) via independent gene elements. Mol. Cell. Endocrinol. 2010, 325, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.L.; Zhuo, M.Q.; Luo, Z.; Pan, Y.X.; Song, Y.F.; Huang, C.; Zhu, Q.L.; Hu, W.; Chen, Q.L. Peroxisome proliferator-activated receptor gamma (PPARγ) in yellow catfish Pelteobagrus fulvidraco: Molecular characterization, mRNA expression and transcriptional regulation by insulin in vivo and in vitro. Gen. Comp. Endocr. 2015, 212, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Feng, Y.; Yang, W.J.; Shu, G.; Jiang, Q.Y.; Wang, X.Q. Effects of dietary thiazolidinedione supplementation on growth performance, intramuscular fat and related genes mRNA abundance in the longissimus dorsi muscle of finishing pigs. Asian Australas. J. Anim. 2013, 26, 1012. [Google Scholar] [CrossRef] [PubMed]
- Opuszynski, K.; Shireman, J.V. Herbivorous Fishes: Culture and Use for Weed Management; CRC Press Inc.: Boca Raton, FL, USA, 1995. [Google Scholar]
- Wang, Y.; Lu, Y.; Zhang, Y.; Ning, Z.; Li, Y.; Zhao, Q.; Lu, H.; Huang, R.; Xia, X.; Feng, Q.; et al. The draft genome of the grass carp (Ctenopharyngodon idellus) provides insights into its evolution and vegetarian adaptation. Nat. Genet. 2015, 47, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Valen, E.; Sandelin, A. Genomic and chromatin signals underlying transcription start-site selection. Trends Genet. 2011, 27, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Batarseh, A.; Barlow, K.D.; Martinez-Arguelles, D.B.; Papadopoulos, V. Functional characterization of the human translocator protein (18kDa) gene promoter in human breast cancer cell lines. BBA-Gene Regul. Mech. 2012, 1819, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Smale, S.T.; Kadonaga, J.T. The RNA polymerase II core promoter. Annu. Rev. Biochem. 2003, 72, 449–479. [Google Scholar] [CrossRef] [PubMed]
- Suske, G. The Sp-family of transcription factors. Gene 1999, 238, 291–300. [Google Scholar] [CrossRef]
- Wierstra, I. Sp1: Emerging roles-beyond constitutive activation of TATA-less housekeeping genes. Biochem. Biophys. Res. Commun. 2008, 372, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bolognese, F.; Pitarque-Martì, M.; Cicero, V.L.; Mantovani, R.; Maier, J.A. Characterization of the human EDF-1 minimal promoter: Involvement of NFY and Sp1 in the regulation of basal transcription. Gene 2006, 374, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Xie, Q.; Ma, J.; Yang, S.; Chen, Q.; Hong, A. Nuclear factor Y is required for basal activation and chromatin accessibility of fibroblast growth factor receptor 2 promoter in osteoblast-like cells. J. Biol. Chem. 2009, 284, 3136–3147. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.M.; Leung, K.S.; Lee, K.H. TFBS identification based on genetic algorithm with combined representations and adaptive post-processing. Bioinformatics 2007, 24, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Hayes, L.; Song, S.; Lavrentyev, E.N.; Jansen, M.S.; Hillgartner, F.B.; Tian, L.; Wood, P.A.; Cook, G.A.; Park, E.A. A thyroid hormone response unit formed between the promoter and first intron of the carnitine palmitoyltransferase-Iα gene mediates the liver-specific induction by thyroid hormone. J. Biol. Chem. 2003, 278, 7964–7972. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Lo, L.J.; Chan, W.K. Temporal expression and T3 induction of thyroid hormone receptors α1 and β1 during early embryonic and larval development in zebrafish, Danio rerio. Mol. Cell. Endocrinol. 2000, 159, 187–195. [Google Scholar] [CrossRef]
- Johnson, K.M.; Lema, S.C. Tissue-specific thyroid hormone regulation of gene transcripts encoding iodothyronine deiodinases and thyroid hormone receptors in striped parrotfish (Scarus iseri). Gen. Comp. Endocrinol. 2011, 172, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.K., III. Peroxisomal β-oxidation, PPARα, and steatohepatitis. Am. J. Physiol. 2001, 281, G1333–G1339. [Google Scholar]
- Lawrence, J.W.; Li, Y.; Chen, S.; DeLuca, J.G.; Berger, J.P.; Umbenhauer, D.R.; Moller, D.E.; Zhou, G. Differential gene regulation in human versus rodent hepatocytes by peroxisome proliferator-activated receptor (PPAR) α: PPARα fails to induce peroxisome proliferation-associated genes in human cells independently of the level of receptor expression. J. Biol. Chem. 2001, 276, 31521–31527. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.H.; Satoh, H.; Herzig, S.; Lee, C.H.; Hedrick, S.; Kulkarni, R.; Evans, R.M.; Olefsky, J.; Montminy, M. PGC-1 promotes insulin resistance in liver through PPAR-α-dependent induction of TRB-3. Nat. Med. 2004, 10, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.C.; Puigserver, P.; Chen, G.; Donovan, J.; Wu, Z.; Rhee, J.; Adelmant, G.; Stafford, J.; Kahn, C.R.; Granner, D.K.; et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 2001, 413, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.M.; Moore, L.B.; Smith-Oliver, T.A.; Wilkison, W.O.; Willson, T.M.; Kliewer, S.A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ). J. Biol. Chem. 1995, 270, 12953–12956. [Google Scholar] [CrossRef] [PubMed]
- Gilde, A.J.; van der Lee, K.A.; Willemsen, P.H.; Chinetti, G.; van der Leij, F.R.; van der Vusse, G.J.; Staels, B.; van Bilsen, M. Peroxisome proliferator-activated receptor (PPAR) α and PPARβ/δ, but not PPARγ, modulate the expression of genes involved in cardiac lipid metabolism. Circ. Res. 2003, 92, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Patsouris, D.; Reddy, J.K.; Muller, M.; Kersten, S. Peroxisome proliferator-activated receptor α mediates the effects of high-fat diet on hepatic gene expression. Endocrinology 2006, 147, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Glass, C.K.; Rosenfeld, M.G. Coactivator and corepressor complexes in nuclear receptor function. Curr. Opin. Genet. Dev. 1999, 9, 140–147. [Google Scholar] [CrossRef]
- Robyr, D.; Wolffe, A.P.; Wahli, W. Nuclear hormone receptor coregulators in action: Diversity for shared tasks. Mol. Endocrinol. 2000, 14, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Stanley, T.B.; Leesnitzer, L.M.; Montana, V.G.; Galardi, C.M.; Lambert, M.H.; Holt, J.A.; Xu, H.E.; Moore, L.B.; Blanchard, S.G.; Stimmel, J.B. Subtype specific effects of peroxisome proliferator-activated receptor ligands on corepressor affinity. Biochemistry 2003, 42, 9278–9287. [Google Scholar] [CrossRef] [PubMed]
- Zianni, M.; Tessanne, K.; Merighi, M.; Laguna, R.; Tabita, F.R. Identification of the DNA bases of a DNase I footprint by the use of dye primer sequencing on an automated capillary DNA analysis instrument. J. Biomol. Tech. 2006, 17, 103–113. [Google Scholar] [PubMed]
- Read, M.A.; Cordle, S.R.; Veach, R.A.; Carlisle, C.D.; Hawiger, J. Cell-free pool of CD14 mediates activation of transcription factor NF-kappa B by lipopolysaccharide in human endothelial cells. Proc. Natl. Acad. Sci. USA 1993, 90, 9887–9891. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.; Fujimoto, N.M.; Goeke, B.J.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]


| Name | Primer | Sequence (5′–3′) |
|---|---|---|
| Primers for promoter construct | ||
| CPT Iα1b | +36R | CCCAAGCTTCTAACAATTCCGATGATGTGG |
| −2695F | CGAGCTCAAGCAAGAAGGCAATAGGGT | |
| CPT Iα2a | +30R | CCCAAGCTTCGTGCCTTTGATACTAAGTGCG |
| −2631F | CGAGCTCGGGCTACCAGTAACTATAAGGG | |
| Primers for deleting PPAR binding sites of promoters | ||
| 1bMut | - PPAR1F | GCACTTTTCTTTTCCAGAATTTTGTAGTTGTGAGTCA |
| - PPAR1R | CTGGAAAAGAAAAGTGCCTTTAATTTCTTGCTC | |
| - PPAR2F | TGTAGTGGCGACATCTCAGTATCATCTGGGTGG | |
| - PPAR2R | TGAGATGTCGCCACTACAGGGAGGTGGAAAGGG | |
| 2aMut | - PPAR1F | GTTTTACAATTTGTTGGAAATTTTGTTTTGTTTAATG |
| - PPAR1R | TCCAACAAATTGTAAAACAAGCATTGCCAACAA | |
| - PPAR2F | GACTTCGGTAACACTAACAAAACAGTGGGGTAAATC | |
| - PPAR2R | GTTAGTGTTACCGAAGTCAACATTCTCACATTG | |
| - PPAR3F | ATGCTCACCGAACAGCTTATGTAAGGCAAGGGA | |
| - PPAR3R | AAGCTGTTCGGTGAGCATGGAACAGGATTTACC | |
| - PPAR4F | GGAAGGGGTGATGGAAAAAATCTGTGGTGTCTG | |
| - PPAR4R | TTTTCCATCACCCCTTCCCTTGCCTTACATAAG | |
| Oligonucleotide for EMSA assay | ||
| CPT Iα1b - PPAR1 | Biotin-probe | Biotin—TAAGCAACTTTGCACTGATTTAC |
| Mutative-competitor | TAAGCAACCCCCCACTGATTTAC | |
| CPT Iα1b - PPAR2 | Biotin-probe | Biotin—ATTTGTTCTTTCCCCCAATGGCC |
| Mutative-competitor | ATTTGTTCCCCCCCCCAATGGCC | |
| CPT Iα2a - PPAR1 | Biotin-probe | Biotin—CGATCAACTATTTCATAGTTGTT |
| Mutative-competitor | CGATCAAGGGGTTCATAGTTGTT | |
| CPT Iα2a - PPAR2 | Biotin-probe | Biotin—AATAATTGTGGGAAAGGTGAAAG |
| Mutative-competitor | AATAATTGTGGGGGGGGTGAAAG | |
| CPT Iα2a - PPAR3 | Biotin-probe | Biotin—TCTTGCTGTGAAATAGGTCAGTT |
| Mutative-competitor | TCTTGCTGTGAAGGGGGTCAGTT | |
| CPT Iα2a - PPAR3 | Biotin-probe | Biotin—GGCTGGGTGGTCTTTTCCCACTT |
| Mutative-competitor | GGCCCCCTGGTCTTTTCCCACTT | |
| Primer for DNase I foot printing assay | ||
| M13F | GTAAAACGACGGCCAGT | |
| M13R-FAM | FAM-CAGGAAACAGCTATGAC | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.-H.; Luo, Z.; Wu, K.; Fan, Y.-F.; You, W.-J.; Zhang, L.-H. Structure and Functional Analysis of Promoters from Two Liver Isoforms of CPT I in Grass Carp Ctenopharyngodon idella. Int. J. Mol. Sci. 2017, 18, 2405. https://doi.org/10.3390/ijms18112405
Xu Y-H, Luo Z, Wu K, Fan Y-F, You W-J, Zhang L-H. Structure and Functional Analysis of Promoters from Two Liver Isoforms of CPT I in Grass Carp Ctenopharyngodon idella. International Journal of Molecular Sciences. 2017; 18(11):2405. https://doi.org/10.3390/ijms18112405
Chicago/Turabian StyleXu, Yi-Huan, Zhi Luo, Kun Wu, Yao-Fang Fan, Wen-Jing You, and Li-Han Zhang. 2017. "Structure and Functional Analysis of Promoters from Two Liver Isoforms of CPT I in Grass Carp Ctenopharyngodon idella" International Journal of Molecular Sciences 18, no. 11: 2405. https://doi.org/10.3390/ijms18112405
APA StyleXu, Y.-H., Luo, Z., Wu, K., Fan, Y.-F., You, W.-J., & Zhang, L.-H. (2017). Structure and Functional Analysis of Promoters from Two Liver Isoforms of CPT I in Grass Carp Ctenopharyngodon idella. International Journal of Molecular Sciences, 18(11), 2405. https://doi.org/10.3390/ijms18112405







