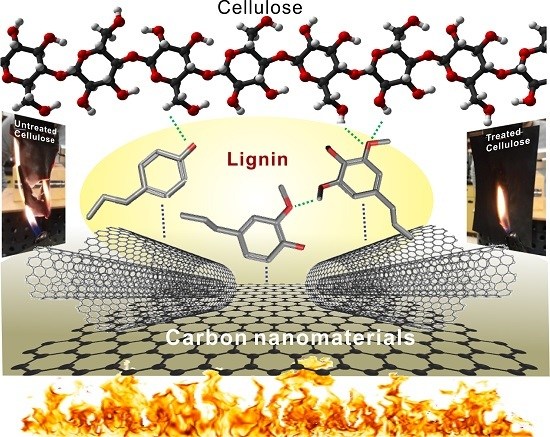
Lignin-Modified Carbon Nanotube/Graphene Hybrid Coating as Efficient Flame Retardant
Abstract
:1. Introduction
2. Results and Discussion
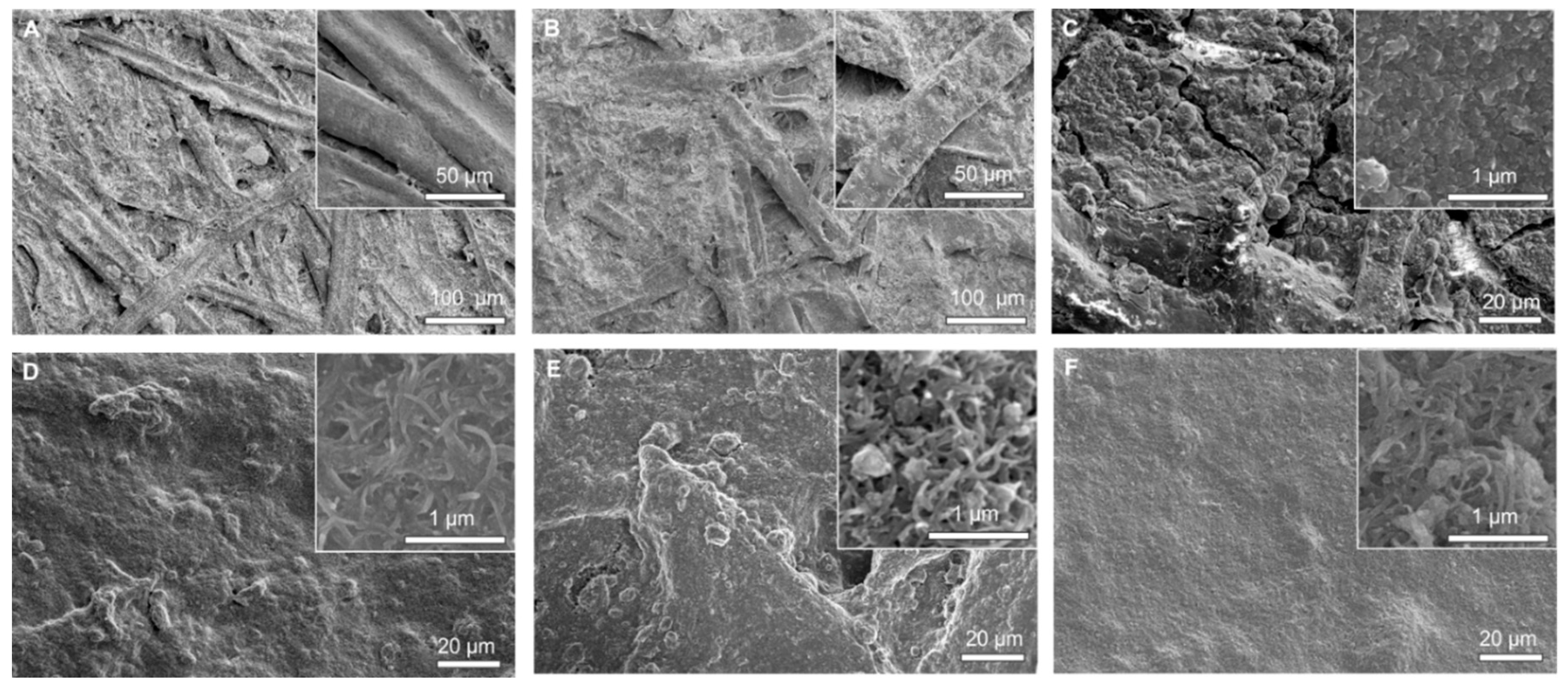
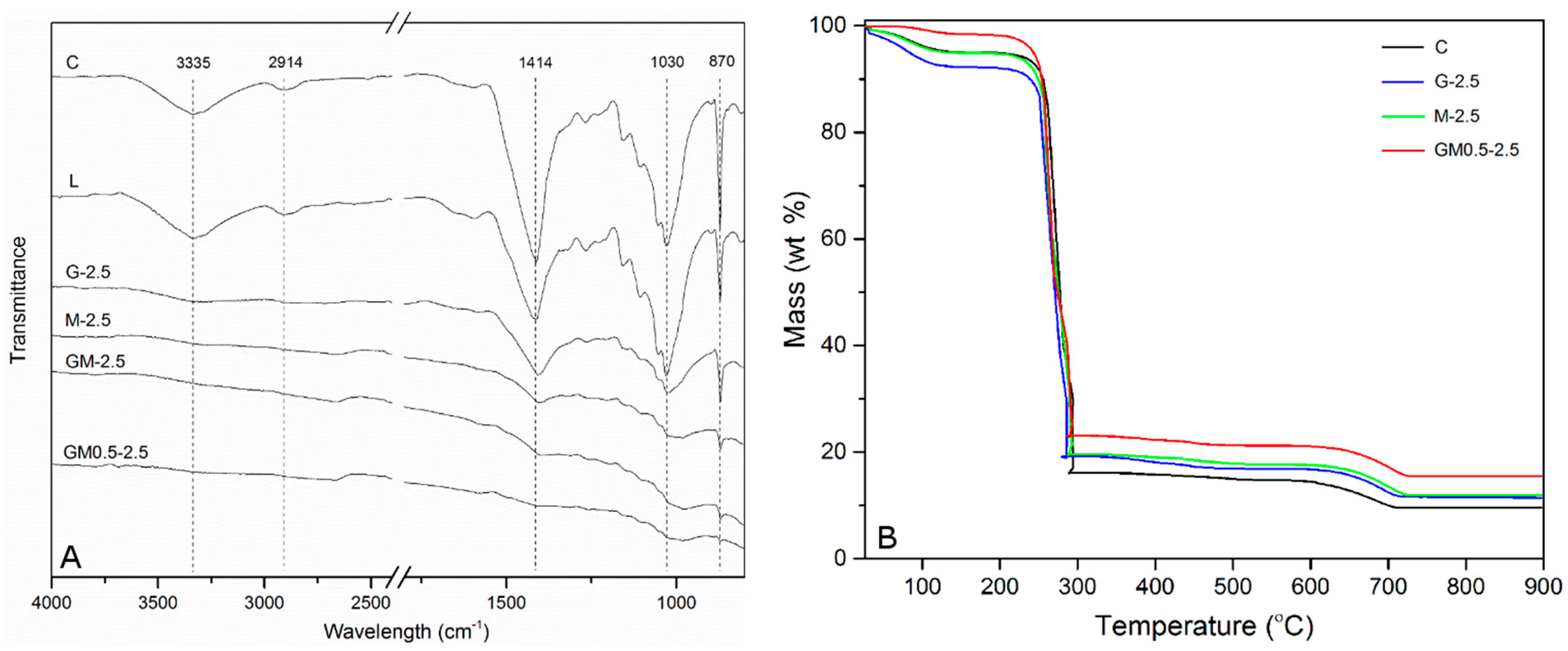
2.1. Characterization of Coated Papers
2.2. Gas Permeability of Coated Papers
2.3. Flame-Retardant Performance
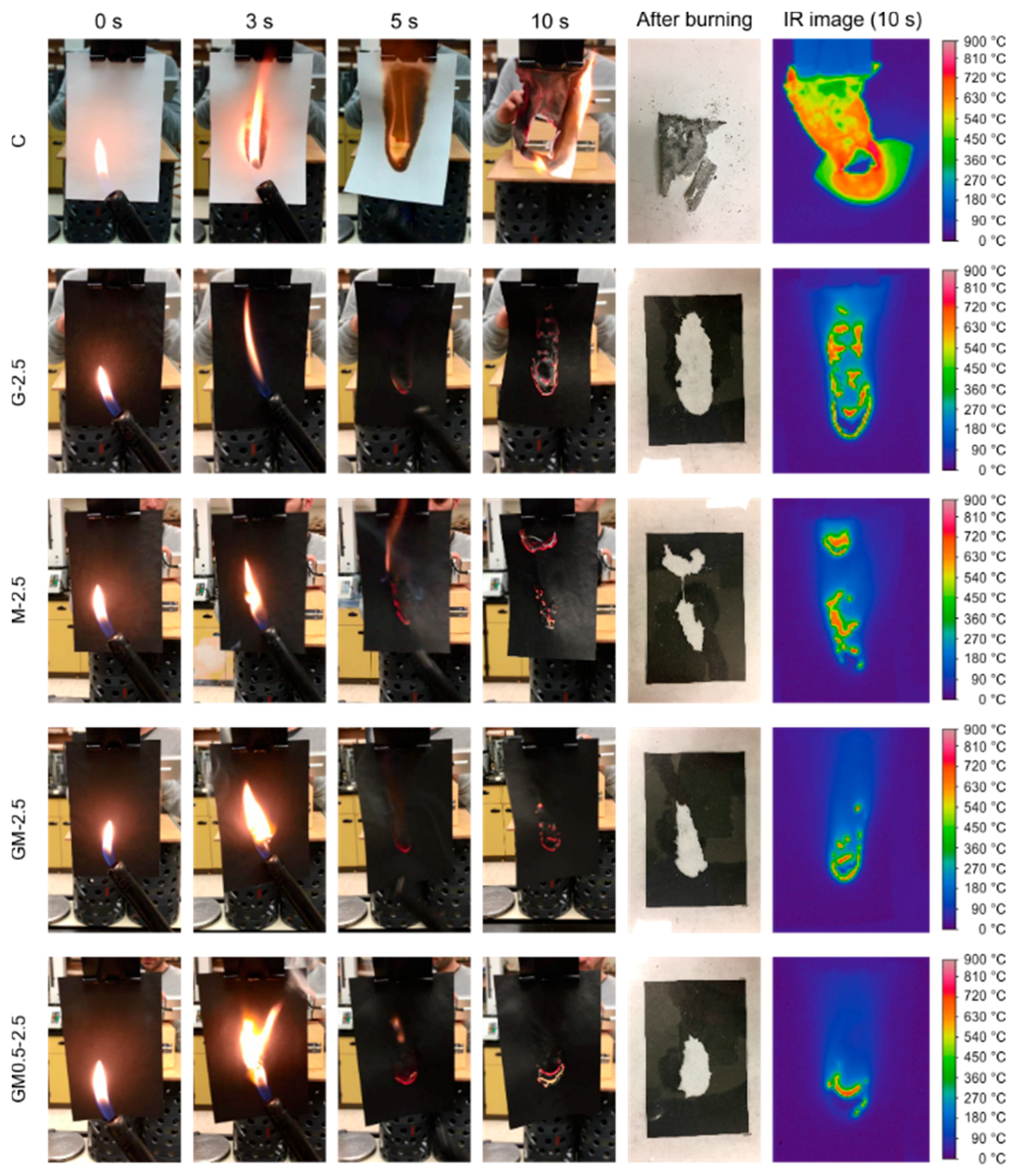
2.3.1. Effect of Carbon Nanomaterials
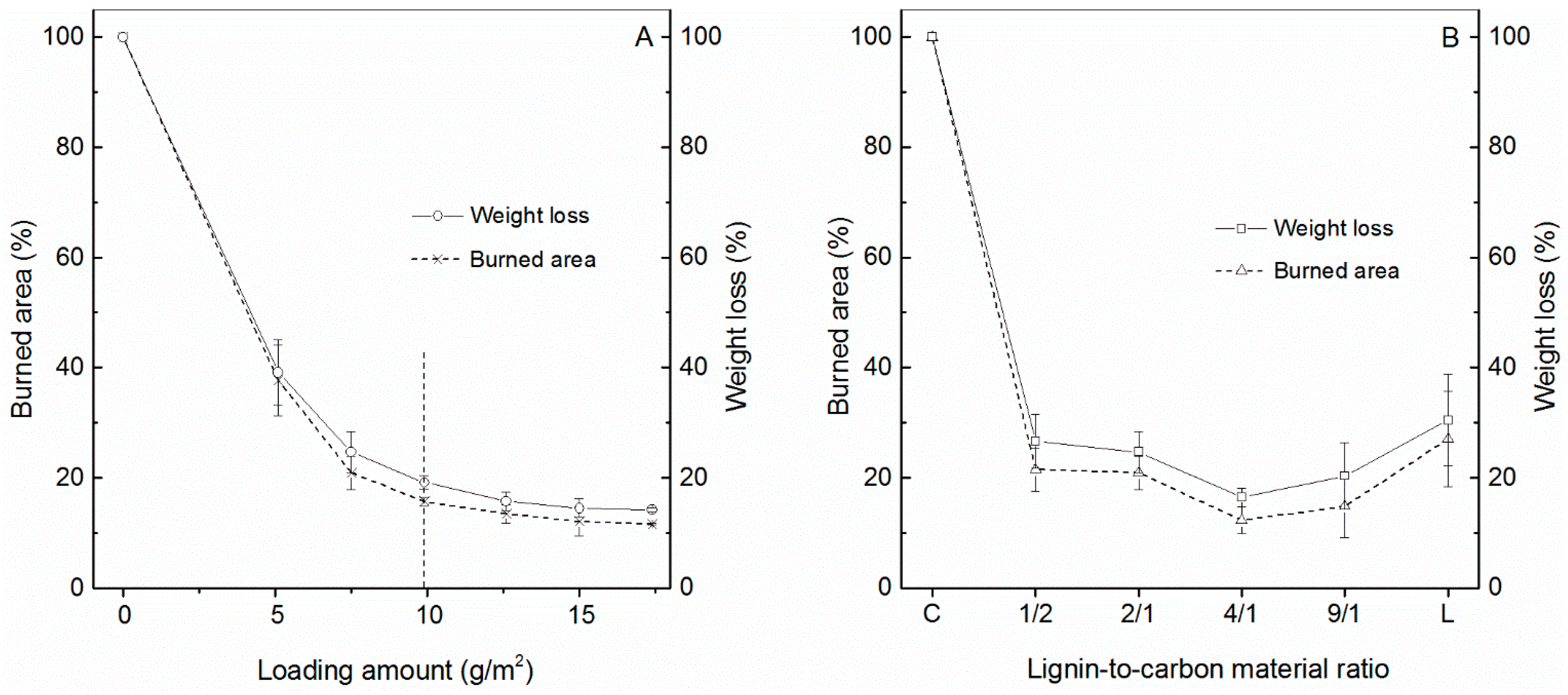
2.3.2. Effect of Coating Density
2.3.3. Effect of the Ratio of Lignin to Carbon Nanomaterials
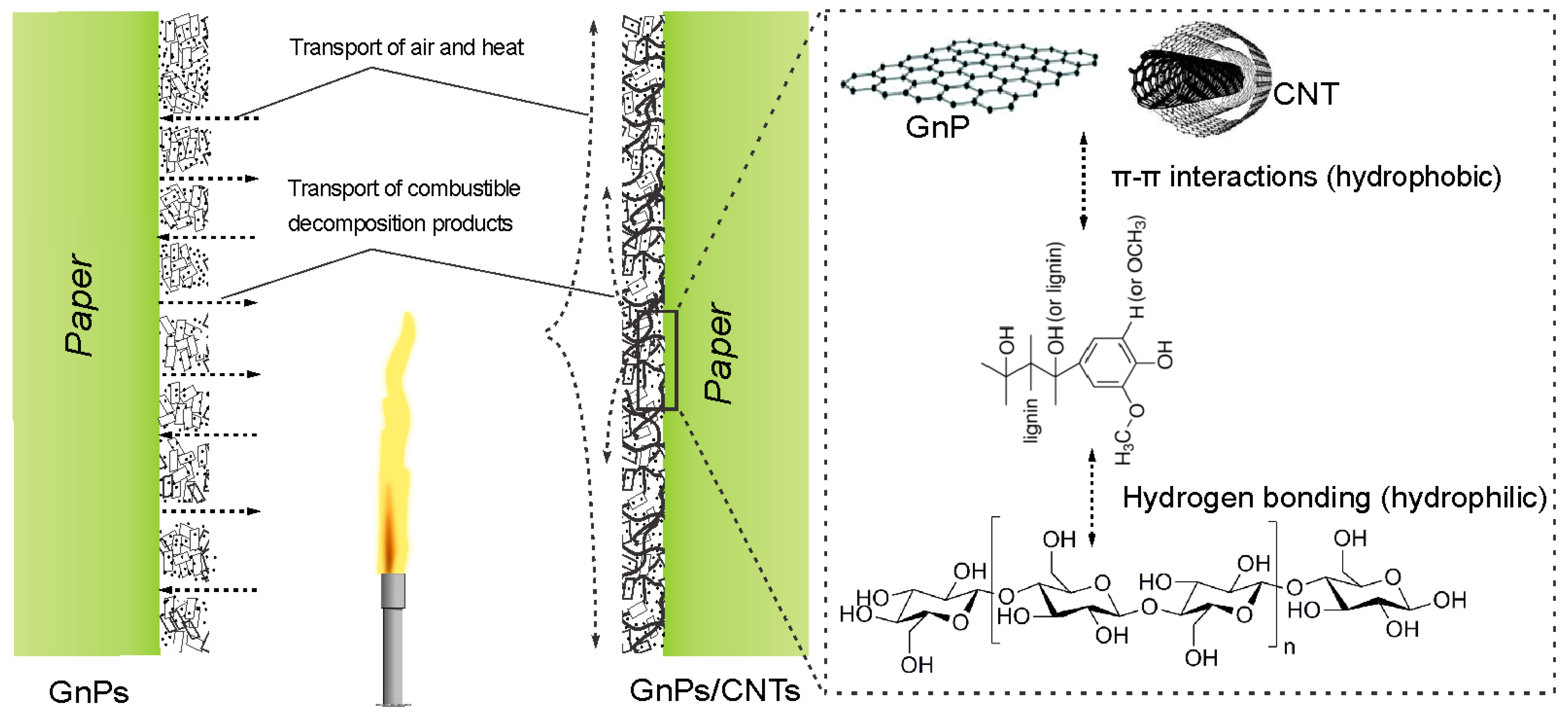
3. Flame-Retardant Mechanism
4. Materials and Methods
4.1. Materials
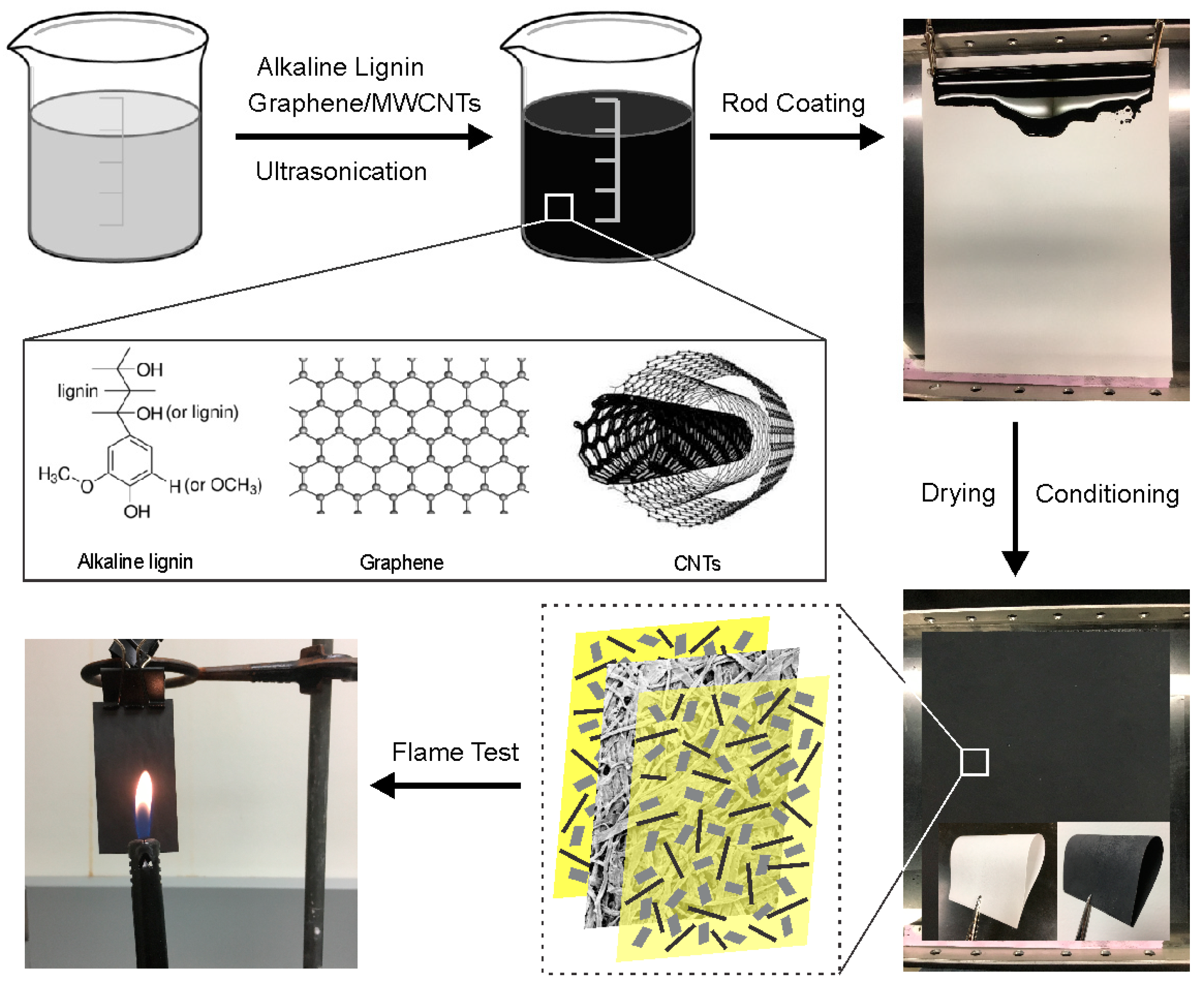
4.2. Preparation of GnP/CNT/Lignin-Coated Paper
4.3. Characterization
4.4. Flame Resistance Test
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haynes, H.J. Fire Loss in the United States during 2015; National Fire Protection Association: Quincy, MA, USA, 2016. [Google Scholar]
- Zhang, T.; Yan, H.; Peng, M.; Wang, L.; Ding, H.; Fang, Z. Construction of flame retardant nanocoating on ramie fabric via layer-by-layer assembly of carbon nanotube and ammonium polyphosphate. Nanoscale 2013, 5, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Alongi, J.; Colleoni, C.; Rosace, G.; Malucelli, G. The role of pre-hydrolysis on multi step sol–gel processes for enhancing the flame retardancy of cotton. Cellulose 2013, 20, 525–535. [Google Scholar] [CrossRef]
- Fang, F.; Tong, B.; Du, T.; Zhang, X.; Meng, Y.; Liu, X.; Tian, X. Unique nanobrick wall nanocoating for flame-retardant cotton fabric via layer-by-layer assembly technique. Cellulose 2016, 23, 3341–3354. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, X.; Meng, Y.; Ding, X.; Bao, C.; Li, S.; Zhang, H.; Tian, X. Boron-containing intumescent multilayer nanocoating for extinguishing flame on cotton fabric. Cellulose 2016, 23, 2161–2172. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Li, Y.; Sun, J. Intumescent flame-retardant and self-healing superhydrophobic coatings on cotton fabric. ACS Nano 2015, 9, 4070–4076. [Google Scholar] [CrossRef] [PubMed]
- Alongi, J.; Carosio, F.; Malucelli, G. Layer by layer complex architectures based on ammonium polyphosphate, chitosan and silica on polyester-cotton blends: Flammability and combustion behaviour. Cellulose 2012, 19, 1041–1050. [Google Scholar] [CrossRef]
- Chang, S.; Slopek, R.P.; Condon, B.; Grunlan, J.C. Surface coating for flame-retardant behavior of cotton fabric using a continuous layer-by-layer process. Ind. Eng. Chem. Res. 2014, 53, 3805–3812. [Google Scholar] [CrossRef]
- Lu, B.Q.; Zhu, Y.J.; Chen, F. Highly flexible and nonflammable inorganic hydroxyapatite paper. Chem. Eur. J. 2014, 20, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Köklükaya, O.; Carosio, F.; Grunlan, J.C.; Wågberg, L. Flame-retardant paper from wood fibers functionalized via layer-by-layer assembly. ACS Appl. Mater. Interfaces 2015, 7, 23750–23759. [Google Scholar] [CrossRef] [PubMed]
- Priegert, A.M.; Siu, P.W.; Hu, T.Q.; Gates, D.P. Flammability properties of paper coated with poly (methylenephosphine), an organophosphorus polymer. Fire Mater. 2015, 39, 647–657. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.-A.; Hofmann, D.; Mülhaupt, R.; Schartel, B. Flame retardancy through carbon nanomaterials: Carbon black, multiwall nanotubes, expanded graphite, multi-layer graphene and graphene in polypropylene. Polym. Degrad. Stab. 2013, 98, 1495–1505. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Grulke, E.; Hilding, J.; Harris, R.; Awad, W.; Douglas, J. Thermal degradation and flammability properties of poly (propylene)/carbon nanotube composites. Macromol. Rapid Commun. 2002, 23, 761–765. [Google Scholar] [CrossRef]
- Zhuo, D.; Wang, R.; Wu, L.; Guo, Y.; Ma, L.; Weng, Z.; Qi, J. Flame retardancy effects of graphene nanoplatelet/carbon nanotube hybrid membranes on carbon fiber reinforced epoxy composites. J. Nanomater. 2013, 2013, 83. [Google Scholar] [CrossRef]
- Huang, G.; Gao, J.; Wang, X.; Liang, H.; Ge, C. How can graphene reduce the flammability of polymer nanocomposites? Mater. Lett. 2012, 66, 187–189. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Du, F.; Douglas, J.F.; Winey, K.I.; Harris, R.H.; Shields, J.R. Nanoparticle networks reduce the flammability of polymer nanocomposites. Nat. Mater. 2005, 4, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Hu, C.; Song, L.; Huang, X.; Chen, N.; Qu, L. A large-area, flexible, and flame-retardant graphene paper. Adv. Funct. Mater. 2016, 26, 1470–1476. [Google Scholar] [CrossRef]
- Zhou, K.; Gui, Z.; Hu, Y. The influence of graphene based smoke suppression agents on reduced fire hazards of polystyrene composites. Compos. Part A 2016, 80, 217–227. [Google Scholar] [CrossRef]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; van der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable atomic membranes from graphene sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.E.; Guan, J.; Ricard, M.; Dubey, G.; Su, J.; Lopinski, G.; Dorris, G.; Bourne, O.; Simard, B. Multifunctional single-walled carbon nanotube–cellulose composite paper. J. Mater. Chem. 2010, 20, 2400–2407. [Google Scholar] [CrossRef]
- Liang, S.; Neisius, N.M.; Gaan, S. Recent developments in flame retardant polymeric coatings. Prog. Org. Coat. 2013, 76, 1642–1665. [Google Scholar] [CrossRef]
- Kim, M.-J.; Jeon, I.-Y.; Seo, J.-M.; Dai, L.; Baek, J.-B. Graphene phosphonic acid as an efficient flame retardant. ACS Nano 2014, 8, 2820–2825. [Google Scholar] [CrossRef] [PubMed]
- Nine, M.J.; Tran, D.N.; Tung, T.T.; Kabiri, S.; Losic, D. Graphene-borate as an efficient fire retardant for cellulosic materials with multiple and synergetic modes of action. ACS Appl. Mater. Interfaces 2017, 9, 10160–10168. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, M.; Fang, Y.; Qiu, T.; Zhang, J.; Zhi, L. Rod-coating: Towards large-area fabrication of uniform reduced graphene oxide films for flexible touch screens. Adv. Mater. 2012, 24, 2874–2878. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Dichiara, A.B.; Bai, J. Carbon nanotube–graphene nanoplatelet hybrids as high-performance multifunctional reinforcements in epoxy composites. Compos. Sci. Technol. 2013, 74, 221–227. [Google Scholar] [CrossRef]
- Dichiara, A.B.; Sherwood, T.J.; Benton-Smith, J.; Wilson, J.C.; Weinstein, S.J.; Rogers, R.E. Free-standing carbon nanotube/graphene hybrid papers as next generation adsorbents. Nanoscale 2014, 6, 6322–6327. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.M.; Ferguson, N.; Dichiara, A.B. Lignin-assisted double acoustic irradiation for concentrated aqueous dispersions of carbon nanotubes. RSC Adv. 2017, 7, 5488–5496. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Basak, S.; Ali, S.W. Sustainable fire retardancy of textiles using bio-macromolecules. Polym. Degrad. Stab. 2016, 133, 47–64. [Google Scholar] [CrossRef]
- De Souza, M.M.; de Sá, S.C.; Zmozinski, A.V.; Peres, R.S.; Ferreira, C.A. Biomass as the carbon source in intumescent coatings for steel protection against fire. Ind. Eng. Chem. Res. 2016, 55, 11961–11969. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, R.; Zhou, D.; Ding, G.; Soah, J.M.; Yue, C.Y.; Lu, X. Lignin-assisted direct exfoliation of graphite to graphene in aqueous media and its application in polymer composites. Carbon 2015, 83, 188–197. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, W.; Zhang, C.; Liang, Z.; Wang, B. Study of fire retardant behavior of carbon nanotube membranes and carbon nanofiber paper in carbon fiber reinforced epoxy composites. Carbon 2010, 48, 1799–1806. [Google Scholar] [CrossRef]
- Yin, Y.; Berglund, L.; Salmén, L. Effect of steam treatment on the properties of wood cell walls. Biomacromolecules 2011, 12, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Åkerholm, M.; Hinterstoisser, B.; Salmén, L. Characterization of the crystalline structure of cellulose using static and dynamic FT-IR spectroscopy. Carbohydr. Res. 2004, 339, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Marchessault, R. Application of infra-red spectroscopy to cellulose and wood polysaccharides. Pure Appl. Chem. 1962, 5, 107–130. [Google Scholar] [CrossRef]
- Ghule, K.; Ghule, A.V.; Chen, B.-J.; Ling, Y.-C. Preparation and characterization of ZnO nanoparticles coated paper and its antibacterial activity study. Green Chem. 2006, 8, 1034–1041. [Google Scholar] [CrossRef]
- Higginbotham, A.L.; Lomeda, J.R.; Morgan, A.B.; Tour, J.M. Graphite oxide flame-retardant polymer nanocomposites. ACS Appl. Mater. Interfaces 2009, 1, 2256–2261. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, T.; Du, F.; Winey, K.I.; Groth, K.M.; Shields, J.R.; Bellayer, S.P.; Kim, H.; Douglas, J.F. Flammability properties of polymer nanocomposites with single-walled carbon nanotubes: Effects of nanotube dispersion and concentration. Polymer 2005, 46, 471–481. [Google Scholar] [CrossRef]
- David, L.; Feldman, A.; Mansfield, E.; Lehman, J.; Singh, G. Evaluating the thermal damage resistance of graphene/carbon nanotube hybrid composite coatings. Sci. Rep. 2014, 4, 4311. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Walther, J.H.; Koumoutsakos, P. Ultrafast cooling by covalently bonded graphene-carbon nanotube hybrid immersed in water. Nanotechnology 2016, 27, 465705. [Google Scholar] [CrossRef] [PubMed]
- Tracton, A.A. Coatings Technology: Fundamentals, Testing, and Processing Techniques; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Hantel, M.M.; Armstrong, M.J.; DaRosa, F.; l’Abee, R. Characterization of tortuosity in polyetherimide membranes based on Gurley and electrochemical impedance spectroscopy. J. Electrochem. Soc. 2017, 164, A334–A339. [Google Scholar] [CrossRef]
| Sample | GnPs (mg/mL) | CNTs (mg/mL) | Lignin (mg/mL) | Coating Density w/–w/o Lignin (g/m2) | Thickness (μm) | Gurley Number (s/100 mL) |
|---|---|---|---|---|---|---|
| C * | 0 | 0 | 0 | 0–0 | 113 ± 6 | 154 ± 3 |
| G-2.5 * | 10 | 0 | 20 | 7.5–2.5 | 168 ± 6 | 255 ± 40 |
| M-2.5 * | 0 | 10 | 20 | 7.5–2.5 | 164 ± 3 | 765 ± 108 |
| GM-2.5 * | 5 | 5 | 20 | 7.5–2.5 | 165 ± 5 | 889 ± 119 |
| GM0.5-2.5 * | 3.3 | 6.7 | 20 | 7.5–2.5 | 169 ± 8 | 903 ± 75 |
| GM0.5-1.7 * | 3.3 | 6.7 | 20 | 5.1–1.7 | 166 ± 3 | 411 ± 47 |
| GM0.5-3.3 * | 3.3 | 6.7 | 20 | 9.9–3.3 | 176 ± 16 | 1199 ± 262 |
| GM0.5-4.2 * | 3.3 | 6.7 | 20 | 12.6–4.2 | 180 ± 10 | 1307 ± 299 |
| GM0.5-5.0 * | 3.3 | 6.7 | 20 | 15.0–5.0 | 184 ± 20 | 1661 ± 172 |
| GM0.5-5.8 * | 3.3 | 6.7 | 20 | 17.4–5.8 | 188 ± 10 | 2368 ± 334 |
| LGM0.5-5.0 * | 6.7 | 13.3 | 10 | 7.5–5.0 | 192 ± 13 | 1717 ± 240 |
| LGM2-2.5 * | 3.3 | 6.7 | 20 | 7.5–2.5 | 169 ± 8 | 903 ± 75 |
| LGM4-1.5 * | 2 | 4 | 24 | 7.5–1.5 | 167 ± 9 | 637 ± 53 |
| LGM9-0.75 * | 1 | 2 | 27 | 7.5–0.75 | 168 ± 6 | 423 ± 53 |
| L * | 0 | 0 | 30 | 7.5–0 | 163 ± 5 | 202 ± 12 |
| Sample | Burned Area (%) | Weight Loss (%) | Maximum Temperature (°C) | |||
|---|---|---|---|---|---|---|
| - | 5 s | 15 s | 5 s | 15 s | 5 s | 15 s |
| C | 100 | 100 | 100 | 100 | 809 ± 71 | 831 ± 53 |
| G-2.5 | 15.7 ± 3.4 | 35.5 ± 1.5 | 18.2 ± 6.0 | 40.4 ± 2.7 | 791 ± 11 | 834 ± 42 |
| M-2.5 | 12.4 ± 2.7 | 21.0 ± 2.6 | 16.05 ± 1.8 | 25.3 ± 2.9 | 766 ± 5 | 705 ± 13 |
| GM-2.5 | 12.0 ± 3.1 | 22.5 ± 2.5 | 14.6 ± 2.9 | 26.4 ± 1.5 | 760 ± 3 | 709 ± 3 |
| GM-0.5-2.5 | 10.7 ± 2.9 | 20.9 ± 3.0 | 13.5 ± 2.7 | 24.7 ± 3.8 | 749 ± 16 | 730 ± 23 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, K.; Ganguly, I.; Eastin, I.; Dichiara, A.B. Lignin-Modified Carbon Nanotube/Graphene Hybrid Coating as Efficient Flame Retardant. Int. J. Mol. Sci. 2017, 18, 2368. https://doi.org/10.3390/ijms18112368
Song K, Ganguly I, Eastin I, Dichiara AB. Lignin-Modified Carbon Nanotube/Graphene Hybrid Coating as Efficient Flame Retardant. International Journal of Molecular Sciences. 2017; 18(11):2368. https://doi.org/10.3390/ijms18112368
Chicago/Turabian StyleSong, Kunlin, Indroneil Ganguly, Ivan Eastin, and Anthony B. Dichiara. 2017. "Lignin-Modified Carbon Nanotube/Graphene Hybrid Coating as Efficient Flame Retardant" International Journal of Molecular Sciences 18, no. 11: 2368. https://doi.org/10.3390/ijms18112368
APA StyleSong, K., Ganguly, I., Eastin, I., & Dichiara, A. B. (2017). Lignin-Modified Carbon Nanotube/Graphene Hybrid Coating as Efficient Flame Retardant. International Journal of Molecular Sciences, 18(11), 2368. https://doi.org/10.3390/ijms18112368