Effect of Saturated Stearic Acid on MAP Kinase and ER Stress Signaling Pathways during Apoptosis Induction in Human Pancreatic β-Cells Is Inhibited by Unsaturated Oleic Acid
Abstract
:1. Introduction
2. Results
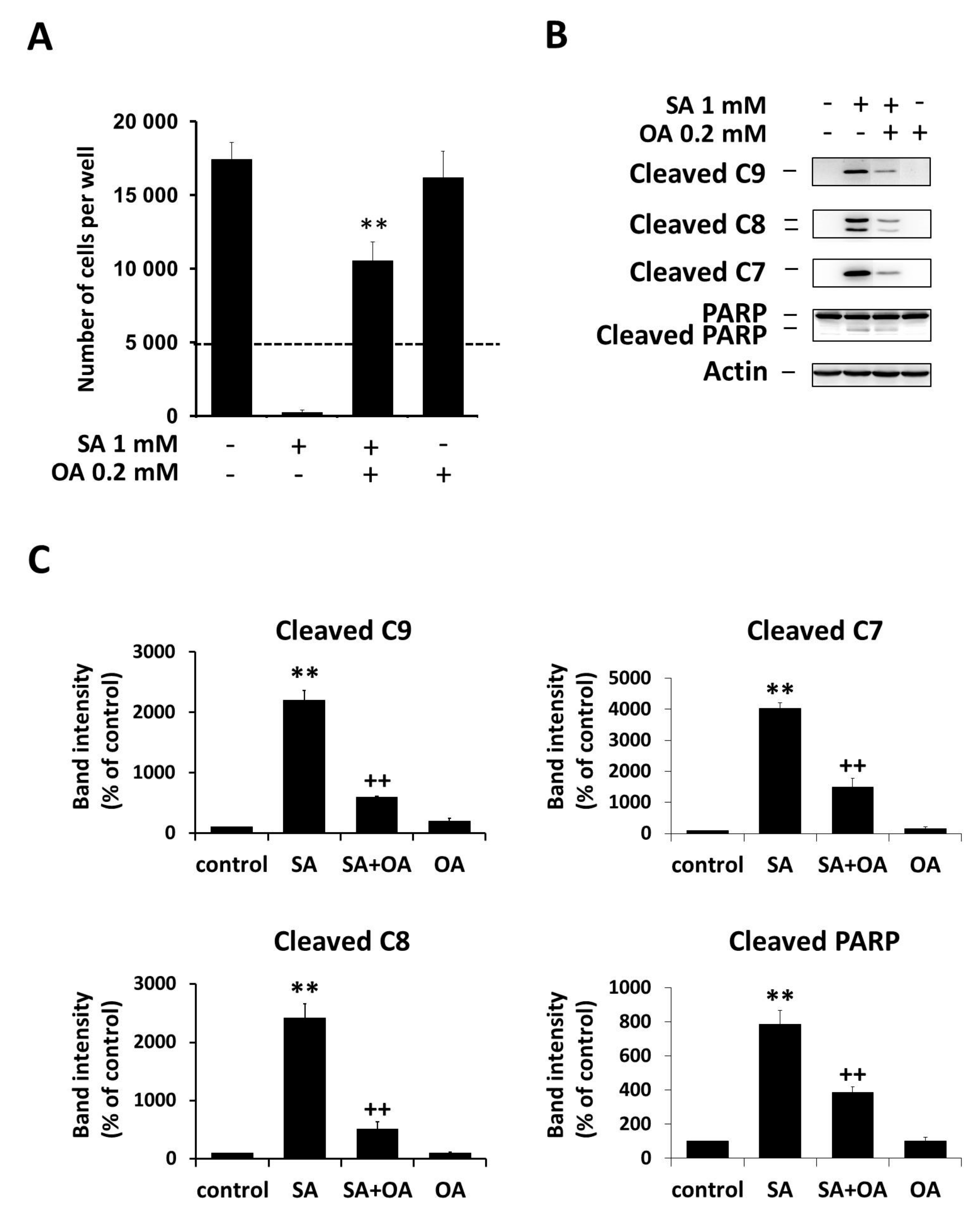
2.1. Effect of Oleic Acid on Stearic Acid-Induced Apoptosis
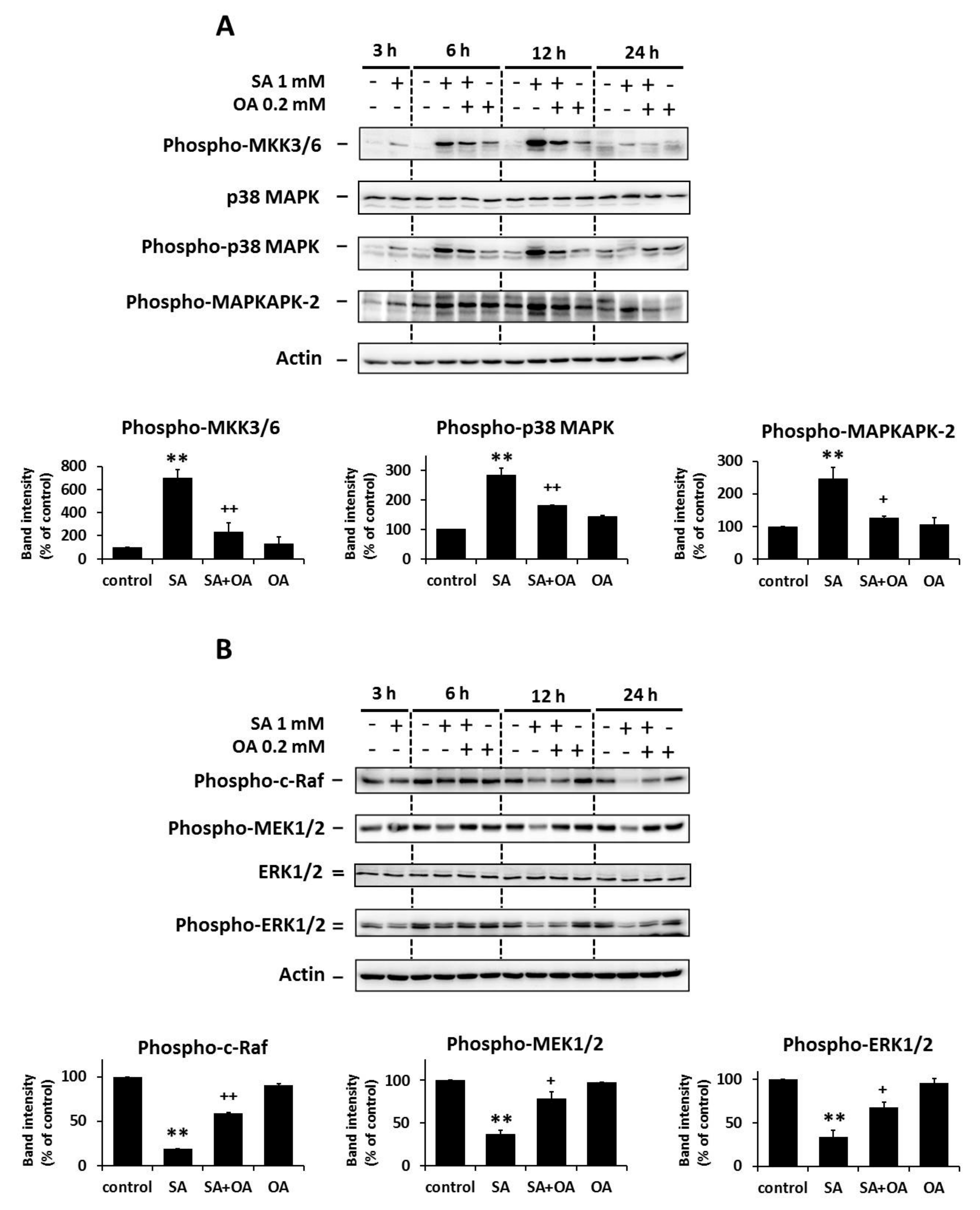
2.2. Effect of Oleic Acid on the Activation of the p38 MAPK and the Inhibition of the ERK Signaling Pathways by Stearic Acids
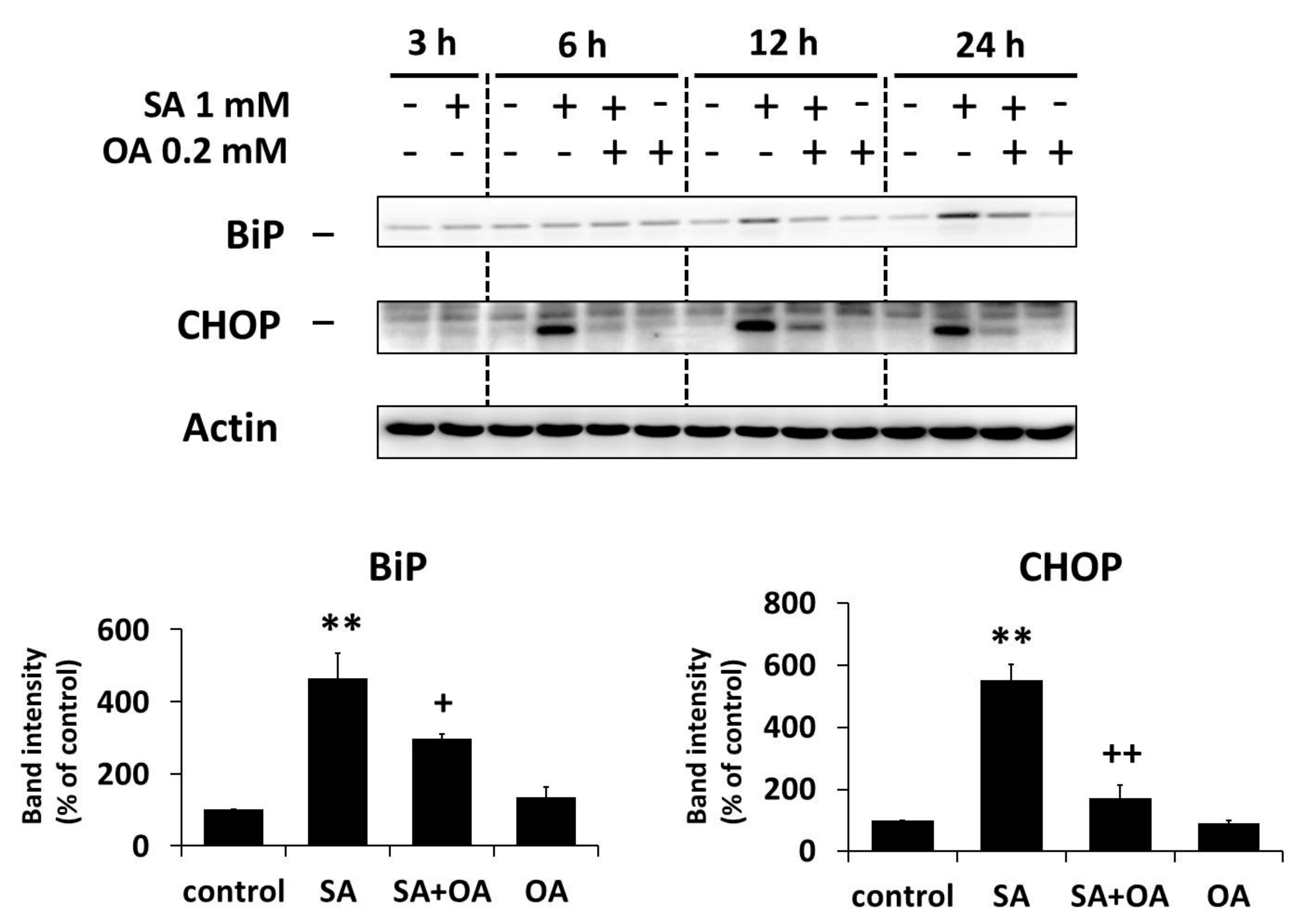
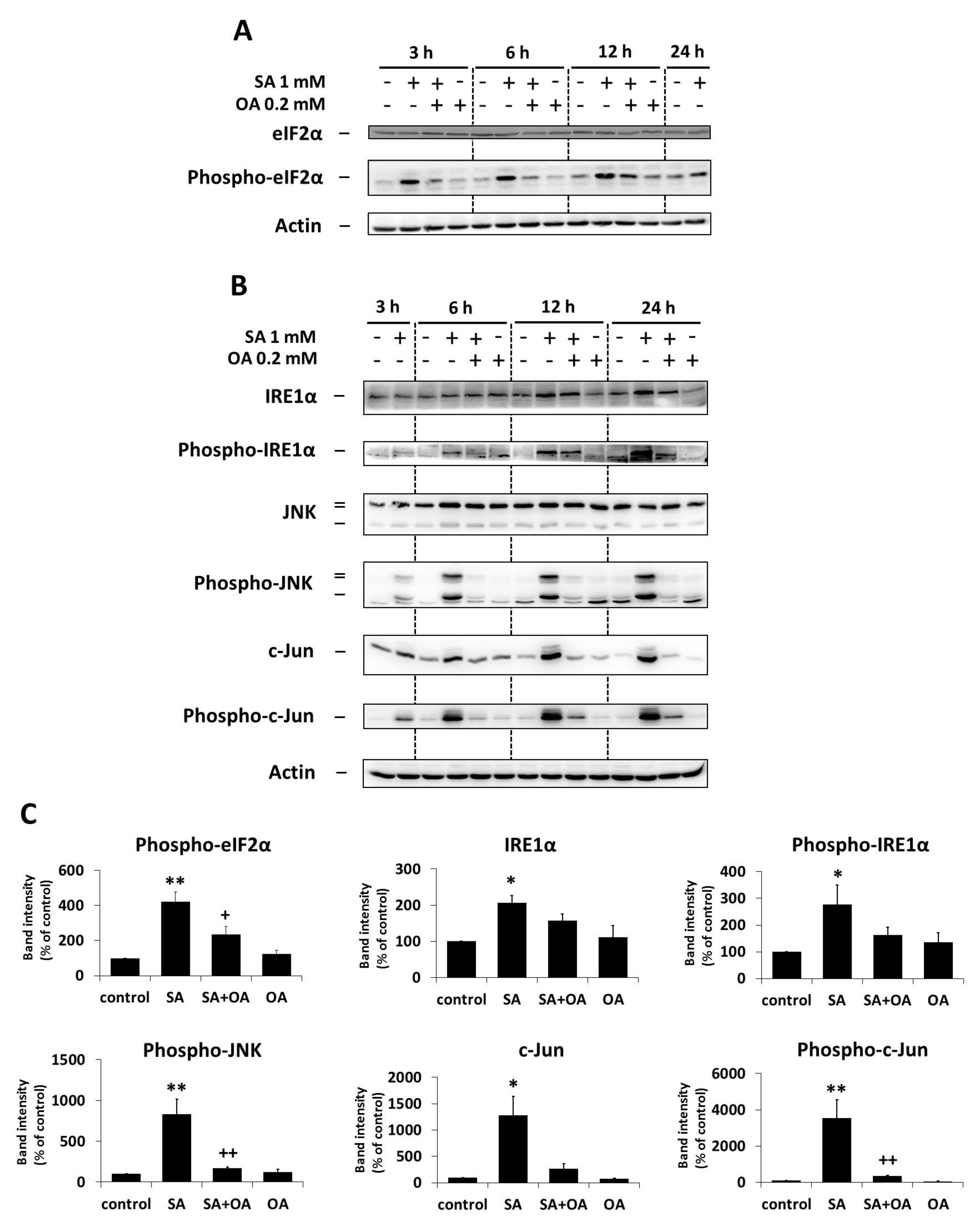
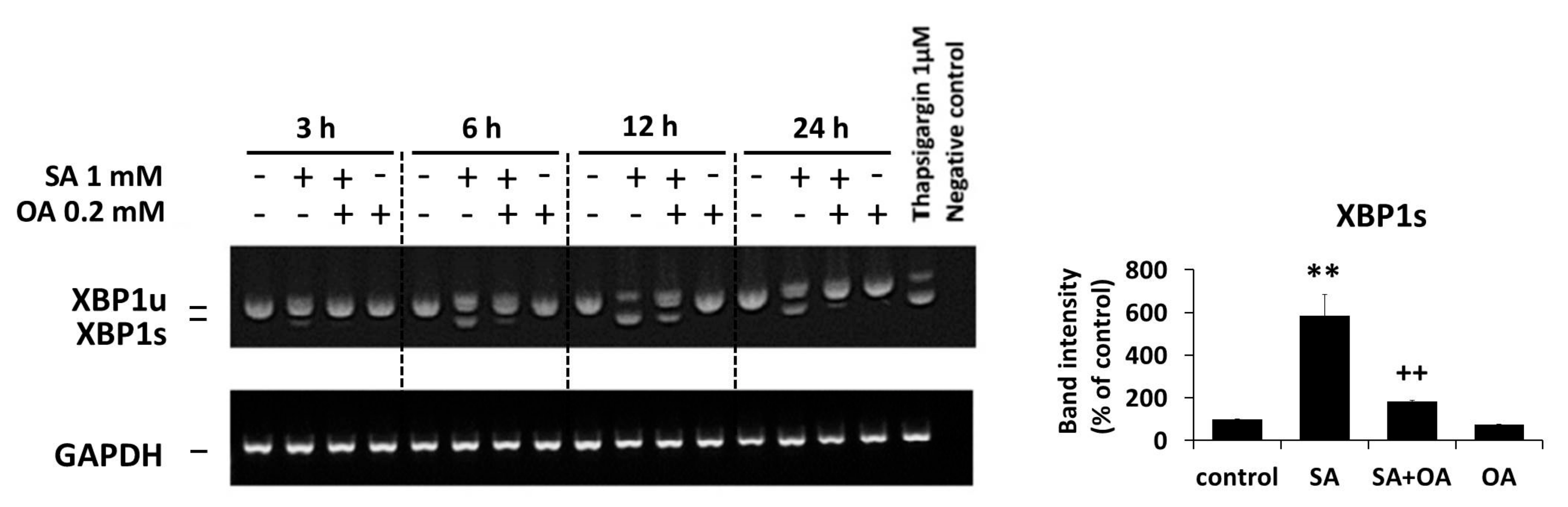
2.3. Effect of Oleic Acid on the Activation of the ER Stress Signaling Pathways by Stearic Acid
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cells and Culture Conditions
4.3. Assessment of the Effect of Oleic Acid on the Effects of Stearic Acid on Cell Growth and Viability
4.4. Western Blot Analysis
4.5. Assessment of XBP1 mRNA Splicing
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ATF6 | Activating transcription factor 6 |
| Bcl-2 | B-cell lymphoma |
| BiP | Immunoglobulin heavy chain-binding protein |
| CHOP | CCAAT-enhancer-binding protein (C/EBP) homologous protein |
| eIF2α | Eukaryotic initiation factor 2α |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| ER | Endoplasmic reticulum |
| FA | Fatty acid |
| Foxo03a | Forkhead box 03a |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| IRE1α | Inositol-requiring protein 1 |
| JNK | c-Jun N-terminal kinase |
| MAP | Mitogen-activated protein |
| MAPK | Mitogen-activated protein kinase |
| MAPKAPK | MAP kinase-activated protein kinase |
| MEK1/2 | Mitogen-activated protein kinase/ERK kinase |
| NF-κB | Nuclear factor kappa B |
| PARP | Poly ADP-ribose polymerase |
| PERK | Protein kinase RNA (PKR)-like ER kinase |
| SA | Stearic acid |
| SEM | Standard error of the mean |
| SOS | Son of sevenless |
| TG | Thapsigargin |
| T2DM | Type 2 diabetes mellitus |
| OA | Oleic acid |
| XBP1 | X-box binding protein 1 |
References
- Lupi, R.; Dotta, F.; Marselli, L.; Del Guerra, S.; Masini, M.; Santangelo, C.; Patane, G.; Boggi, U.; Piro, S.; Anello, M.; et al. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: Evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes 2002, 51, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Maedler, K.; Oberholzer, J.; Bucher, P.; Spinas, G.A.; Donath, M.Y. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes 2003, 52, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Azevedo-Martins, A.K.; Monteiro, A.P.; Lima, C.L.; Lenzen, S.; Curi, R. Fatty acid-induced toxicity and neutral lipid accumulation in insulin-producing RINm5F cells. Toxicol. In Vitro 2006, 20, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Welters, H.J.; Diakogiannaki, E.; Mordue, J.M.; Tadayyon, M.; Smith, S.A.; Morgan, N.G. Differential protective effects of palmitoleic acid and cAMP on caspase activation and cell viability in pancreatic beta-cells exposed to palmitate. Apoptosis 2006, 11, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Fürstova, V.; Kopska, T.; James, R.F.; Kovar, J. Comparison of the effect of individual saturated and unsaturated fatty acids on cell growth and death induction in the human pancreatic β-cell line NES2Y. Life Sci. 2008, 82, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Welters, H.J.; Tadayyon, M.; Scarpello, J.H.; Smith, S.A.; Morgan, N.G. Mono-unsaturated fatty acids protect against beta-cell apoptosis induced by saturated fatty acids, serum withdrawal or cytokine exposure. FEBS Lett. 2004, 560, 103–108. [Google Scholar] [CrossRef]
- Diakogiannaki, E.; Welters, H.J.; Morgan, N.G. Differential regulation of the endoplasmic reticulum stress response in pancreatic beta-cells exposed to long-chain saturated and monounsaturated fatty acids. J. Endocrinol. 2008, 197, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Nemcova-Fürstova, V.; James, R.F.L.; Kovar, J. Inhibitory effect of unsaturated fatty acids on saturated fatty acids-induced apoptosis in human pancreatic β-cells: Activation of caspases and ER stress induction. Cell. Physiol. Biochem. 2011, 27, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Biden, T.J.; Boslem, E.; Chu, K.Y.; Sue, N. Lipotoxic endoplasmic reticulum stress, β cell failure, and type 2 diabetes mellitus. Trends Endocrinol. Metab. 2014, 25, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.N.; Azevedo-Martins, A.K.; Amanso, A.M.; Carvalho, C.R.O.; Curim, R. Persistent activation of Akt or ERK prevents the toxicity induced by saturated and polyunsaturated fatty acids in RINm5F beta-cells. Toxicol. In Vitro 2008, 22, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Cvjeticanin, T.; Stojanovic, I.; Timotijevic, G.; Stosic-Grujicic, S.; Miljkovic, D. T cells cooperate with palmitic acid in induction of beta cell apoptosis. BMC Immunol. 2009, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Plaisance, V.; Perret, V.; Favrea, D.; Abderrahmania, A.; Yanga, J.-Y.; Widmanna, C.; Regazzi, R. Role of the transcriptional factor C/EBP in free fatty acid-elicited beta cell failure. Mol. Cell. Endocrinol. 2009, 305, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, X.; Huang, X.; Lu, Y.; Tang, W.; Man, Y.; Wang, S.; Xi, J.; Li, J. NADPH Oxidase 2-Derived Reactive Oxygen Species Mediate FFAs-Induced Dysfunction and Apoptosis of β-Cells via JNK, p38 MAPK and p53 Pathways. PLoS ONE 2010, 5, e15726. [Google Scholar] [CrossRef] [PubMed]
- Sramek, J.; Nemcova-Fürstova, V.; Balusikova, K.; Daniel, P.; Jelinek, M.; James, R.F.; Kovar, J. p38 MAPK is activated but does not play a key role during apoptosis induction by saturated fatty Acid in human pancreatic β-cells. Int. J. Mol. Sci. 2016, 17, 159. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M.; Ladrière, L.; Igoillo-Esteve, M.; Moura, R.F.; Cunha, D.A. Causes and cures for endoplasmic reticulum stress in lipotoxic β-cell dysfunction. Diabetes Obes. Metab. 2010, 12, S76–S82. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.G.; Gromada, J.; Urano, F. Endoplasmic reticulum stress and pancreatic β-cell death. Trends Endocrinol. Metab. 2011, 22, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Ben-Levy, R.; Hooper, S.; Wilson, R.; Paterson, H.F.; Marshall, C.J. Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr. Biol. 1998, 8, 1049–1057. [Google Scholar] [CrossRef]
- Sakai, N.; Wada, T.; Furuichi, K.; Iwata, Y.; Yoshimoto, K.; Kitagawa, K.; Kokubo, S.; Kobayashi, M.; Takeda, S.; Kida, H.; et al. p38 MAPK phosphoryloation and NF-κB activation in human crescentic glomerulonephris. Nephrol. Dial. Transplant. 2002, 17, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Demirs, J.T.; Kochevar, I.E. p38 mitogen-activated protein kinase mediates bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen but not by hydrogen peroxide. J. Biol. Chem. 2000, 275, 25939–25948. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Steelman, L.S.; Lee, J.T.; Shelton, J.G.; Navolanic, P.M.; Blalock, W.L.; Franklin, R.A.; McCubrey, J.A. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: Potential targeting for therapeutic intervention. Leukemia 2003, 17, 1263–1293. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Xu, S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 2006, 58, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Martinon, F.; Rodriguez, D.; Glimcher, L.H. The unfolded protein response: Integrating stress signals through the stress sensor IRE1α. Physiol. Rev. 2011, 91, 1219–1243. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell. Biol. 2011, 13, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Nemcova-Fürstova, V.; Balusikova, K.; Sramek, J.; James, R.F.; Kovar, J. Caspase-2 and JNK Activated by Saturated Fatty Acids are Not Involved in Apoptosis Induction but Modulate ER Stress in Human Pancreatic β-cells. Cell. Physiol. Biochem. 2013, 31, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Keane, D.; Welters, H.J.; Morgan, N.G. Life and death decisions of the pancreatic beta-cell: The role of fatty acids. Clin. Sci. 2007, 112, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Morgan, N.G. Fatty acids and beta-cell toxicity. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Sommerweiss, D.; Gorski, T.; Richter, S.; Garten, A.; Kiess, W. Oleate rescues INS-1E β-cells from palmitate-induced apoptosis by preventing activation of the unfolded protein response. Biochem. Biophys. Res. Commun. 2013, 441, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Vacaresse, N.; Lajoie-Mazenc, I.; Auge, N.; Suc, I.; Frisach, M.-F.; Salvayre, R.; Nègre-Salvayre, A. Activation of epithelial growth factor receptor pathway by unsaturated fatty acids. Circ. Res. 1995, 85, 892–899. [Google Scholar] [CrossRef]
- Holzer, R.G.; Park, E.J.; Li, N.; Tran, H.; Chen, M.; Choi, C.; Solinas, G.; Karin, M. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell 2011, 147, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Karnovsky, M.J.; Kleinfeld, A.M.; Hoover, R.L.; Klausner, R.D. The concept of lipid domains in membranes. J. Cell. Biol. 1982, 94, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, W.M.; Cragg, H.; Docherty, H.M.; Read, M.L.; James, R.F.; Aynsley-Green, A.; Docherty, K. Impaired expression of transcription factor IUF1 in a pancreatic beta-cell line derived from a patient with persistent hyperinsulinaemic hypoglycaemia of infancy (nesidioblastosis). FEBS Lett. 1997, 413, 304–308. [Google Scholar] [CrossRef]
- Musilkova, J.; Kovar, J. Additive stimulatory effect of extracellular calcium and potassium on non-transferrin ferric iron uptake by HeLa and K562 cells. Biochim. Biophys. Acta 2001, 1514, 117–126. [Google Scholar] [CrossRef]
- Kovar, J.; Franek, F. Growth-stimulating effect of transferrin on a hybridoma cell line: Relation to transferrin iron-transporting function. Exp. Cell. Res. 1989, 182, 358–369. [Google Scholar] [CrossRef]
- Cnop, M.; Hannaert, J.C.; Hoorens, A.; Eizirik, D.L.; Pipeleers, D.G. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes 2001, 50, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Lagerstedt, S.A.; Hinrichs, D.R.; Batt, S.M.; Magera, M.J.; Rinaldo, P.; McConnell, J.P. Quantitative determination of plasma c8-c26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders. Mol. Genet. Metab. 2001, 73, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Abdelmagid, S.A.; Clarke, S.E.; Nielsen, D.E.; Badawi, A.; El-Sohemy, A.; Mutch, D.M.; Ma, D.W.L. Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. PLoS ONE 2015, 10, e0116195. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Steyn, N.P.; Mann, J.; Bennett, P.H.; Temple, N.; Zimmet, P.; Tuomilehto, J.; Lindstrom, J.; Louheranta, A. Diet, nutrition and the prevention of type 2 diabetes. Public Health Nutr. 2004, 7, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Y.; Liu, B.Q.; Niu, X.F.; Zhuang, Y.; Wang, H.Q. Resveratrol-induced cytotoxicity in human Burkitt’s lymphoma cells is coupled to the unfolded protein response. BMC Cancer 2010, 10, 445. [Google Scholar]
- Balusikova, K.; Neubauerova, J.; Dostalikova-Cimburova, M.; Horak, J.; Kovar, J. Differing expression of genes involved in non-transferrin iron transport across plasma membrane in various cell types under iron deficiency and excess. Mol. Cell. Biochem. 2009, 321, 123–133. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šrámek, J.; Němcová-Fürstová, V.; Pavlíková, N.; Kovář, J. Effect of Saturated Stearic Acid on MAP Kinase and ER Stress Signaling Pathways during Apoptosis Induction in Human Pancreatic β-Cells Is Inhibited by Unsaturated Oleic Acid. Int. J. Mol. Sci. 2017, 18, 2313. https://doi.org/10.3390/ijms18112313
Šrámek J, Němcová-Fürstová V, Pavlíková N, Kovář J. Effect of Saturated Stearic Acid on MAP Kinase and ER Stress Signaling Pathways during Apoptosis Induction in Human Pancreatic β-Cells Is Inhibited by Unsaturated Oleic Acid. International Journal of Molecular Sciences. 2017; 18(11):2313. https://doi.org/10.3390/ijms18112313
Chicago/Turabian StyleŠrámek, Jan, Vlasta Němcová-Fürstová, Nela Pavlíková, and Jan Kovář. 2017. "Effect of Saturated Stearic Acid on MAP Kinase and ER Stress Signaling Pathways during Apoptosis Induction in Human Pancreatic β-Cells Is Inhibited by Unsaturated Oleic Acid" International Journal of Molecular Sciences 18, no. 11: 2313. https://doi.org/10.3390/ijms18112313
APA StyleŠrámek, J., Němcová-Fürstová, V., Pavlíková, N., & Kovář, J. (2017). Effect of Saturated Stearic Acid on MAP Kinase and ER Stress Signaling Pathways during Apoptosis Induction in Human Pancreatic β-Cells Is Inhibited by Unsaturated Oleic Acid. International Journal of Molecular Sciences, 18(11), 2313. https://doi.org/10.3390/ijms18112313




