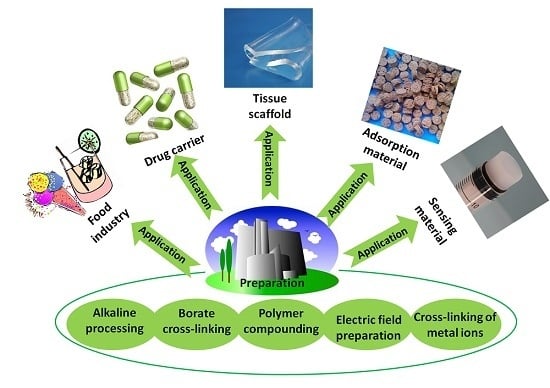
A Review on Konjac Glucomannan Gels: Microstructure and Application
Abstract
1. Introduction
2. Preparation of Konjac Glucomannan (KGM) Gel and Its Microstructure
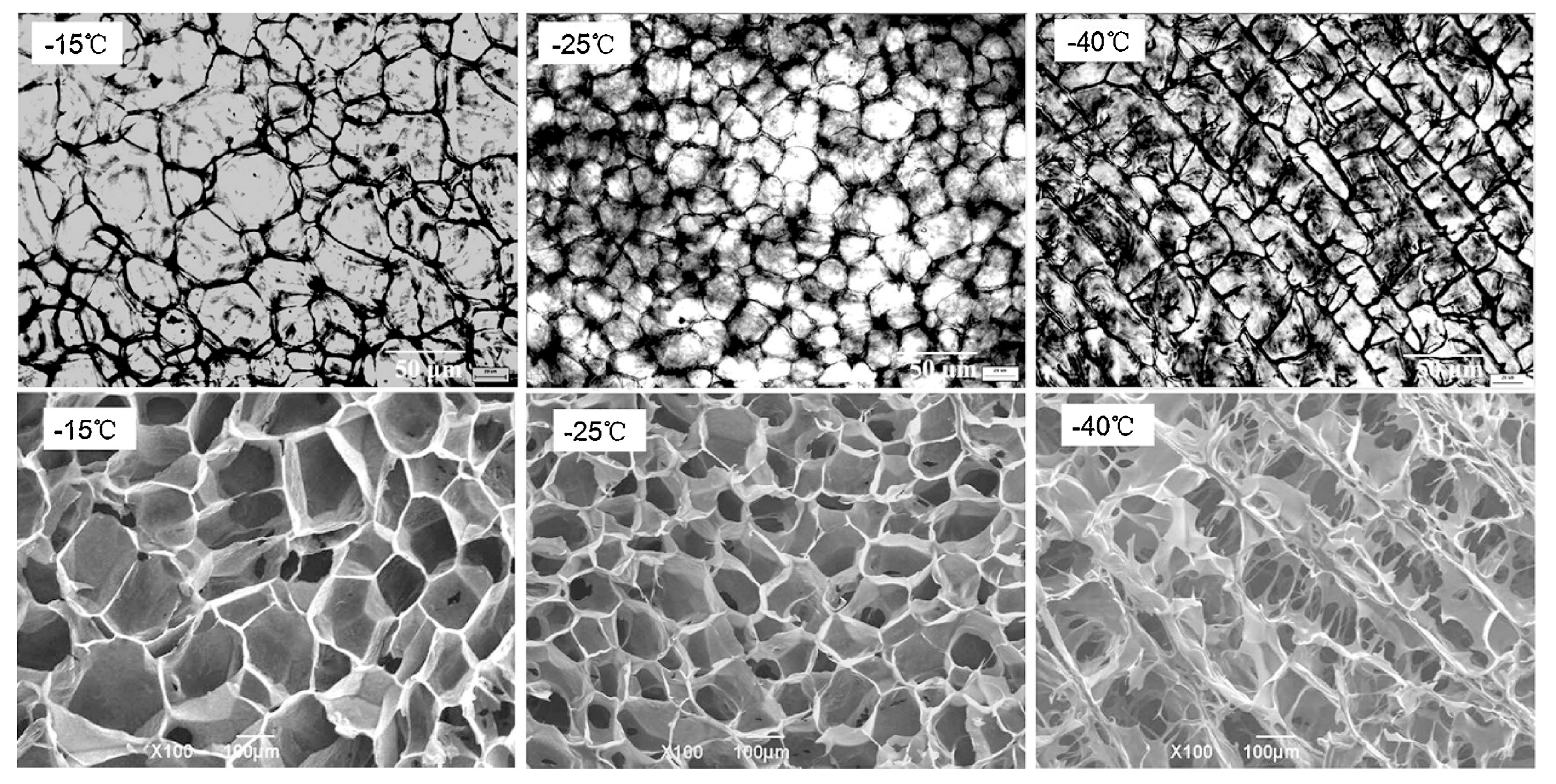
2.1. Alkaline Processing
2.2. Borate Cross-Linking
2.3. Polymer Compounding
2.3.1. KGM-Polysaccharide Compounding
2.3.2. KGM-Protein Compounding
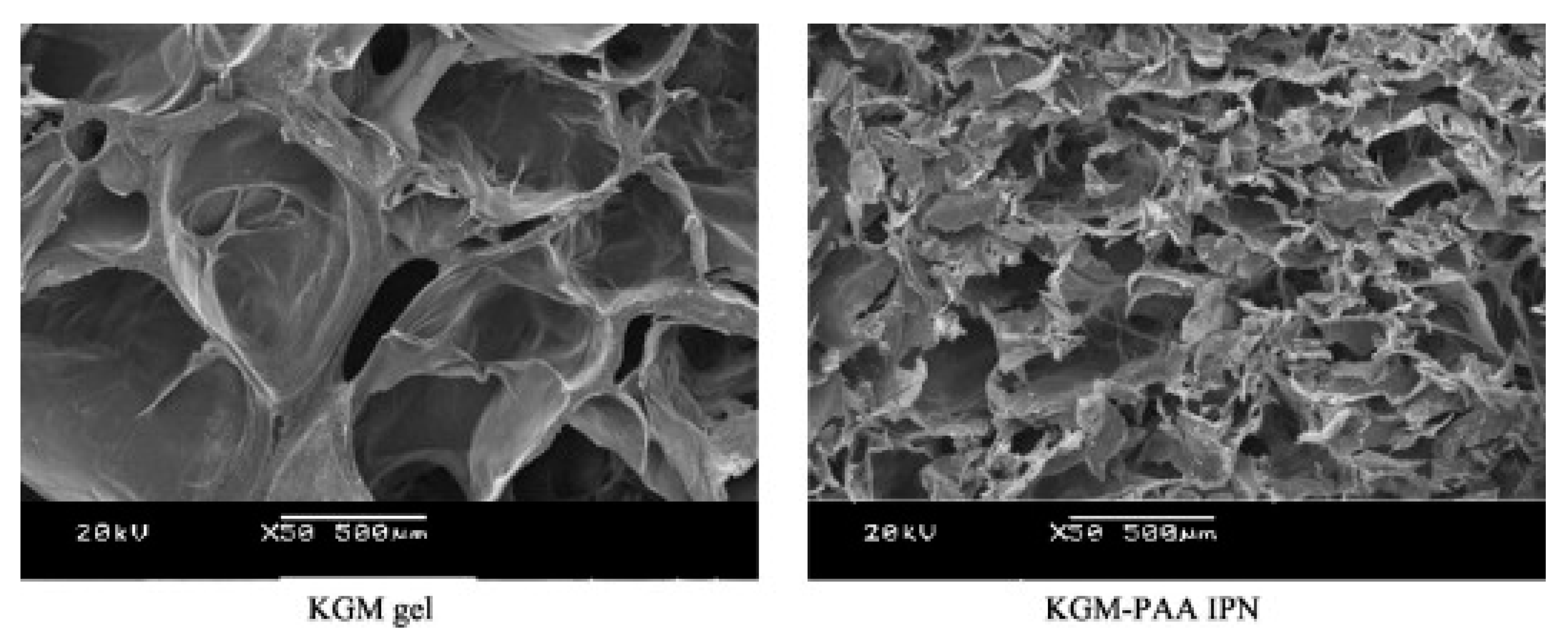
2.3.3. KGM-Synthetic Polymer Compounding
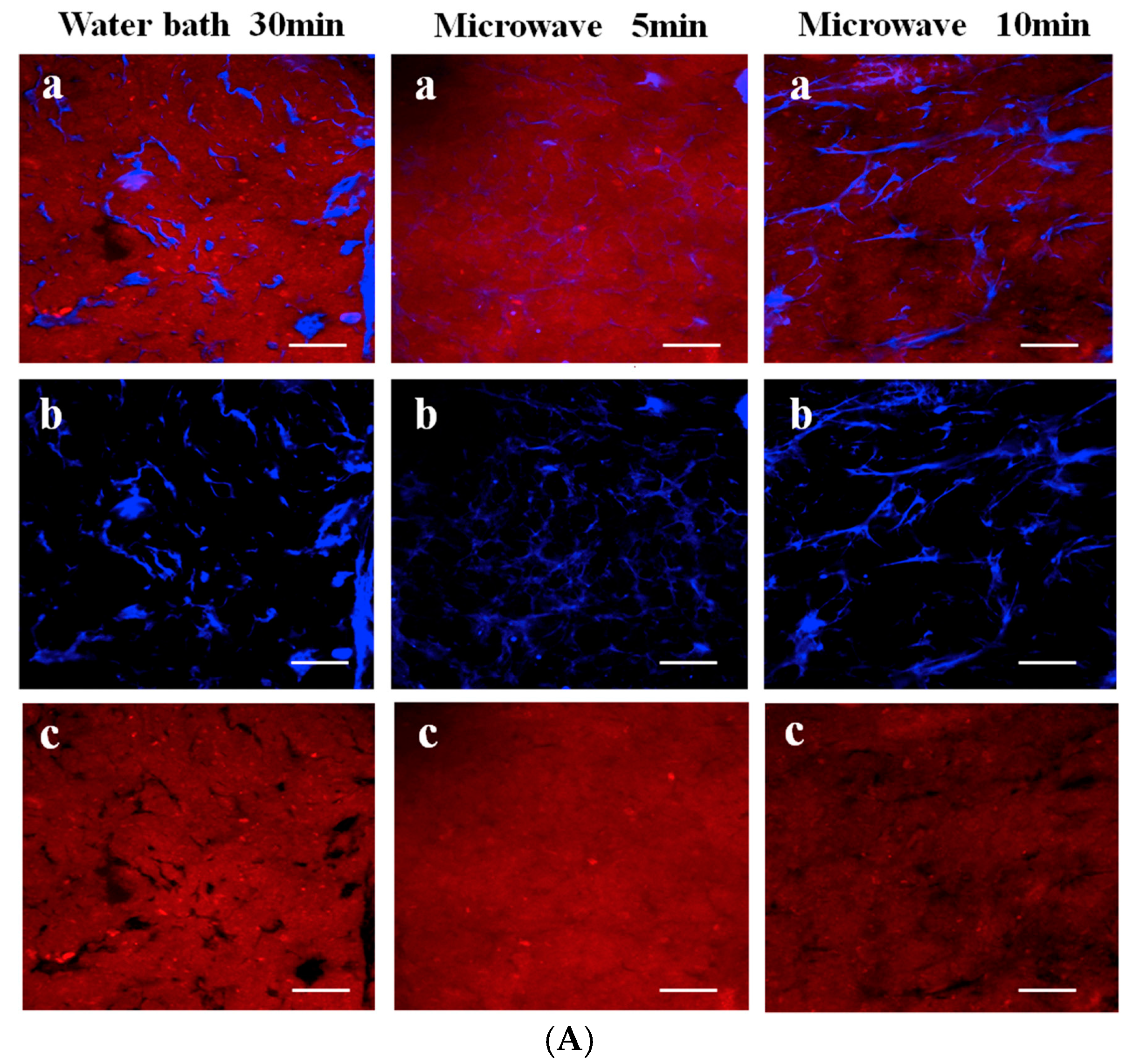
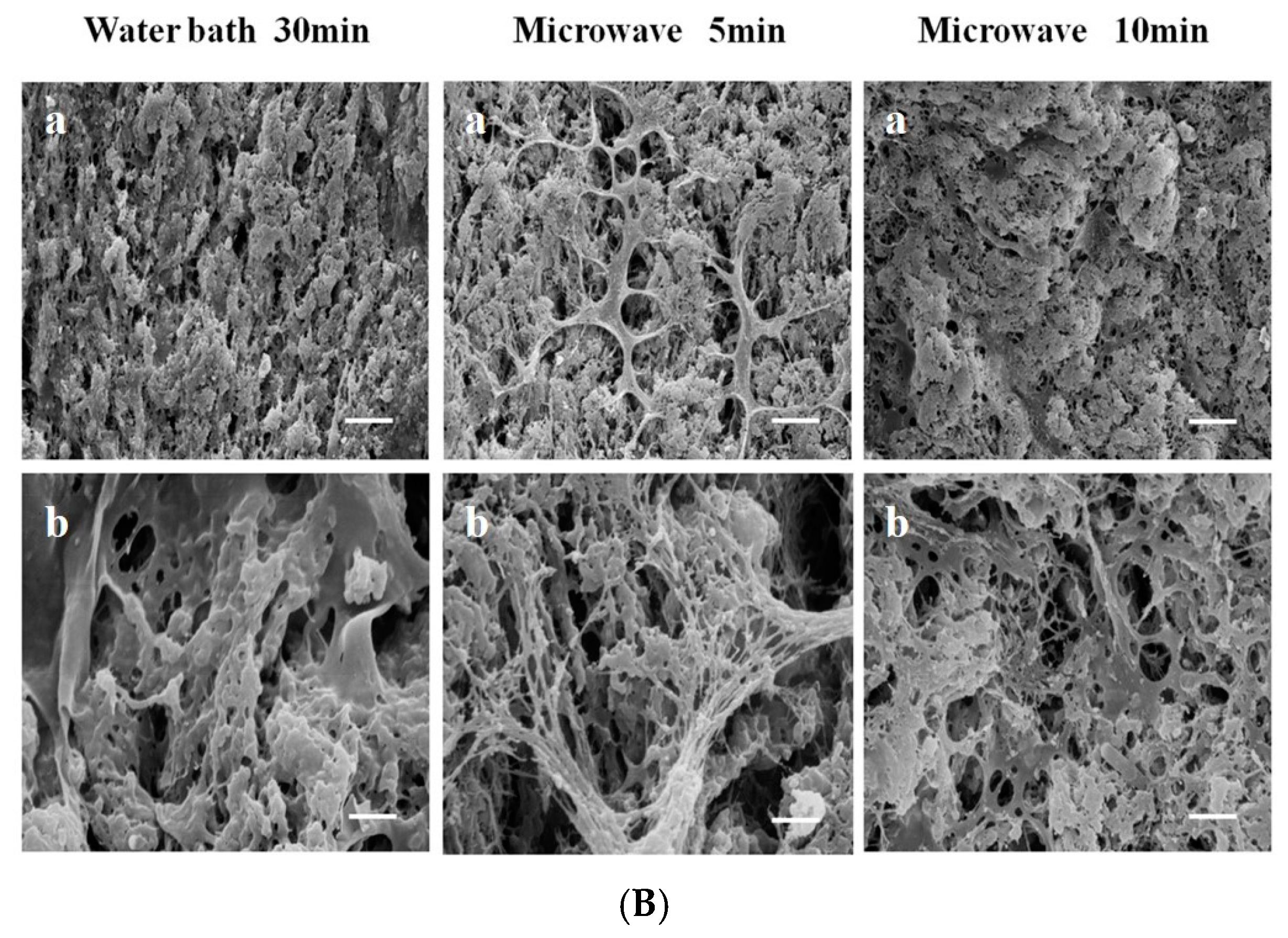
2.4. Electric Field Preparation
2.5. Cross-Linking of Metal Ions after Modification
3. Application of KGM Gel
3.1. Food Industry
3.1.1. Food Additives
3.1.2. KGM Gel Food
3.1.3. Meat and Fish Industry
3.2. Drug Carrier
3.3. Tissue Scaffold
3.4. Absorption Material
3.5. Sensing Material
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Zalewski, B.M.; Chmielewska, A.; Szajewska, H. The effect of glucomannan on body weight in overweight or obese children and adults: A systematic review of randomized controlled trials. Nutrition 2015, 31, 437.e2–442.e2. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Zhang, W.Y.; Li, X. Adsorption of Cu(II) ion from aqueous solutions on hydrogel prepared from konjac glucomannan. Polym. Bull. 2016, 73, 1965–1984. [Google Scholar] [CrossRef]
- Zhu, W.K.; Cong, H.P.; Yao, H.B.; Mao, L.B.; Asiri, A.M.; Alamry, K.A.; Marwani, H.M.; Yu, S.H. Bioinspired, Ultrastrong, Highly Biocompatible, and Bioactive Natural Polymer/Graphene Oxide Nanocomposite Films. Small 2015, 34, 4298–4302. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.G.; Luo, X.G.; Lin, X.Y.; Zhuo, X.R.; Liang, L.L. Preparation and characterization of polylactide/thermoplastic konjac glucomannan blends. Polymer 2009, 50, 3698–3705. [Google Scholar] [CrossRef]
- Xiong, Z.D.; Zhou, W.Q.; Sun, L.J.; Li, N.X.; Zhao, D.W.; Chen, Y.; Li, J.; Ma, G.H.; Su, Z.G. Konjac glucomannan microspheres for low-cost desalting of protein solution. Carbohydr. Polym. 2014, 111, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Song, R.; Xu, W.; Wang, Y.; Li, J.; Shah, B.R.; Li, Y.; Li, B. Analysis of deacetylated konjac glucomannan and xanthan gum phase separation by film forming. Food Hydrocoll. 2015, 48, 320–326. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C. Konjac glucomannan, a promising polysaccharide of Amorphophallus konjac, K. Koch in health care. Int. J. Biol. Macromol. 2016, 92, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.; Siu, K.C.; Wu, J.Y. Effects of pH and temperature on colloidal properties and molecular characteristics of konjac glucomannan. Carbohydr. Polym. 2015, 134, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Takahashi, R.; Kobayashi, S.; Kawase, T.; Nishinari, K. Gelation Behavior of Native and Acetylated konjac glucomannan. Biomacromolecules 2002, 3, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, J.; Chen, J.; Li, B. Effect of degree of deacetylation on physicochemical and gelation properties of konjac glucomannanc. Food Res. Int. 2012, 46, 270–278. [Google Scholar] [CrossRef]
- Gao, S.; Guo, J.; Wu, L.; Wang, S. Gelation of konjac glucomannan crosslinked by organic borate. Carbohydr. Polym. 2008, 73, 498–505. [Google Scholar] [CrossRef]
- Gao, S.; Guo, J.; Nishinari, K. Thermoreversible konjac glucomannan gel crosslinked by borax. Carbohydr. Polym. 2008, 72, 315–325. [Google Scholar] [CrossRef]
- Li, Z.Y.; Su, Y.L.; Haq, M.A.; Xie, B.Q.; Wang, D.J. Konjac glucomannan/polyacrylamide bicomponent hydrogels: Self-healing originating from semi-interpenetrating network. Polymer 2016, 103, 146–151. [Google Scholar] [CrossRef]
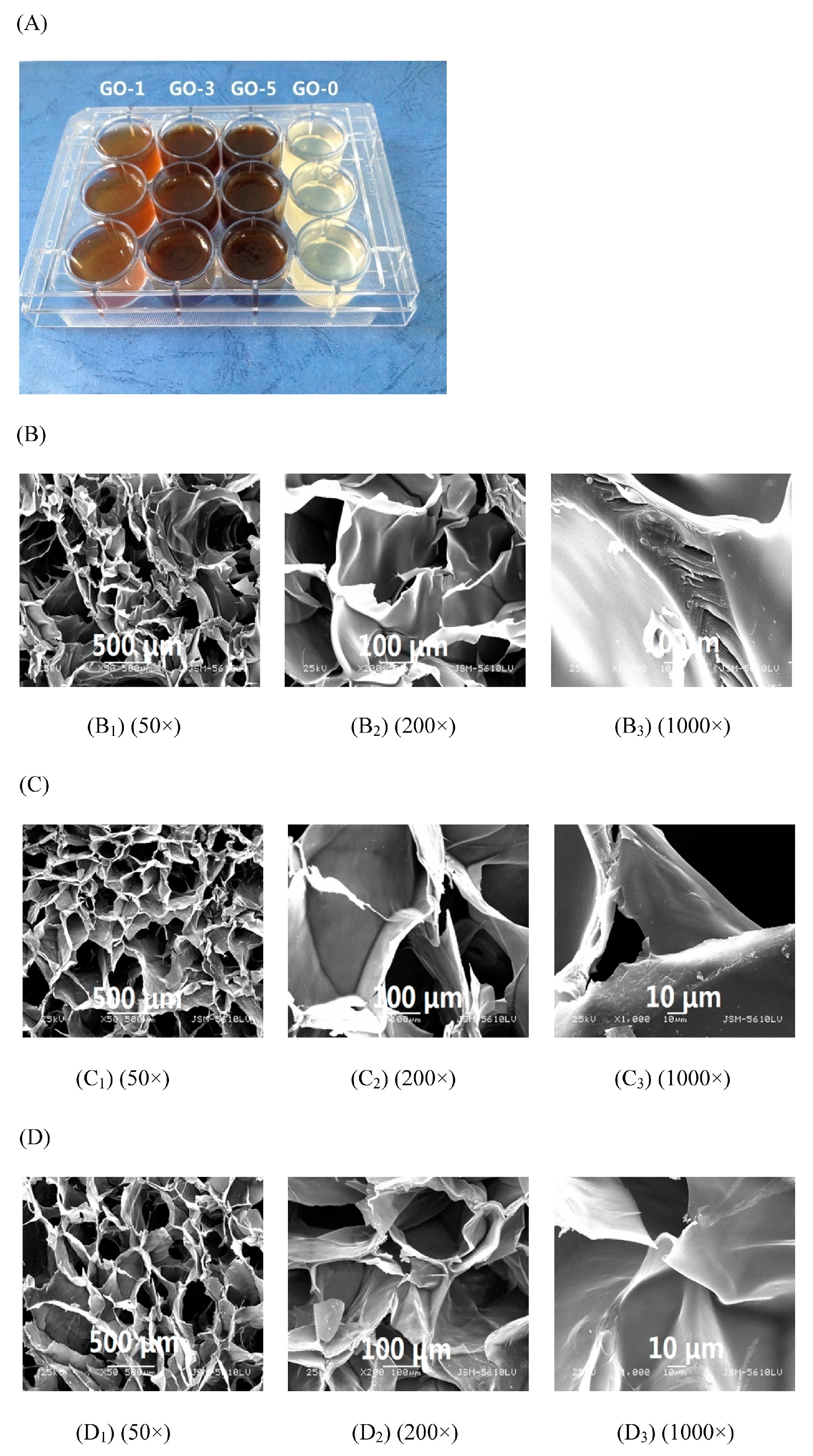
- Fan, L.H.; Yi, J.Y.; Tong, J.; Zhou, X.Y.; Ge, H.Y.; Zou, S.Q.; Wen, H.G.; Nie, M. Preparation and characterization of Oxidized konjac glucomannan/Carboxymethyl Chitosan/Graphene Oxide hydrogel. Int. J. Biol. Macromol. 2016, 91, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Jiang, Y.P.; Lin, Y.H.; Pang, J.; Liu, X.Y. Rheological properties and formation mechanism of DC electric fields induced konjac glucomannan-tungsten gels. Carbohydr. Polym. 2016, 142, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Zhuang, Y.H.; Li, J.L.; Pang, J.; Liu, X.Y. The textural properties and microstructure of konjac glucomannan–tungsten gels induced by DC electric fields. Food Chem. 2016, 212, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.P.; Lin, X.Y.; Wu, J.J.; Zhou, X.B.; Luo, X.G. Adsorption behavior of carboxymethyl konjac glucomannan microspheres for fluoride from aqueous solution. RSC Adv. 2016, 6, 89417–89429. [Google Scholar] [CrossRef]
- Wang, B.; Liao, L.M.; Huang, Q.H.; Cheng, Y.X. Adsorption Behaviors of Benzonic Acid by Carboxyl Methyl konjac glucomannan Gel Micropheres Cross-Linked with Fe3+. J. Chem. Eng. Data 2011, 57, 72–77. [Google Scholar] [CrossRef]
- Ji, L.; Xue, Y.; Zhang, T.; Li, Z.J.; Xue, C.H. The effects of microwave processing on the structure and various quality parameters of Alaska pollock surimi protein-polysaccharide gels. Food Hydrocoll. 2017, 63, 77–84. [Google Scholar] [CrossRef]
- Moreno, H.M.; Herranz, B.; Borderías, A.J.; Tovar, C.A. Effect of high pressure treatment on the structural, mechanical and rheological properties of glucomannan gels. Food Hydrocoll. 2016, 60, 437–444. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Z.J.; Wang, Y.M.; Xue, Y.; Xue, C.H. Effects of konjac glucomannan on heat-induced changes of physicochemical and structural properties of surimi gels. Food Res. Int. 2016, 83, 152–161. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C. Nutritional and potential health benefits of konjac glucomannan, a promising polysaccharide of elephant foot yam, Amorphophallus konjac K. Koch: A review. Food Rev. Int. 2016, 33, 22–43. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, L.; Pang, J.; Hong, X.; Mu, R.J.; Wang, W.H.; Xie, B.Q. A Review of the Development of Properties and Structures Based on konjac glucomannan as Functional Materials. Chin. J. Struct. Chem. 2017, 36, 346–360. [Google Scholar]
- Nair, S.B.; Jyothi, A.N.; Sajeev, M.S. Chitosan-Konjac glucomannan-cassava starch-nanosilver composite films with moisture resistant and antimicrobial properties for food packaging applications. Starch Stärke 2016, 69, 1600210. [Google Scholar] [CrossRef]
- Jian, W.J.; Wu, H.Y.; Wu, L.L.; Wu, Y.H.; Jia, L.N.; Pang, J.; Sun, Y.M. Effect of molecular characteristics of konjac glucomannan on gelling and rheological properties of Tilapia myofibrillar protein. Carbohydr. Polym. 2016, 150, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, C.C.; Shuai, Y.; Cui, X.Y.; Nie, L. Controlled release of anticancer drug using graphene oxide as a drug-binding effector in konjac glucomannan/sodium alginate hydrogels. Colloids Surf. B 2014, 113, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Weska, R.F.; Achilli, M.; Beppu, M.M.; Mantovani, D. Improvement of Collagen Hydrogel Scaffolds Properties by the Addition of konjac glucomannan. Adv. Mater. Res. 2011, 409, 187–192. [Google Scholar] [CrossRef]
- Kondo, T.; Shinozaki, T.; Oku, H.; Takigami, S.; Takagishi, K. Konjac glucomannan-based hydrogel with hyaluronic acid as a candidate for a novel scaffold for chondrocyte culture. J. Tissue Eng. Regen. Med. 2009, 3, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zou, H.L.; Peng, J.B.; Hu, J.W.; Liu, H.B.; Chen, Y.W.; Lu, F.H. Removal of copper (II) using deacetylated konjac glucomannan conjugated soy protein isolate. Int. J. Biol. Macromol. 2016, 86, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, J.; Li, B. Identification of molecular driving forces involved in the gelation of konjac glucomannan: Effect of degree of deacetylation on hydrophobic association. Carbohydr. Polym. 2011, 86, 865–871. [Google Scholar] [CrossRef]
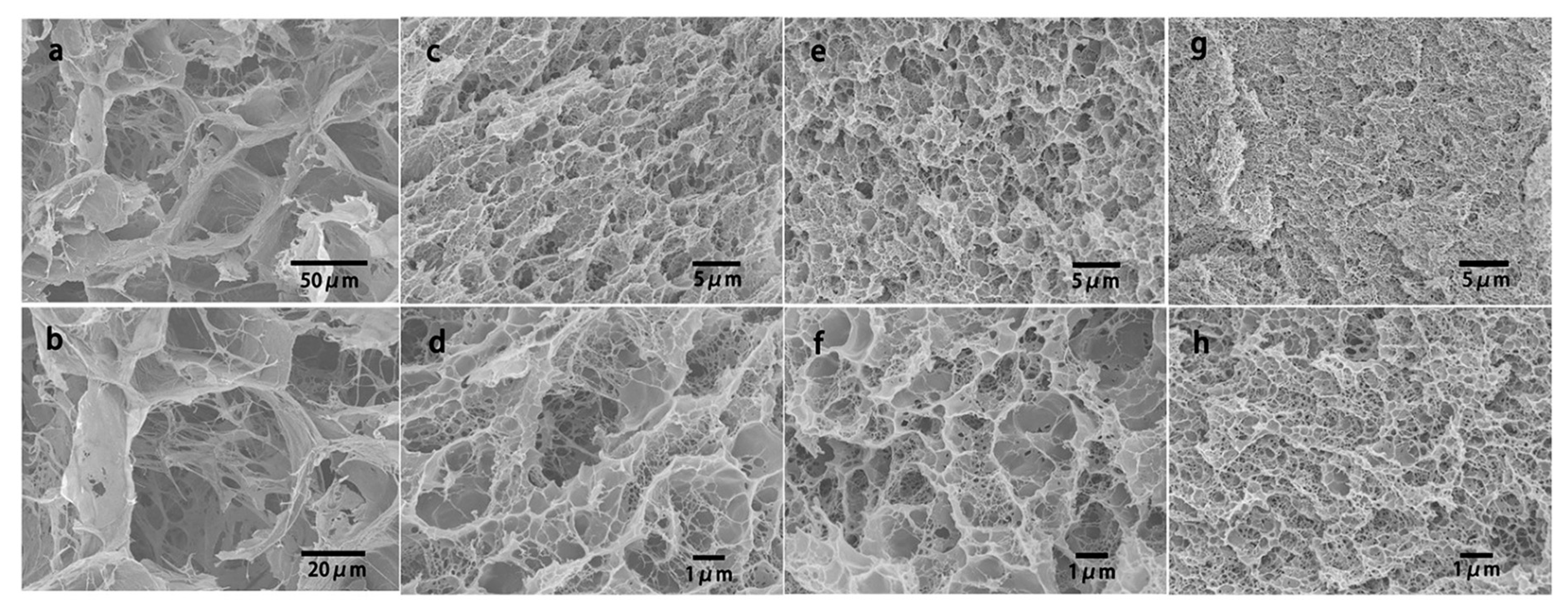
- Ni, X.W.; Ke, F.; Xiao, M.; Wu, K.; Kuang, Y.; Corke, H.; Jiang, F.T. The control of ice crystal growth and effect on porous structure of konjac glucomannan-based aerogels. Int. J. Biol. Macromol. 2016, 92, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yan, Z.M.; Mu, R.J.; Wang, L.; Gong, J.N.; Hong, X.; Haruna, H.M.; Pang, J. The effects of graphene oxide on the properties and drug delivery of konjac glucomannan hydrogel. J. Appl. Polym. Sci. 2017, 38, 1–10. [Google Scholar] [CrossRef]
- Ye, S.X.; Jin, W.P.; Huang, Q.; Hu, Y.; Shah, B.R.; Li, Y.; Li, B. Development of Mag-FMBO in clay-reinforced KGM aerogels for arsenite removal. Int. J. Biol. Macromol. 2016, 87, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.X.; Jin, W.P.; Huang, Q.; Hu, Y.; Li, Y.; Li, B. KGM-based magnetic carbon aerogels matrix for the uptake of methylene blue and methyl orange. Int. J. Biol. Macromol. 2016, 92, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.X.; Jin, W.P.; Huang, Q.; Hu, Y.; Li, Y.; Li, J.; Li, B. Da-KGM based GO-reinforced FMBO-loaded aerogels for efficient arsenic removal in aqueous solution. Int. J. Biol. Macromol. 2017, 94, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.X.; Jin, W.P.; Huang, Q.; Hu, Y.; Shah, B.R.; Liu, S.L.; Li, Y.; Li, B. Fabrication and characterization of KGM-based FMBO-containing aerogels for removal of arsenite in aqueous solution. RSC Adv. 2015, 5, 41877–41886. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, D.; Winkworth-Smith, C.G.; Foster, T.J.; Nirasawa, S.; Tatsumi, E.; Cheng, Y.Q. Effect of a small amount of sodium carbonate on konjac glucomannan-induced changes in wheat starch gel. Carbohydr. Polym. 2015, 116, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xue, Y.; Li, Z.J.; Wang, Y.M.; Xue, C.H. Effects of deacetylation of konjac glucomannan on Alaska Pollock surimi gels subjected to high-temperature (120 °C) treatment. Food Hydrocoll. 2015, 43, 125–131. [Google Scholar] [CrossRef]
- Kohyama, K.; Sano, Y.; Nishinari, K. A mixed system composed of different molecular weights konjac glucomannan and κ-carrageenan. II. Molecular weight dependence of viscoelasticity and thermal properties. Food Hydrocoll. 1996, 10, 229–238. [Google Scholar] [CrossRef]
- Kohyama, K.; Iida, H.; Nishinari, K. A mixed system composed of different molecular weights konjac glucomannan and κ carrageenan: Large deformation and dynamic viscoelastic study. Food Hydrocoll. 1993, 7, 213–226. [Google Scholar] [CrossRef]
- Alvarez-Manceñido, F.; Braeckmans, K.; Smedt, S.C.D.; Demeester, J.; Landin, M.; Martínez-Pacheco, R. Characterization of diffusion of macromolecules in konjac glucomannan solutions and gels by fluorescence recovery after photobleaching technique. Int. J. Pharm. 2006, 316, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Agoub, A.A.; Smith, A.M.; Giannouli, P.; Richardson, R.K.; Morris, E.R. “Melt-in-the-mouth” gels from mixtures of xanthan and konjac glucomannan under acidic conditions: A rheological and calorimetric study of the mechanism of synergistic gelation. Carbohydr. Polym. 2007, 69, 713–724. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Xiong, W.D.; Mi, Z.Y.; Ma, Z.; Li, X.L. Adhesive and In Vitro Release Properties of the konjac glucomannan and Xanthan Gum Mixture Gel Film. In Proceedings of the 4th International Conference on Bioinformatics & Biomedical Engineering, Chengdu, China, 18–20 June 2010; IEEE: New York, NY, USA, 2010; pp. 1–4. [Google Scholar]
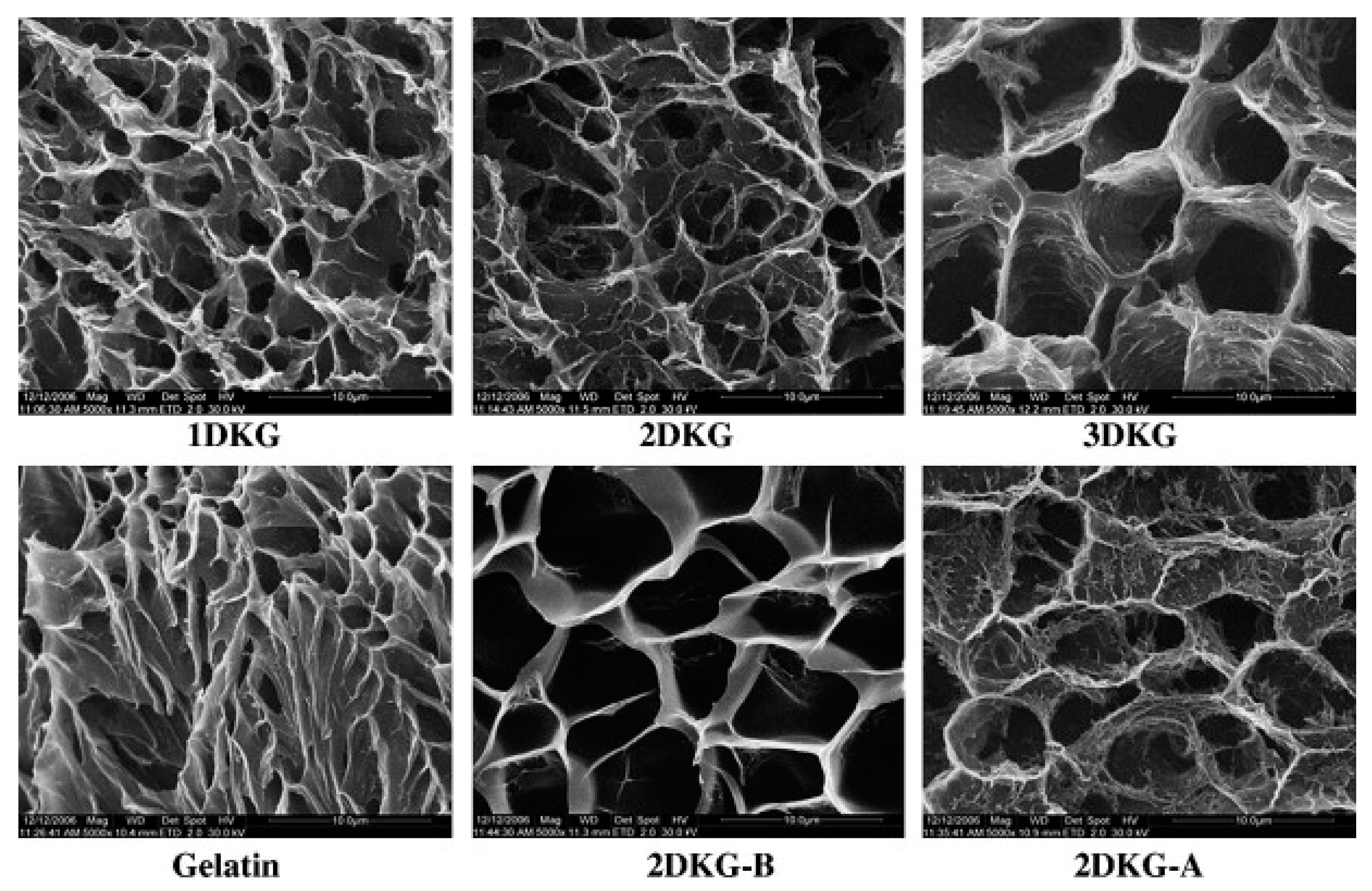
- Yu, H.; Xiao, C. Synthesis and properties of novel hydrogels from oxidized konjac glucomannan crosslinked gelatin for in vitro drug delivery. Carbohydr. Polym. 2008, 72, 489–499. [Google Scholar] [CrossRef]
- Schwartz, J.M.; Bail, K.L.; Garnier, C.; Llamas, G.; Queveau, D.; Pontoire, B.; Srzednicki, G.; Baila, P.L. Available water in konjac glucomannan–starch mixtures. Influence on the gelatinization, retrogradation and complexation properties of two starches. Food Hydrocoll. 2014, 41, 71–78. [Google Scholar] [CrossRef]
- Lafarge, C.; Journaux, L.; Bonnotte, A.; Lherminier, J.; Lee, J.A.; Bail, P.L.; Cayot, N. Trapping of carvacrol by konjac glucomannan-potato starch gels: Stability from macroscopic to microscopic scale, using image processing. Food Hydrocoll. 2017, 66, 216–226. [Google Scholar] [CrossRef]
- Charoenrein, S.; Tatirat, O.; Rengsutthi, K.; Thongngam, M. Effect of konjac glucomannan on syneresis, textural properties and the microstructure of frozen rice starch gels. Carbohydr. Polym. 2011, 83, 291–296. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhu, K.K.; Ye, T.; Wan, S.L.; Wang, Y.T.; Wang, D.; Li, B.; Wang, C. Influence of konjac glucomannan on gelling properties and water state in egg white protein gel. Food Res. Int. 2013, 51, 437–443. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.H.; Zhou, Y.; Nirasawa, S.; Tatsumi, E.; Li, X.T.; Cheng, Y.Q. Effects of konjac glucomannan on heat-induced changes of wheat gluten structure. Food Chem. 2017, 229, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.Q.; Cheng, W.; Ye, L.X.; Du, X.; Zhou, M.; Lin, R.T.; Geng, S.R.; Chen, M.L.; Corke, H.; Cai, Y.Z. Effects of konjac glucomannan on phys icochemical properties of myofibrillar protein and surimi gels from grass carp (Ctenopharyngodon idella). Food Chem. 2009, 116, 413–418. [Google Scholar] [CrossRef]
- Li, Z.Y.; Su, Y.L.; Xie, B.Q.; Liu, X.G.; Gao, X.; Wang, D.J. A novel biocompatible double network hydrogel consisting of konjac glucomannan with high mechanical strength and ability to be freely shaped. J. Mater. Chem. B 2015, 3, 1769–1778. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Chen, J. Synthesis and characteristics of pH-sensitive semi-interpenetrating polymer network hydrogels based on konjac glucomannan and poly (aspartic acid) for in vitro drug delivery. Carbohydr. Polym. 2010, 79, 500–506. [Google Scholar] [CrossRef]
- Wen, X.; Cao, X.L.; Yin, Z.H.; Wang, T.; Zhao, C.S. Preparation and characterization of konjac glucomannan–poly(acrylic acid) IPN hydrogels for controlled release. Carbohydr. Polym. 2009, 78, 193–198. [Google Scholar] [CrossRef]
- Chen, X.D.; Wang, S.S.; Lu, M.L.; Chen, Y.Y.; Zhao, L.H.; Li, W.; Yuan, Q.P.; Norde, W.; Li, Y. Formation and Characterization of Light-Responsive TEMPO-Oxidized Konjac Glucomannan Microspheres. Biomacromolecules 2014, 15, 2166–2171. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Colmenero, F.; Cofrades, S.; Herrero, A.M.; Solas, M.T.; Ruiz-Capillas, C. Konjac gel for use as potential fat analogue for healthier meat product development: Effect of chilled and frozen storage. Food Hydrocoll. 2013, 30, 351–357. [Google Scholar] [CrossRef]
- Felix da Silva, D.; Barbosa de Souza Ferreira, S.; Bruschi, M.L.; Britten, M.; Matumoto-Pintro, P.T. Effect of commercial konjac glucomannan and konjac flours on textural, rheological and microstructural properties of low fat processed cheese. Food Hydrocoll. 2016, 60, 308–316. [Google Scholar] [CrossRef]
- Dai, S.; Corke, H.; Shah, N.P. Utilization of Konjac glucomannan as a fat replacer in low-fat and skimmed yogurt. J. Dairy Sci. 2016, 99, 7063–7074. [Google Scholar] [CrossRef] [PubMed]
- Borreani, J.; Llorca, E.; Larrea, V.; Hernando, I. Adding neutral or anionic hydrocolloids to dairy proteins under in vitro gastric digestion conditions. Food Hydrocoll. 2016, 57, 169–177. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Jin, W.; Zhou, B.; Li, B. Application of micronized konjac gel for fat analogue in mayonnaise. Food Hydrocoll. 2014, 35, 375–382. [Google Scholar] [CrossRef]
- Chen, X.; Lin, D.L. Physicochemical and Pharmacological Properties of KGM and Its Application. J. Chongqing Inst. Technol. Nat. Sci. 2009, 23, 36–39. [Google Scholar]
- Hu, Y.; Liang, H.; Xu, W.; Wang, Y.; An, Y.; Yan, X.; Ye, S.; Huang, Q.; Liu, J.; Li, B. Synergistic effects of small amounts of konjac glucomannan on functional properties of egg white protein. Food Hydrocoll. 2016, 52, 213–220. [Google Scholar] [CrossRef]
- Kohyama, K.; Hayakawa, F.; Gao, Z.; Ishihara, S.; Funami, T.; Nishinari, K. Natural eating behavior of two types of hydrocolloid gels as measured by electromyography: Quantitative analysis of mouthful size effects. Food Hydrocoll. 2016, 52, 243–252. [Google Scholar] [CrossRef]
- Akesowan, A. Optimization of Textural Properties of Konjac Gels Formed with κ-Carrageenan or Xanthan and Xylitol as Ingredients in Jelly Drink Processing. J. Food Process. Preserv. 2015, 39, 1735–1743. [Google Scholar] [CrossRef]
- Han, L.; Cheng, Y.; Zhang, Q.; Ma, H.; Tatsumi, E.; Li, L. Synergistic Effects of Calcium Hydroxide and Konjac Glucomannan (KGM) on the Thermomechanical Properties of Buckwheat Flour and the Quality of Buckwheat Noodles. J. Texture Stud. 2015, 45, 420–429. [Google Scholar] [CrossRef]
- Herranz, B.; Tovar, C.A.; Solo-de-Zaldívar, B.; Borderias, A.J. Effect of alkalis on konjac glucomannan gels for use as potential gelling agents in restructured seafood products. Food Hydrocoll. 2012, 27, 145–153. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, G.; Peng, Y.B.; Wu, W.; Li, X.; Wang, J.; Qiao, Y.; Liao, L.; Ding, A. The Cryoprotective Effect of Different Konjac Glucomannan (KGM) Hydrolysates on the Glass Carp (Ctenopharyngodon idella) Myofibrillar During Frozen Storage. Food Bioprocess Technol. 2014, 7, 3398–3406. [Google Scholar] [CrossRef]
- Iglesiasotero, M.A.; Borderías, J.; Asunción Tovar, C. Use of konjac glucomannan as additive to reinforce the gels from low-quality squid surimi. J. Food Eng. 2010, 101, 281–288. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Ding, Y. Optimization of adding konjac glucomannan to improve gel properties of low-quality surimi. Carbohydr. Polym. 2013, 92, 484–489. [Google Scholar]
- Andrés-Bello, A.; Iborra-Bernad, C.; García-Segovia, P.; Martínez-Monzó, J. Effect of Konjac glucomannan (KGM) and Carboxymethylcellulose (CMC) on some Physico-Chemical and Mechanical Properties of Restructured Gilthead Sea Bream (Sparus aurata) Products. Food Bioprocess Technol. 2013, 6, 133–145. [Google Scholar] [CrossRef]
- Berry, B.W.; Bigner, M.E. Use of carrageenan and konjac flour gel in low-fat restructured pork nuggets. Food Res. Int. 1996, 29, 355–362. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Cofrades, S.; López-López, I.; Ruiz-Capillas, C.; Pintado, T.; Solas, M.T. Technological and sensory characteristics of reduced/low-fat, low-salt frankfurters as affected by the addition of konjac and seaweed. Meat Sci. 2010, 84, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Kao, W.T.; Lin, K.W. Quality of reduced-fat frankfurter modified by konjac-starch mixed gels. J. Food Sci. 2006, 71, S326–S332. [Google Scholar] [CrossRef]
- Salcedo-Sandoval, L.; Cofrades, S.; Ruiz-Capillas, P.C.; Solas, M.T.; Jiménez-Colmenero, F. Healthier oils stabilized in konjac matrix as fat replacers in n-3 PUFA enriched frankfurters. Meat Sci. 2013, 93, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Salcedo-Sandoval, L.; Ruiz-Capillas, C.; Cofrades, S.; Triki, M.; Jimenez-Colmenero, F. Shelf-life of n-3 PUFA enriched frankfurters formulated with a konjac-based oil bulking agent. LWT Food Sci. Technol. 2015, 62, 711–717. [Google Scholar] [CrossRef]
- Chin, K.B.; Keeton, J.T.; Longnecker, M.T.; Lamkey, J.W. Functional, Textural and Microstructural Properties of Low-fat Bologna (Model System) with a Konjac Blend. J. Food Sci. 1998, 63, 801–807. [Google Scholar] [CrossRef]
- Chin, K.B.; Keeton, J.T.; Miller, R.K.; Longnecker, M.T.; Lamkey, J.W. Evaluation of Konjac Blends and Soy Protein Isolate as Fat Replacements in Low-fat Bologna. J. Food Sci. 2000, 65, 756–763. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Triki, M.; Herrero, A.M.; Rodriguez-Salas, L.; Jiménez-Colmenero, F. Konjac gel as pork backfat replacer in dry fermented sausages: Processing and quality characteristics. Meat Sci. 2012, 92, 144–150. [Google Scholar] [CrossRef] [PubMed]
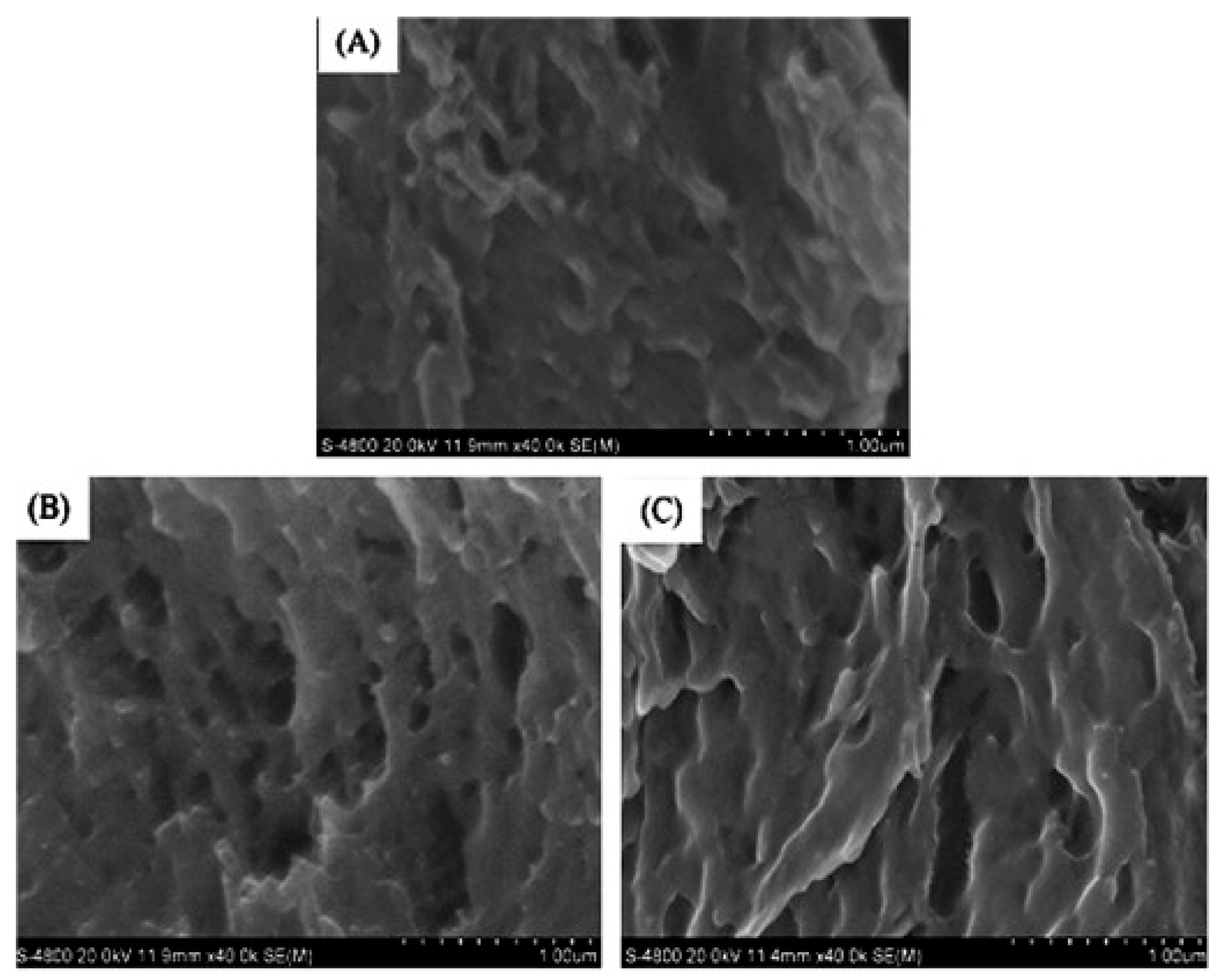
- Xiao, M.; Dai, S.H.; Wang, L.; Ni, X.W.; Yan, W.L.; Fang, Y.P.; Corke, H.; Jiang, F.T. Carboxymethyl modification of konjac glucomannan affects water binding properties. Carbohydr. Polym. 2015, 130, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiao, M.; Dai, S.H.; Song, J.; Ni, X.W.; Fang, Y.P.; Corke, H.; Jiang, F.T. Interactions between carboxymethyl konjac glucomannan and soy protein isolate in blended films. Carbohydr. Polym. 2014, 101, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xia, B.; Branham, M.; Ha, W.; Wu, H.; Peng, S.L.; Ding, L.S.; Li, B.J.; Zhang, S. Self-assembly of carboxymethyl konjac glucomannan-g-poly (ethylene glycol) and (α-cyclodextrin) to biocompatible hollow nanospheres for glucose oxidase encapsulation. Carbohydr. Polym. 2011, 86, 120–126. [Google Scholar] [CrossRef]
- Ha, W.; Wu, H.; Wang, X.L.; Peng, S.L.; Ding, L.S.; Zhang, S.; Li, B.J. Self-aggregates of cholesterol-modified carboxymethyl Konjac glucomannan conjugate: Preparation, characterization, and preliminary assessment as a carrier of etoposide. Carbohydr. Polym. 2011, 86, 513–519. [Google Scholar] [CrossRef]
- Alvarez-Manceñido, F.; Landin, M.; Martínez-Pacheco, R. Konjac glucomannan/xanthan gum enzyme sensitive binary mixtures for colonic drug delivery. Eur. J. Pharm. Biopharm. 2008, 69, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; He, Z.M. Alginate-Konjac glucomannan-chitosan beads as controlled release matrix. Int. J. Pharm. 2002, 244, 117–126. [Google Scholar] [CrossRef]
- Du, J.; Dai, J.; Liu, J.L.; Dankovich, T. Novel pH-sensitive polyelectrolyte carboxymethyl konjac glucomannan-chitosan beads as drug carriers. React. Funct. Polym. 2006, 66, 1055–1061. [Google Scholar] [CrossRef]
- Wen, X.; Wang, T.; Wang, Z.Y.; Li, L.; Zhao, C.S. Preparation of konjac glucomannan hydrogels as DNA-controlled release matrix. Int. J. Biol. Macromol. 2008, 42, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Liu, Z.L.; Zhuo, R.X. Synthesis and properties of degradable hydrogels of konjac glucomannan grafted acrylic acid for colon-specific drug delivery. Polymer 2005, 46, 6274–6281. [Google Scholar] [CrossRef]
- Lu, M.L.; Li, Z.J.; Liang, H.; Shi, M.X.; Zhao, L.H.; Li, W.; Chen, Y.Y.; Wu, J.D.; Wang, S.S.; Chen, X.D.; et al. Controlled release of anthocyanins from oxidized konjac glucomannan microspheres stabilized by chitosan oligosaccharides. Food Hydrocoll. 2015, 51, 476–485. [Google Scholar] [CrossRef]
- Marzuke, A.B.; Wan Kamarul Zaman, W.; Shahbuddin, M.; Aung, S.W. The Effects of KGM, Mannose and Co-Supplementation of KGM and Mannose on Mammalian Cells Cultured at Inside and Outside Incubator Conditions. In Proceedings of the International Conference for Innovation in Biomedical Engineering and Life Sciences, Putrajaya, Malaysia, 6–8 December 2015; Springer: Singapore, 2016; pp. 208–211. [Google Scholar]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Aerogel insulation for building applications: A state-of-the-art review. Energy Build. 2011, 43, 761–769. [Google Scholar] [CrossRef]
- Chen, H.; Mu, R.J.; Pang, J.; Tan, X.D.; Wang, M.; Chiang, W.Y. Structure and Potential Application of Konjac glucomannan Nano Microfibril Aerogel. Chin. J. Struct. Chem. 2016, 35, 166–168. [Google Scholar]
- Gan, L.; Shang, S.; Hu, E.; Yuen, C.W.M.; Jiang, S. Konjac glucomannan/graphene oxide hydrogel with enhanced dyes adsorption capability for methyl blue and methyl orange. Appl. Surf. Sci. 2015, 357, 866–872. [Google Scholar] [CrossRef]
- Yang, S.; Wang, X.Q.; Yan, C.C.; Yang, L.; Yu, J.Y.; Ding, B. Ultralight Biomass-Derived Carbonaceous Nanofibrous Aerogels with Superelasticity and High Pressure-Sensitivity. Adv. Mater. 2016, 28, 9512–9518. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Yuan, Y.; Wang, L.; Wang, X.; Mu, R.; Pang, J.; Xiao, J.; Zheng, Y. A Review on Konjac Glucomannan Gels: Microstructure and Application. Int. J. Mol. Sci. 2017, 18, 2250. https://doi.org/10.3390/ijms18112250
Yang D, Yuan Y, Wang L, Wang X, Mu R, Pang J, Xiao J, Zheng Y. A Review on Konjac Glucomannan Gels: Microstructure and Application. International Journal of Molecular Sciences. 2017; 18(11):2250. https://doi.org/10.3390/ijms18112250
Chicago/Turabian StyleYang, Dan, Yi Yuan, Lin Wang, Xiaoshan Wang, Ruojun Mu, Jie Pang, Jianbo Xiao, and Yafeng Zheng. 2017. "A Review on Konjac Glucomannan Gels: Microstructure and Application" International Journal of Molecular Sciences 18, no. 11: 2250. https://doi.org/10.3390/ijms18112250
APA StyleYang, D., Yuan, Y., Wang, L., Wang, X., Mu, R., Pang, J., Xiao, J., & Zheng, Y. (2017). A Review on Konjac Glucomannan Gels: Microstructure and Application. International Journal of Molecular Sciences, 18(11), 2250. https://doi.org/10.3390/ijms18112250