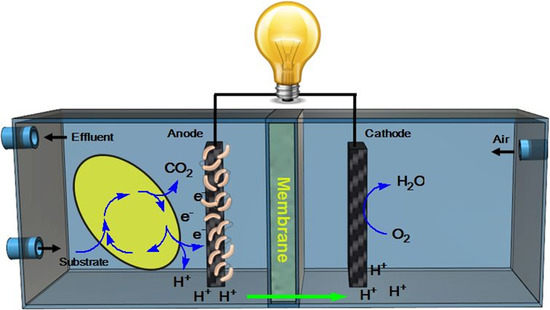
Metal-Free Carbon-Based Materials: Promising Electrocatalysts for Oxygen Reduction Reaction in Microbial Fuel Cells
Abstract
:1. Introduction
2. Mechanism of Oxygen Reduction Reaction in Cathode
3. Cathode Control Energy Loss in Microbial Fuel Cells
4. Requirements of an Ideal Oxygen Reduction Reaction Catalyst
4.1. High Catalytic Activity
4.2. Cost Effectiveness
4.3. Long-Term Stability
5. Limitations of Metal-Based Catalysts
6. Why Carbon-Based Catalysts?
6.1. High Catalytic Activity and Durability
6.2. Cost Effectiveness
7. Carbon-Based Oxygen Reduction Reaction Catalysts
7.1. Carbon Black
7.2. Activated Carbon
7.3. Carbon Nanomaterials
7.3.1. Graphene
7.3.2. Carbon Nanotubes
7.4. Heteroatom-Doped Carbon Materials
7.4.1. N-Doped Carbon
7.4.2. Other Heteroatom-Doped Carbon Materials
7.5. Carbon Materials from Sustainable Precursor
8. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AC | Activated carbon |
| CB | Carbon black |
| CBr | Carbon brush |
| CC | Carbon cloth |
| CF | Carbon felt |
| CG | Carbon granule |
| CM | Carbon mesh |
| CNFs | Carbon nanofibers |
| CoNPc | Cobalt naphthalocyanine |
| CNTs | Carbon nanotubes |
| COD | Chemical oxygen demand |
| CP | Carbon paper |
| CuPc | Copperphthalocyanine |
| CVD | Chemical vapor deposition |
| DC | Direct current |
| FePc | Iron phthalocyanine |
| GBr | Graphite brush |
| GC | Glassy carbon |
| ITO | Indium tin oxide |
| MFC | Microbial fuel cell |
| mpg-C3N4 | Mesoporous carbon nitride |
| MOFs | Metal-organic frameworks |
| MWCNTs | Multi-walled carbon nanotubes |
| OCP | Open circuit potential |
| ORR | Oxygen reduction reaction |
| PANI | Polyaniline |
| PDDA | Polydiallyldimethylammonium chloride |
| PDMS | Poly(dimethylsiloxane) |
| PEDOT | Poly (3,4-ethylenedioxythiophene) |
| PEM | Proton-exchange membrane |
| PEPU | Poly[bis(2-chloroethyl)ether-alt-1,3-bis[3-(dimethylamino)propyl]urea] |
| PPy | Polypyrrole |
| PTFE | Polytetrafluoroethylene |
| PVDF | Poly(vinylidene fluoride) |
| PVDF-HFP | Poly(vinylidene fluoride-co-hexafluoropropylene) |
| RDE | Rotating disk electrode |
| r-Graphene oxide | Reduced graphene oxide |
| RRDE | Rotating ring-disk electrode |
| SEM | Scanning electron microscope |
| SS | Stainless steel |
| SWCNTs | Single-walled carbon nanotubes |
| WP | Wipe-based |
References
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Electromicrobiology. Annu. Rev. Microbiol. 2012, 66, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-W.; Yu, H.-Q.; He, Z. Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ. Sci. 2014, 7, 911–924. [Google Scholar] [CrossRef]
- Yuan, H.Y.; Hou, Y.; Abu-Reesh, I.M.; Chen, J.H.; He, Z. Oxygen reduction reaction catalysts used in microbial fuel cells for energy-efficient wastewater treatment: A review. Mater. Horiz. 2016, 3, 382–401. [Google Scholar] [CrossRef]
- Logan, B.E.; Wallack, M.J.; Kim, K.-Y.; He, W.; Feng, Y.; Saikaly, P.E. Assessment of microbial fuel cell configurations and power densities. Environ. Sci. Technol. Lett. 2015, 2, 206–214. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Ghadge, A.N.; Mondal, D.; Ghangrekar, M.M. Comparison of oxygen and hypochlorite as cathodic electron acceptor in microbial fuel cells. Bioresour. Technol. 2014, 154, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Gil, G.-C.; Chang, I.-S.; Kim, B.H.; Kim, M.; Jang, J.-K.; Park, H.S.; Kim, H.J. Operational parameters affecting the performance of a mediator-less microbial fuel cell. Biosens. Bioelectron. 2003, 18, 327–334. [Google Scholar] [CrossRef]
- Qu, L.; Liu, Y.; Baek, J.-B.; Dai, L. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.A.; Hamelers, H.V.M.; Rabaey, K.; Keller, J.; Buisman, C.J.N. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008, 26, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Ben Liew, K.; Daud, W.R.W.; Ghasemi, M.; Leong, J.X.; Lim, W.S.; Ismail, M. Non-Pt catalyst as oxygen reduction reaction in microbial fuel cells: A review. Int. J. Hydrog. Energy 2014, 39, 4870–4883. [Google Scholar] [CrossRef]
- Morris, J.M.; Jin, S.; Wang, J.; Zhu, C.; Urynowicz, M.A. Lead dioxide as an alternative catalyst to platinum in microbial fuel cells. Electrochem. Commun. 2007, 9, 1730–1734. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, M.; Lu, Y.; Liu, R.; Zhao, J. Ethylene glycol stabilized NaBH4 reduction for preparation carbon-supported Pt-Co alloy nanoparticles used as oxygen reduction electrocatalysts for microbial fuel cells. J. Solid State Electrochem. 2014, 18, 1087–1097. [Google Scholar] [CrossRef]
- Dong, H.; Yu, H.; Wang, X.; Zhou, Q.; Sun, J. Carbon-supported perovskite oxides as oxygen reduction reaction catalyst in single chambered microbial fuel cells. J. Chem. Technol. Biotechnol. 2013, 88, 774–778. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, S.G.; Zhuang, L. Polypyrrole/carbon black composite as a novel oxygen reduction catalyst for microbial fuel cells. J. Power Sources 2010, 195, 3490–3493. [Google Scholar] [CrossRef]
- Wang, Z.J.; Cao, C.L.; Zheng, Y.; Chen, S.L.; Zhao, F. Abiotic oxygen reduction reaction catalysts used in microbial fuel cells. ChemElectroChem 2014, 1, 1813–1821. [Google Scholar] [CrossRef]
- Merino-Jimenez, I.; Santoro, C.; Rojas-Carbonell, S.; Greenman, J.; Ieropoulos, I.; Atanassov, P. Carbon-based air-breathing cathodes for microbial fuel cells. Catalysts 2016, 6, 127. [Google Scholar] [CrossRef]
- Watson, V.J.; Delgado, C.N.; Logan, B.E. Influence of chemical and physical properties of activated carbon powders on oxygen reduction and microbial fuel cell performance. Environ. Sci. Technol. 2013, 47, 6704–6710. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dai, L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016. [Google Scholar] [CrossRef]
- He, Y.; Han, X.; Du, Y.; Zhang, B.; Xu, P. Heteroatom-doped carbon nanostructures derived from conjugated polymers for energy applications. Polymers 2016, 8, 366. [Google Scholar] [CrossRef]
- Liu, J.; Song, P.; Ning, Z.; Xu, W. Recent advances in heteroatom-doped metal-free electrocatalysts for highly efficient oxygen reduction reaction. Electrocatalysis 2015, 6, 132–147. [Google Scholar] [CrossRef]
- Wang, B. Recent development of non-platinum catalysts for oxygen reduction reaction. J. Power Sources 2005, 152, 1–15. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.W.; Li, W.W.; Yu, H.Q. Cathodic catalysts in bioelectrochemical systems for energy recovery from wastewater. Chem. Soc. Rev. 2014, 43, 7718–7745. [Google Scholar] [CrossRef] [PubMed]
- Erable, B.; Féron, D.; Bergel, A. Microbial catalysis of the oxygen reduction reaction for microbial fuel cells: A review. ChemSusChem 2012, 5, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yan, Y.; Gong, K.; Mao, L.; Guo, Z.; Chen, Y. Electrostatic layer-by-layer assembled carbon nanotube multilayer film and its electrocatalytic activity for O2 reduction. Langmuir 2004, 20, 8781–8785. [Google Scholar] [CrossRef] [PubMed]
- Hiep, H.T.; Parveen, N.; Ansari, S.A.; Shim, J.H.; Nguyen, A.T.N.; Cho, M.H. Electrochemically synthesized sulfur-doped graphene as a superior metal-free cathodic catalyst for the oxygen reduction reaction in microbial fuel cell. RSC Adv. 2016, 6, 103446–103454. [Google Scholar]
- Yue, G.Z.; Meng, K.; Liu, Q. One-step synthesis of N-doped carbon and its application as a cost-efficient catalyst for the oxygen reduction reaction in microbial fuel cells. ChemPlusChem 2015, 80, 1133–1138. [Google Scholar] [CrossRef]
- You, S.; Gong, X.; Wang, W.; Qi, D.; Wang, X.; Chen, X.; Ren, N. Enhanced cathodic oxygen reduction and power production of microbial fuel cell based on noble-metal-free electrocatalyst derived from metal-organic frameworks. Adv. Energy Mater. 2016, 6, 1501497. [Google Scholar] [CrossRef]
- Popat, S.C.; Ki, D.; Rittmann, B.E.; Torres, C.I. Importance of OH− transport from cathodes in microbial fuel cells. ChemSusChem 2012, 5, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Gojković, S.L.; Gupta, S.; Savinell, R.F. Heat-treated iron(III) tetramethoxyphenyl porphyrin supported on high-area carbon as an electrocatalyst for oxygen reduction: I. Characterization of the electrocatalyst. J. Electrochem. Soc. 1998, 145, 3493–3499. [Google Scholar] [CrossRef]
- Son, J.; Cho, S.; Lee, C.; Lee, Y.; Shim, J.H. Sponge-like nanoporous Pd and Pd/Au structures: Facile synthesis and enhanced electrocatalytic activity. Langmuir 2014, 30, 3579–3588. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.L.; Xu, L.; Ding, L.; Shi, P.H.; Zhang, L.; Baker, R.; Zhang, J.J. Effect of KOH concentration on the oxygen reduction kinetics catalyzed by heat-treated Co-pyridine/C electrocatalysts. Int. J. Electrochem. Sci. 2013, 8, 1189–1208. [Google Scholar]
- Davis, R.E.; Horvath, G.L.; Tobias, C.W. The solubility and diffusion coefficient of oxygen in potassium hydroxide solutions. Electrochim. Acta 1967, 12, 287–297. [Google Scholar] [CrossRef]
- Gubbins, K.E.; Walker, R.D., Jr. The solubility and diffusivity of oxygen in electrolytic solutions. J. Electrochem. Soc. 1965, 112, 469–471. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, L.; Shi, G.; Liu, J.; Chen, J.; Dai, L. N,P-codoped carbon networks as efficient metal-free bifunctional catalysts for oxygen reduction and hydrogen evolution reactions. Angew. Chem. Int. Ed. 2016, 55, 2230–2234. [Google Scholar] [CrossRef] [PubMed]
- Birry, L.; Mehta, P.; Jaouen, F.; Dodelet, J.P.; Guiot, S.R.; Tartakovsky, B. Application of iron-based cathode catalysts in a microbial fuel cell. Electrochim. Acta 2011, 56, 1505–1511. [Google Scholar] [CrossRef]
- Roche, I.; Scott, K. Carbon-supported manganese oxide nanoparticles as electrocatalysts for oxygen reduction reaction (ORR) in neutral solution. J. Appl. Electrochem. 2009, 39, 197–204. [Google Scholar] [CrossRef]
- Chen, Y.F.; Lv, Z.S.; Xu, J.M.; Peng, D.Q.; Liu, Y.X.; Chen, J.X.; Sun, X.B.; Feng, C.H.; Wei, C.H. Stainless steel mesh coated with MnO2/carbon nanotube and polymethylphenyl siloxane as low-cost and high-performance microbial fuel cell cathode materials. J. Power Sources 2012, 201, 136–141. [Google Scholar] [CrossRef]
- Li, S.Z.; Hu, Y.Y.; Xu, Q.; Sun, J.; Hou, B.; Zhang, Y.P. Iron- and nitrogen-functionalized graphene as a non-precious metal catalyst for enhanced oxygen reduction in an air-cathode microbial fuel cell. J. Power Sources 2012, 213, 265–269. [Google Scholar] [CrossRef]
- Rismani-Yazdi, H.; Carver, S.M.; Christy, A.D.; Tuovinen, O.H. Cathodic limitations in microbial fuel cells: An overview. J. Power Sources 2008, 180, 683–694. [Google Scholar] [CrossRef]
- Logan, B.E.; Regan, J.M. Microbial fuel cells—Challenges and applications. Environ. Sci. Technol. 2006, 40, 5172–5180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, X.; Ivanov, I.; Huang, X.; Logan, B.E. Enhanced activated carbon cathode performance for microbial fuel cell by blending carbon black. Environ. Sci. Technol. 2014, 48, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Watson, V.J.; Delgado, C.N.; Logan, B.E. Improvement of activated carbons as oxygen reduction catalysts in neutral solutions by ammonia gas treatment and their performance in microbial fuel cells. J. Power Sources 2013, 242, 756–761. [Google Scholar] [CrossRef]
- Barsukov, V.Z.; Khomenko, V.G.; Katashinskii, A.S.; Motronyuk, T.I. Catalytic activity of polyaniline in the molecular oxygen reduction: Its nature and mechanism. Russ. J. Electrochem. 2004, 40, 1170–1173. [Google Scholar] [CrossRef]
- Wen, Q.; Wang, S.Y.; Yan, J.; Cong, L.J.; Chen, Y.; Xi, H.Y. Porous nitrogen-doped carbon nanosheet on graphene as metal-free catalyst for oxygen reduction reaction in air-cathode microbial fuel cells. Bioelectrochemistry 2014, 95, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Shahgaldi, S.; Ismail, M.; Kim, B.H.; Yaakob, Z.; Daud, W.R.W. Activated carbon nanofibers as an alternative cathode catalyst to platinum in a two-chamber microbial fuel cell. Int. J. Hydrog. Energy 2011, 36, 13746–13752. [Google Scholar] [CrossRef]
- Dong, H.; Yu, H.; Wang, X. Catalysis kinetics and porous analysis of rolling activated carbon-PTFE air-cathode in microbial fuel cells. Environ. Sci. Technol. 2012, 46, 13009–13015. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Murano, C.; Scott, K.; Gray, N.D.; Head, I.M. Electricity generation from cysteine in a microbial fuel cell. Water Res. 2005, 39, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Liu, H.; Logan, B.E. Power densities using different cathode catalysts (Pt and CoTMPP) and polymer binders (Nafion and PTFE) in single chamber microbial fuel cells. Environ. Sci. Technol. 2006, 40, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Paulus, U.A.; Gasteiger, H.A.; Behm, R.J. The oxygen reduction reaction on a Pt/carbon fuel cell catalyst in the presence of chloride anions. J. Electroanal. Chem. 2001, 508, 41–47. [Google Scholar] [CrossRef]
- Zhao, F.; Harnisch, F.; Schröder, U.; Scholz, F.; Bogdanoff, P.; Herrmann, I. Challenges and constraints of using oxygen cathodes in microbial fuel cells. Environ. Sci. Technol. 2006, 40, 5193–5199. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Li, X.Y.; Zuo, J.E.; Ling, A.; Logan, B.E. Power generation using an activated carbon fiber felt cathode in an upflow microbial fuel cell. J. Power Sources 2010, 195, 1130–1135. [Google Scholar] [CrossRef]
- Feng, L.Y.; Yan, Y.Y.; Chen, Y.G.; Wang, L.J. Nitrogen-doped carbon nanotubes as efficient and durable metal-free cathodic catalysts for oxygen reduction in microbial fuel cells. Energy Environ. Sci. 2011, 4, 1892–1899. [Google Scholar] [CrossRef]
- Xiao, L.; Damien, J.; Luo, J.Y.; Jang, H.D.; Huang, J.X.; He, Z. Crumpled graphene particles for microbial fuel cell electrodes. J. Power Sources 2012, 208, 187–192. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Stadlhofer, A.; Hacker, V.; Squadrito, G.; Schroder, U.; Li, B. Activated carbon nanofibers (ACNF) as cathode for single chamber microbial fuel cells (SCMFCs). J. Power Sources 2013, 243, 499–507. [Google Scholar] [CrossRef]
- Zhang, B.; Wen, Z.H.; Ci, S.Q.; Mao, S.; Chen, J.H.; He, Z. Synthesizing nitrogen-doped activated carbon and probing its active sites for oxygen reduction reaction in microbial fuel cells. ACS Appl. Mater. Interfaces 2014, 6, 7464–7470. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-W.; Wu, Z.-Y.; Chen, L.-F.; Li, C.; Yu, S.-H. Bacterial cellulose derived nitrogen-doped carbon nanofiber aerogel: An efficient metal-free oxygen reduction electrocatalyst for zinc-air battery. Nano Energy 2015, 11, 366–376. [Google Scholar] [CrossRef]
- Song, M.Y.; Park, H.Y.; Yang, D.-S.; Bhattacharjya, D.; Yu, J.-S. Seaweed-derived heteroatom-doped highly porous carbon as an electrocatalyst for the oxygen reduction reaction. ChemSusChem 2014, 7, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yin, J.; Wang, X.; Wang, H.; Yang, X. Microorganism-derived heteroatom-doped carbon materials for oxygen reduction and supercapacitors. Adv. Funct. Mater. 2013, 23, 1305–1312. [Google Scholar] [CrossRef]
- Chen, P.; Wang, L.-K.; Wang, G.; Gao, M.-R.; Ge, J.; Yuan, W.-J.; Shen, Y.-H.; Xie, A.-J.; Yu, S.-H. Nitrogen-doped nanoporous carbon nanosheets derived from plant biomass: An efficient catalyst for oxygen reduction reaction. Energy Environ. Sci. 2014, 7, 4095–4103. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Chen, S.L.; Wang, Z.J.; Hou, H.Q.; Zhao, F. Cellulose-derived nitrogen and phosphorus dual-doped carbon as high performance oxygen reduction catalyst in microbial fuel cell. J. Power Sources 2015, 273, 1189–1193. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Xu, Y.; Yang, Q.; Chen, Y.W.; Zhu, S.M.; Shen, S.B. Power generation using polyaniline/multi-walled carbon nanotubes as an alternative cathode catalyst in microbial fuel cells. Int. J. Energy Res. 2014, 38, 1416–1423. [Google Scholar] [CrossRef]
- Freguia, S.; Rabaey, K.; Yuan, Z.; Keller, J. Non-catalyzed cathodic oxygen reduction at graphite granules in microbial fuel cells. Electrochim. Acta 2007, 53, 598–603. [Google Scholar] [CrossRef]
- Yuan, H.Y.; Hou, Y.; Wen, Z.H.; Guo, X.R.; Chen, J.H.; He, Z. Porous carbon nanosheets codoped with nitrogen and sulfur for oxygen reduction reaction in microbial fuel cells. ACS Appl. Mater. Interfaces 2015, 7, 18672–18678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Pant, D.; Zhang, F.; Liu, J.; He, W.H.; Logan, B.E. Long-term performance of chemically and physically modified activated carbons in air cathodes of microbial fuel cells. ChemElectroChem 2014, 1, 1859–1866. [Google Scholar] [CrossRef]
- Martin, E.; Tartakovsky, B.; Savadogo, O. Cathode materials evaluation in microbial fuel cells: A comparison of carbon, Mn2O3, Fe2O3 and platinum materials. Electrochim. Acta 2011, 58, 58–66. [Google Scholar] [CrossRef]
- Antolini, E. Carbon supports for low-temperature fuel cell catalysts. Appl. Catal. B 2009, 88, 1–24. [Google Scholar] [CrossRef]
- Mshoperi, E.; Fogel, R.; Limson, J. Application of carbon black and iron phthalocyanine composites in bioelectricity production at a brewery wastewater fed microbial fuel cell. Electrochim. Acta 2014, 128, 311–317. [Google Scholar] [CrossRef]
- Yang, C.; Liu, P.; Wang, T.M. Well-defined core-shell carbon black/polypyrrole nanocomposites for electrochemical energy storage. ACS Appl. Mater. Interfaces 2011, 3, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Kim, H.J.; Kim, S. Polyaniline nanofiber/carbon black composite as oxygen reduction catalyst for air cathode microbial fuel cells. J. Electrochem. Soc. 2012, 159, B497–B501. [Google Scholar] [CrossRef]
- Kim, J.R.; Kim, J.Y.; Han, S.B.; Park, K.W.; Saratale, G.D.; Oh, S.E. Application of Co-naphthalocyanine (CoNPc) as alternative cathode catalyst and support structure for microbial fuel cells. Bioresour. Technol. 2011, 102, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Mecheri, B.; Iannaci, A.; D'Epifanio, A.; Mauri, A.; Licoccia, S. Carbon-supported zirconium oxide as a cathode for microbial fuel cell applications. ChemPlusChem 2016, 81, 80–85. [Google Scholar] [CrossRef]
- Yuan, Y.; Ahmed, J.; Kim, S. Polyaniline/carbon black composite-supported iron phthalocyanine as an oxygen reduction catalyst for microbial fuel cells. J. Power Sources 2011, 196, 1103–1106. [Google Scholar] [CrossRef]
- Yang, W.L.; Zhang, F.; He, W.H.; Liu, J.; Hickner, M.A.; Logan, B.E. Poly(vinylidene fluoride-co-hexafluoropropylene) phase inversion coating as a diffusion layer to enhance the cathode performance in microbial fuel cells. J. Power Sources 2014, 269, 379–384. [Google Scholar] [CrossRef]
- Qiu, Z.Z.; Wei, L.L.; Wang, G.; Su, M.; Shen, J.Q. Stainless steel felt as diffusion backing for high-performance microbial fuel cell cathodes. RSC Adv. 2015, 5, 46210–46217. [Google Scholar] [CrossRef]
- Wu, G.; Li, L.; Li, J.-H.; Xu, B.-Q. Polyaniline-carbon composite films as supports of Pt and PtRu particles for methanol electrooxidation. Carbon 2005, 43, 2579–2587. [Google Scholar] [CrossRef]
- Ghasemi, M.; Daud, W.R.W.; Rahimnejad, M.; Rezayi, M.; Fatemi, A.; Jafari, Y.; Somalu, M.R.; Manzour, A. Copper-phthalocyanine and nickel nanoparticles as novel cathode catalysts in microbial fuel cells. Int. J. Hydrog. Energy 2013, 38, 9533–9540. [Google Scholar] [CrossRef]
- Duteanu, N.; Erable, B.; Kumar, S.M.S.; Ghangrekar, M.M.; Scott, K. Effect of chemically modified Vulcan XC-72R on the performance of air-breathing cathode in a single-chamber microbial fuel cell. Bioresour. Technol. 2010, 101, 5250–5255. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.Y.; Chen, Y.G.; Chen, L. Easy-to-operate and low-temperature synthesis of gram-scale nitrogen-doped graphene and its application as cathode catalyst in microbial fuel cells. ACS Nano 2011, 5, 9611–9618. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; He, Z. An effective dipping method for coating activated carbon catalyst on the cathode electrodes of microbial fuel cells. RSC Adv. 2015, 5, 36933–36937. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, S.; Pant, D.; Bogaert, G.V.; Logan, B.E. Power generation using an activated carbon and metal mesh cathode in a microbial fuel cell. Electrochem. Commun. 2009, 11, 2177–2179. [Google Scholar] [CrossRef]
- Wang, X.; Gao, N.S.J.; Zhou, Q.X.; Dong, H.; Yu, H.B.; Feng, Y.J. Acidic and alkaline pretreatments of activated carbon and their effects on the performance of air-cathodes in microbial fuel cells. Bioresour. Technol. 2013, 144, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.A.; Wu, J.C. Air-cathode preparation with activated carbon as catalyst, PTFE as binder and nickel foam as current collector for microbial fuel cells. Bioelectrochemistry 2013, 92, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, C.J.; Ding, N.; Zhang, Q.R.; Li, N.; Li, X.J.; Zhang, Y.Y.; Zhou, Q.X. Accelerated OH− transport in activated carbon air cathode by modification of quaternary ammonium for microbial fuel cells. Environ. Sci. Technol. 2014, 48, 4191–4198. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, Y.N.; An, J.K.; Feng, C.J.; Wang, X. Bifunctional quaternary ammonium compounds to inhibit biofilm growth and enhance performance for activated carbon air-cathode in microbial fuel cells. J. Power Sources 2014, 272, 895–899. [Google Scholar] [CrossRef]
- Gajda, I.; Stinchcombe, A.; Greenman, J.; Melhuish, C.; Ieropoulos, I. Ceramic MFCs with internal cathode producing sufficient power for practical applications. Int. J. Hydrog. Energy 2015, 40, 14627–14631. [Google Scholar] [CrossRef]
- Wei, B.; Tokash, J.C.; Chen, G.; Hickner, M.A.; Logan, B.E. Development and evaluation of carbon and binder loading in low-cost activated carbon cathodes for air-cathode microbial fuel cells. RSC Adv. 2012, 2, 12751–12758. [Google Scholar] [CrossRef]
- Yang, W.L.; Kim, K.Y.; Logan, B.E. Development of carbon free diffusion layer for activated carbon air cathode of microbial fuel cells. Bioresour. Technol. 2015, 197, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; He, W.H.; Yang, W.L.; Liu, J.; Wang, Q.Y.; Liang, P.; Huang, X.; Logan, B.E. Diffusion layer characteristics for increasing the performance of activated carbon air cathodes in microbial fuel cells. Environ. Sci. 2016, 2, 266–273. [Google Scholar] [CrossRef]
- Li, D.; Liu, J.; Wang, H.M.; Qu, Y.P.; Feng, Y.J. Effect of long-term operation on stability and electrochemical response under water pressure for activated carbon cathodes in microbial fuel cells. Chem. Eng. J. 2016, 299, 314–319. [Google Scholar] [CrossRef]
- Li, X.J.; Wang, X.; Zhang, Y.Y.; Gao, N.S.; Li, D.S.; Zhou, Q.X. Effects of catalyst layer and gas diffusion layer thickness on the performance of activated carbon air-cathode for microbial fuel cells. Int. J. Electrochem. Sci. 2015, 10, 5086–5100. [Google Scholar]
- Li, X.J.; Wang, X.; Zhang, Y.Y.; Ding, N.; Zhou, Q.X. Opening size optimization of metal matrix in rolling-pressed activated carbon air-cathode for microbial fuel cells. Appl. Energy 2014, 123, 13–18. [Google Scholar] [CrossRef]
- Li, D.; Qu, Y.P.; Liu, J.; He, W.H.; Wang, H.M.; Feng, Y.J. Using ammonium bicarbonate as pore former in activated carbon catalyst layer to enhance performance of air cathode microbial fuel cell. J. Power Sources 2014, 272, 909–914. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Shi, J.; Liang, P.; Wei, J.C.; Huang, X.; Zhang, C.Y.; Logan, B.E. Power generation by packed-bed air-cathode microbial fuel cells. Bioresour. Technol. 2013, 142, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Yu, H.; Yu, H.B.; Gao, N.S.J.; Wang, X. Enhanced performance of activated carbon-polytetrafluoroethylene air-cathode by avoidance of sintering on catalyst layer in microbial fuel cells. J. Power Sources 2013, 232, 132–138. [Google Scholar] [CrossRef]
- Khilari, S.; Pandit, S.; Ghangrekar, M.M.; Pradhan, D.; Das, D. Graphene oxide-impregnated PVA-STA composite polymer electrolyte membrane separator for power generation in a single-chambered microbial fuel cell. Ind. Eng. Chem. Res. 2013, 52, 11597–11606. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Hsu, W.H.; Huang, Y.C. Characterization of carbon nanotube/graphene on carbon cloth as an electrode for air-cathode microbial fuel cells. J. Nanomater. 2015, 2015, 7. [Google Scholar] [CrossRef]
- Sawant, S.Y.; Somani, R.S.; Cho, M.H.; Bajaj, H.C. A low temperature bottom-up approach for the synthesis of few layered graphene nanosheets via C–C bond formation using a modified Ullmann reaction. RSC Adv. 2015, 58, 46589–46597. [Google Scholar] [CrossRef]
- Sawant, S.Y.; Somani, R.S.; Sharma, S.S.; Bajaj, H.C. Solid-state dechlorination pathway for the synthesis of few layered functionalized carbon nanosheets and their greenhouse gas adsorptivity over CO and N2. Carbon 2014, 68, 210–220. [Google Scholar] [CrossRef]
- ElMekawy, A.; Hegab, H.M.; Losic, D.; Saint, C.P.; Pant, D. Applications of graphene in microbial fuel cells: The gap between promise and reality. Renew. Sustain. Energy Rev. 2016. [Google Scholar] [CrossRef]
- Kou, R.; Shao, Y.; Wang, D.; Engelhard, M.H.; Kwak, J.H.; Wang, J.; Viswanathan, V.V.; Wang, C.; Lin, Y.; Wang, Y.; et al. Enhanced activity and stability of Pt catalysts on functionalized graphene sheets for electrocatalytic oxygen reduction. Electrochem. Commun. 2009, 11, 954–957. [Google Scholar] [CrossRef]
- Ren, Y.P.; Pan, D.Y.; Li, X.F.; Fu, F.; Zhao, Y.N.; Wang, X.H. Effect of polyaniline-graphene nanosheets modified cathode on the performance of sediment microbial fuel cell. J. Chem. Technol. Biotechnol. 2013, 88, 1946–1950. [Google Scholar] [CrossRef]
- Khilari, S.; Pandit, S.; Ghangrekar, M.M.; Das, D.; Pradhan, D. Graphene supported alpha-MnO2 nanotubes as a cathode catalyst for improved power generation and wastewater treatment in single-chambered microbial fuel cells. RSC Adv. 2013, 3, 7902–7911. [Google Scholar] [CrossRef]
- Ghasemi, M.; Ismail, M.; Kamarudin, S.K.; Saeedfar, K.; Daud, W.R.W.; Hassan, S.H.A.; Heng, L.Y.; Alam, J.; Oh, S.E. Carbon nanotube as an alternative cathode support and catalyst for microbial fuel cells. Appl. Energy 2013, 102, 1050–1056. [Google Scholar] [CrossRef]
- Wang, H.M.; Wu, Z.C.; Plaseied, A.; Jenkins, P.; Simpson, L.; Engtrakul, C.; Ren, Z.Y. Carbon nanotube modified air-cathodes for electricity production in microbial fuel cells. J. Power Sources 2011, 196, 7465–7469. [Google Scholar] [CrossRef]
- Lotowska, W.A.; Rutkowska, I.A.; Seta, E.; Szaniawska, E.; Wadas, A.; Sek, S.; Raczkowska, A.; Brzostek, K.; Kulesza, P.J. Bacterial-biofilm enhanced design for improved electrocatalytic reduction of oxygen in neutral medium. Electrochim. Acta 2016, 213, 314–323. [Google Scholar] [CrossRef]
- Ghasemi, M.; Daud, W.R.W.; Hassan, S.H.A.; Jafary, T.; Rahimnejad, M.; Ahmad, A.; Yazdi, M.H. Carbon nanotube/polypyrrole nanocomposite as a novel cathode catalyst and proper alternative for Pt in microbial fuel cell. Int. J. Hydrog. Energy 2016, 41, 4872–4878. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Sun, J.; Hu, Y.Y.; Li, S.Z.; Xu, Q. Carbon nanotube-coated stainless steel mesh for enhanced oxygen reduction in biocathode microbial fuel cells. J. Power Sources 2013, 239, 169–174. [Google Scholar] [CrossRef]
- Zhu, D.W.; Wang, D.B.; Song, T.S.; Guo, T.; Ouyang, P.K.; Wei, P.; Xie, J.J. Effect of carbon nanotube modified cathode by electrophoretic deposition method on the performance of sediment microbial fuel cells. Biotechnol. Lett. 2015, 37, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.C.; Woo, J.H.; Yoo, K.; Chung, J.W.; Lee, C.Y. Dual layered CNT structure air cathode for power generation from microbial fuel cells. KSCE J. Civ. Eng. 2013, 17, 646–650. [Google Scholar] [CrossRef]
- Song, T.S.; Wang, D.B.; Wang, H.Q.; Li, X.X.; Liang, Y.Y.; Xie, J.J. Cobalt oxide/nanocarbon hybrid materials as alternative cathode catalyst for oxygen reduction in microbial fuel cell. Int. J. Hydrog. Energy 2015, 40, 3868–3874. [Google Scholar] [CrossRef]
- Tan, L.; Liu, Z.Q.; Li, N.; Zhang, J.Y.; Zhang, L.; Chen, S. CuSe decorated carbon nanotubes as a high performance cathode catalyst for microbial fuel cells. Electrochim. Acta 2016, 213, 283–290. [Google Scholar] [CrossRef]
- Wang, S.; Yu, D.; Dai, L. Polyelectrolyte functionalized carbon nanotubes as efficient metal-free electrocatalysts for oxygen reduction. J. Am. Chem. Soc. 2011, 133, 5182–5185. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.N.; Lu, M.; Guo, L.; Bay, L.G.H.; Zhang, Z.; Li, S.F.Y. Polyelectrolyte-single wall carbon nanotube composite as an effective cathode catalyst for air-cathode microbial fuel cells. Water Sci. Technol. 2014, 70, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.G.; Kirubaharan, C.J.; Yoo, D.J.; Kim, A.R. Graphene/poly(3,4-ethylenedioxythiophene)/Fe3O4 nanocomposite—An efficient oxygen reduction catalyst for the continuous electricity production from wastewater treatment microbial fuel cells. Int. J. Hydrog. Energy 2016, 41, 13208–13219. [Google Scholar] [CrossRef]
- Garino, N.; Sacco, A.; Castellino, M.; Munoz-Tabares, J.A.; Chiodoni, A.; Agostino, V.; Margaria, V.; Gerosa, M.; Massaglia, G.; Quaglio, M. Microwave-assisted synthesis of reduced graphene oxide/SnO2 nanocomposite for oxygen reduction reaction in microbial fuel cells. ACS Appl. Mater. Interfaces 2016, 8, 4633–4643. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Wang, S.Y.; Yan, J.; Cong, L.J.; Pan, Z.C.; Ren, Y.M.; Fan, Z.J. MnO2-graphene hybrid as an alternative cathodic catalyst to platinum in microbial fuel cells. J. Power Sources 2012, 216, 187–191. [Google Scholar] [CrossRef]
- Lu, M.; Kharkwal, S.; Ng, H.Y.; Li, S.F.Y. Carbon nanotube supported MnO2 catalysts for oxygen reduction reaction and their applications in microbial fuel cells. Biosens. Bioelectron. 2011, 26, 4728–4732. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.H.; Wang, M.; Huang, B.X.; Zhao, J.S.; Liu, R.M. Carboxyl multi-wall carbon nanotubes supported Pt-Ni alloy nanoparticles as cathode catalyst for microbial fuel cells. Int. J. Electrochem. Sci. 2012, 7, 10825–10834. [Google Scholar]
- Zhu, N.W.; Huang, J.J.; Shen, W.H.; Tu, L.X.; Wu, P.X.; Ma, H.Q. Carboxylic carbon nanotube: Catalyst support material and oxygen reduction reaction of microbial fuel cells. Int. J. Electrochem. Sci. 2015, 10, 2634–2645. [Google Scholar]
- Song, T.S.; Peng, X.; Wu, X.Y.; Zhou, C.C. Electrophoretic deposition of multi-walled carbon nanotube on a stainless steel electrode for use in sediment microbial fuel cells. Appl. Biochem. Biotechnol. 2013, 170, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Hu, Y.Y.; Li, S.Z.; Sun, J.; Hou, B. Manganese dioxide-coated carbon nanotubes as an improved cathodic catalyst for oxygen reduction in a microbial fuel cell. J. Power Sources 2011, 196, 9284–9289. [Google Scholar] [CrossRef]
- Matter, P.H.; Wang, E.; Arias, M.; Biddinger, E.J.; Ozkan, U.S. Oxygen reduction reaction activity and surface properties of nanostructured nitrogen-containing carbon. J. Mol. Catal. A 2007, 264, 73–81. [Google Scholar] [CrossRef]
- Erable, B.; Duteanu, N.; Kumar, S.M.S.; Feng, Y.J.; Ghangrekar, M.M.; Scott, K. Nitric acid activation of graphite granules to increase the performance of the non-catalyzed oxygen reduction reaction (ORR) for MFC applications. Electrochem. Commun. 2009, 11, 1547–1549. [Google Scholar] [CrossRef]
- Chen, S.L.; Chen, Y.; He, G.H.; He, S.J.; Schroder, U.; Hou, H.Q. Stainless steel mesh supported nitrogen-doped carbon nanofibers for binder-free cathode in microbial fuel cells. Biosens. Bioelectron. 2012, 34, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.Y.; Yang, L.Q.; Huang, Z.J.; Luo, J.Y.; Li, M.; Wang, D.B.; Chen, Y.G. Enhancing electrocatalytic oxygen reduction on nitrogen-doped graphene by active sites implantation. Sci. Rep. 2013, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Wang, C.; Hou, S.X.; Yang, N.A. Sustainable energy recovery in wastewater treatment by microbial fuel cells: Stable power generation with nitrogen-doped graphene cathode. Environ. Sci. Technol. 2013, 47, 13889–13895. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Zou, W.J.; Su, Y.H.; Zhu, Y.H.; Jiang, H.L.; Shen, J.H.; Li, C.Z. Activated nitrogen-doped carbon nanofibers with hierarchical pore as efficient oxygen reduction reaction catalyst for microbial fuel cells. J. Power Sources 2014, 266, 36–42. [Google Scholar] [CrossRef]
- Shi, X.X.; Feng, Y.J.; Wang, X.; Lee, H.; Liu, J.; Qu, Y.P.; He, W.H.; Kumar, S.M.S.; Ren, N.Q. Application of nitrogen-doped carbon powders as low-cost and durable cathodic catalyst to air-cathode microbial fuel cells. Bioresour. Technol. 2012, 108, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.Y.; Zheng, R.P.; Peng, H.L.; Liang, H.G.; Liao, S.J. Nitrogen-doped graphene prepared by a transfer doping approach for the oxygen reduction reaction application. J. Power Sources 2014, 245, 801–807. [Google Scholar] [CrossRef]
- Fu, S.F.; Zhu, C.Z.; Zhou, Y.Z.; Yang, G.H.; Jeon, J.W.; Lemmon, J.; Du, D.; Nune, S.K.; Lin, Y.H. Metal-organic framework derived hierarchically porous nitrogen-doped carbon nanostructures as novel electrocatalyst for oxygen reduction reaction. Electrochim. Acta 2015, 178, 287–293. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.; Hu, Z. Boron-doped carbon nanotubes as metal-free electrocatalysts for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2011, 50, 7132–7135. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-W.; Peng, F.; Wang, H.-J.; Yu, H.; Zheng, W.-X.; Yang, J. Phosphorus-doped graphite layers with high electrocatalytic activity for the O2 reduction in an alkaline medium. Angew. Chem. Int. Ed. 2011, 50, 3257–3261. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-S.; Bhattacharjya, D.; Inamdar, S.; Park, J.; Yu, J.-S. Phosphorus-doped ordered mesoporous carbons with different lengths as efficient metal-free electrocatalysts for oxygen reduction reaction in alkaline media. J. Am. Chem. Soc. 2012, 134, 16127–16130. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.; Huang, S. Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction. ACS Nano 2012, 6, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Li, K.X.; Liu, Y.; Pu, L.T.; Chen, Z.H.; Deng, S.G. The high-performance and mechanism of P-doped activated carbon as a catalyst for air-cathode microbial fuel cells. J. Mater. Chem. A 2015, 3, 21149–21158. [Google Scholar] [CrossRef]
- Chen, Z.H.; Li, K.X.; Pu, L.T. The performance of phosphorus (P)-doped activated carbon as a catalyst in air-cathode microbial fuel cells. Bioresour. Technol. 2014, 170, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, S.L.; Zhou, Y.; Zheng, S.Q.; Hou, H.Q.; Zhao, F. Phosphorus-doped carbon derived from cellulose phosphate as efficient catalyst for air-cathode in microbial fuel cells. J. Power Sources 2014, 261, 245–248. [Google Scholar] [CrossRef]
- Zhong, S.K.; Zhou, L.H.; Wu, L.; Tang, L.F.; He, Q.Y.; Ahmed, J. Nitrogen- and boron-co-doped core shell carbon nanoparticles as efficient metal-free catalysts for oxygen reduction reactions in microbial fuel cells. J. Power Sources 2014, 272, 344–350. [Google Scholar] [CrossRef]
- Meng, K.; Liu, Q.; Huang, Y.Y.; Wang, Y.B. Facile synthesis of nitrogen and fluorine co-doped carbon materials as efficient electrocatalysts for oxygen reduction reactions in air-cathode microbial fuel cells. J. Mater. Chem. A 2015, 3, 6873–6877. [Google Scholar] [CrossRef]
- Abbas, S.C.; Ding, K.; Liu, Q.; Huang, Y.Y.; Bu, Y.K.; Wu, J.; Lv, J.Q.; Ghausi, M.A.; Wang, Y.B. Si-C-F decorated porous carbon materials: A new class of electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2016, 4, 7924–7929. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, Z. Mechanisms of oxygen reduction reaction on nitrogen-doped graphene for fuel cells. J. Phys. Chem. C 2011, 115, 11170–11176. [Google Scholar] [CrossRef]
- Zhou, L.H.; Fu, P.; Cai, X.X.; Zhou, S.G.; Yuan, Y. Naturally derived carbon nanofibers as sustainable electrocatalysts for microbial energy harvesting: A new application of spider silk. Appl. Catal. B-Environ. 2016, 188, 31–38. [Google Scholar] [CrossRef]
- Feng, Y.J.; Shi, X.X.; Wang, X.; Lee, H.; Liu, J.; Qu, Y.P.; He, W.H.; Kumar, S.M.S.; Kim, B.H.; Ren, N.Q. Effects of sulfide on microbial fuel cells with platinum and nitrogen-doped carbon powder cathodes. Biosens. Bioelectron. 2012, 35, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Zhu, Y.H.; Yang, X.L.; Shen, J.H.; Lu, J.D.; Zhang, X.Y.; Chen, J.D.; Li, C.Z. A highly efficient catalyst toward oxygen reduction reaction in neutral media for microbial fuel cells. Ind. Eng. Chem. Res. 2013, 52, 6076–6082. [Google Scholar] [CrossRef]
- Pan, Y.J.; Mo, X.P.; Li, K.X.; Pu, L.T.; Liu, D.; Yang, T.T. Iron-nitrogen-activated carbon as cathode catalyst to improve the power generation of single-chamber air-cathode microbial fuel cells. Bioresour. Technol. 2016, 206, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Ivanov, I.; Nagaiah, T.C.; Bordoloi, A.; Logan, B.E. Mesoporous nitrogen-rich carbon materials as cathode catalysts in microbial fuel cells. J. Power Sources 2014, 269, 212–215. [Google Scholar] [CrossRef]
- Liu, L.; Xiong, Q.; Li, C.G.; Feng, Y.; Chen, S.L. Conversion of straw to nitrogen doped carbon for efficient oxygen reduction catalysts in microbial fuel cells. RSC Adv. 2015, 5, 89771–89776. [Google Scholar] [CrossRef]
- Yuan, H.R.; Deng, L.F.; Qi, Y.J.; Kobayashi, N.; Tang, J.H. Nonactivated and activated biochar derived from bananas as alternative cathode catalyst in microbial fuel cells. Sci. World J. 2014, 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.H.; Fu, P.; Wen, D.H.; Yuan, Y.; Zhou, S.G. Self-constructed carbon nanoparticles-coated porous biocarbon from plant moss as advanced oxygen reduction catalysts. Appl. Catal. B 2016, 181, 635–643. [Google Scholar] [CrossRef]
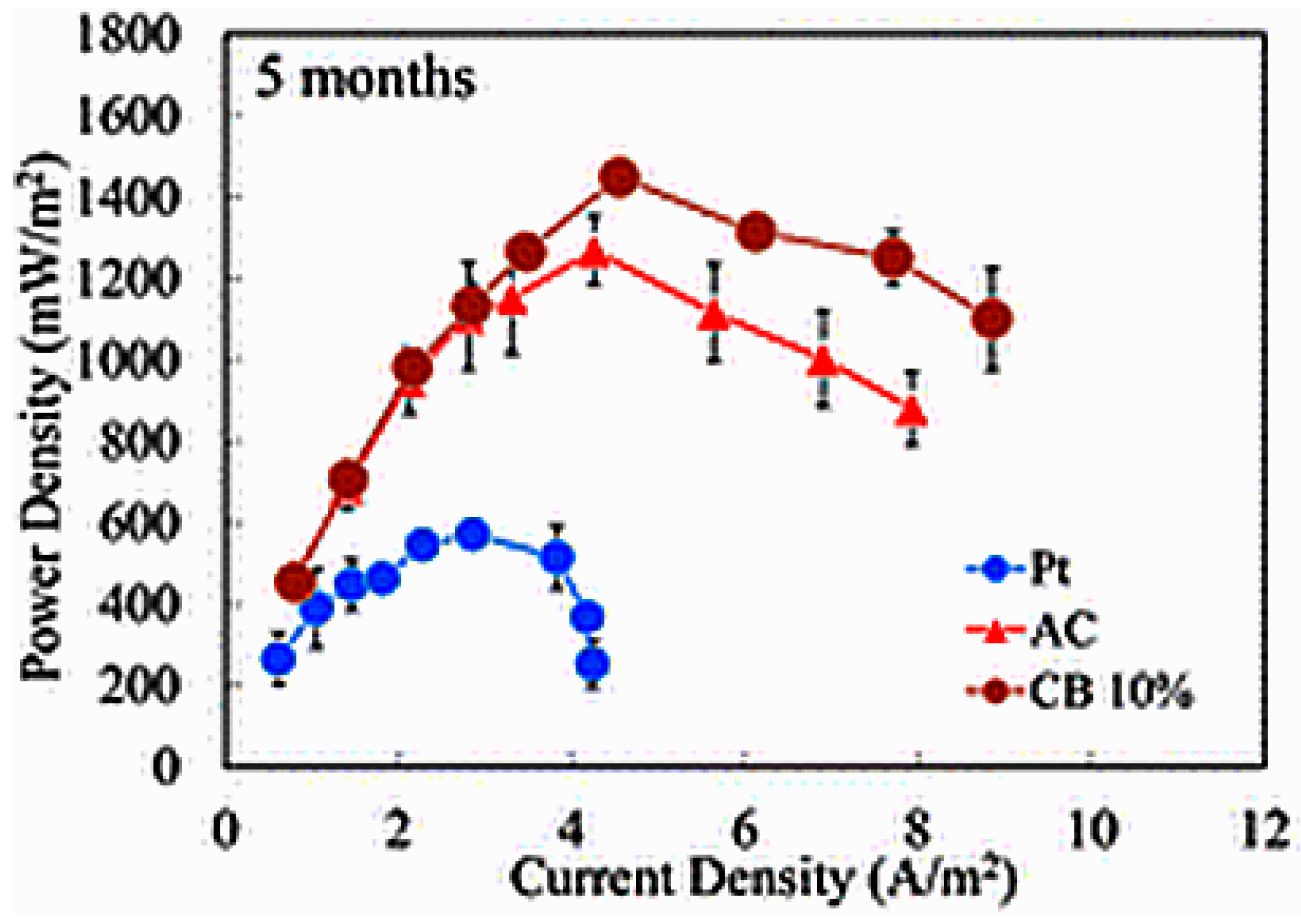
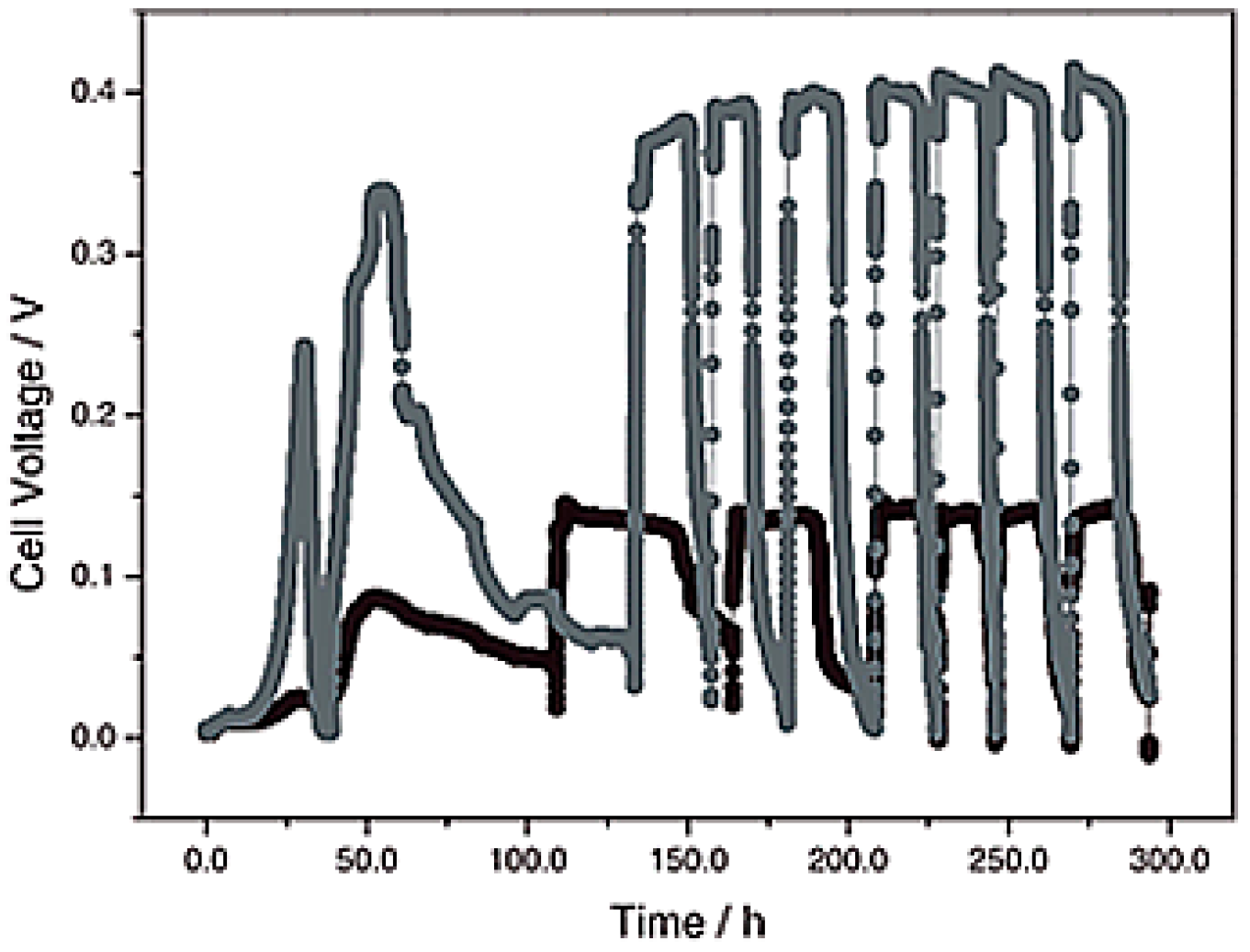
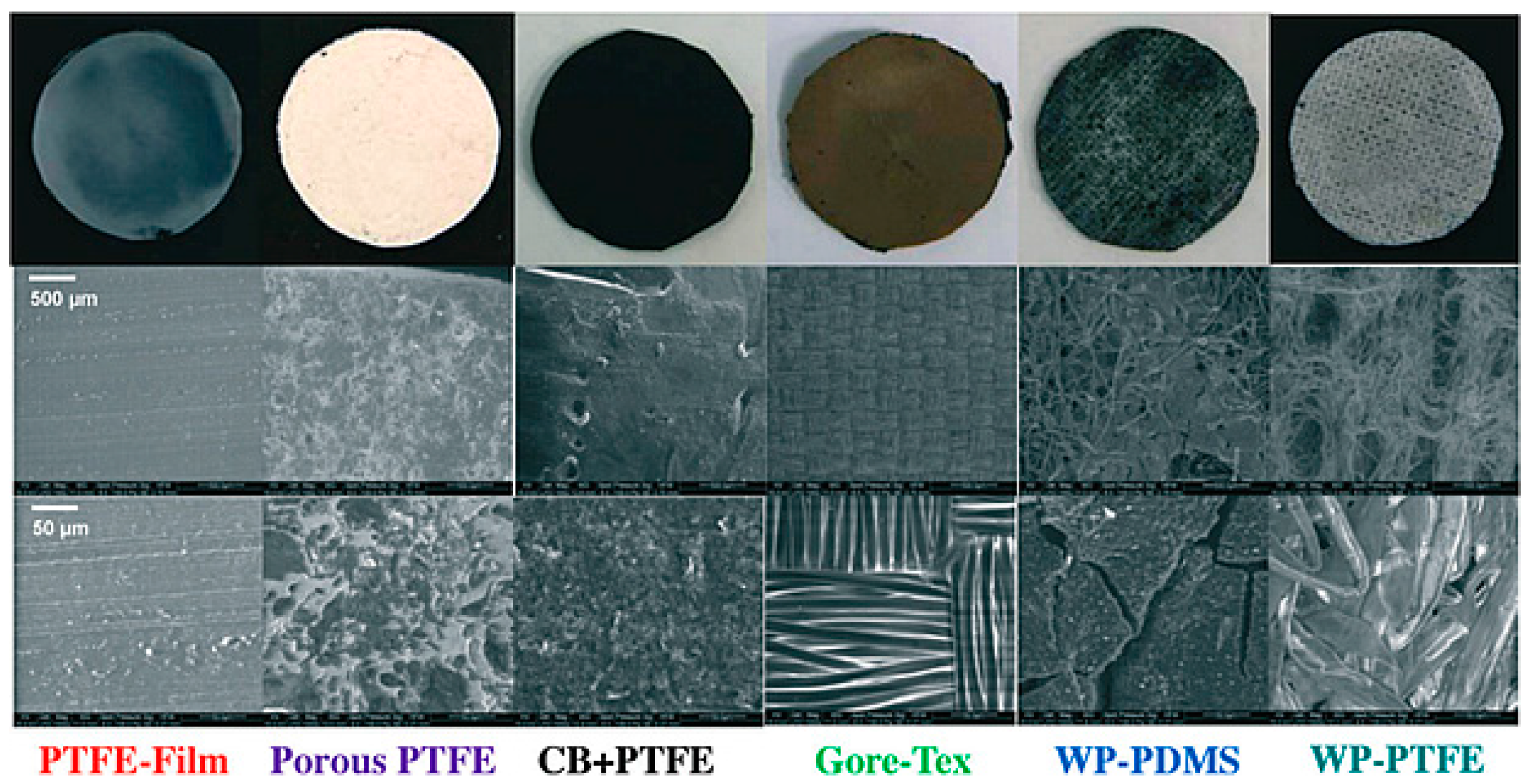
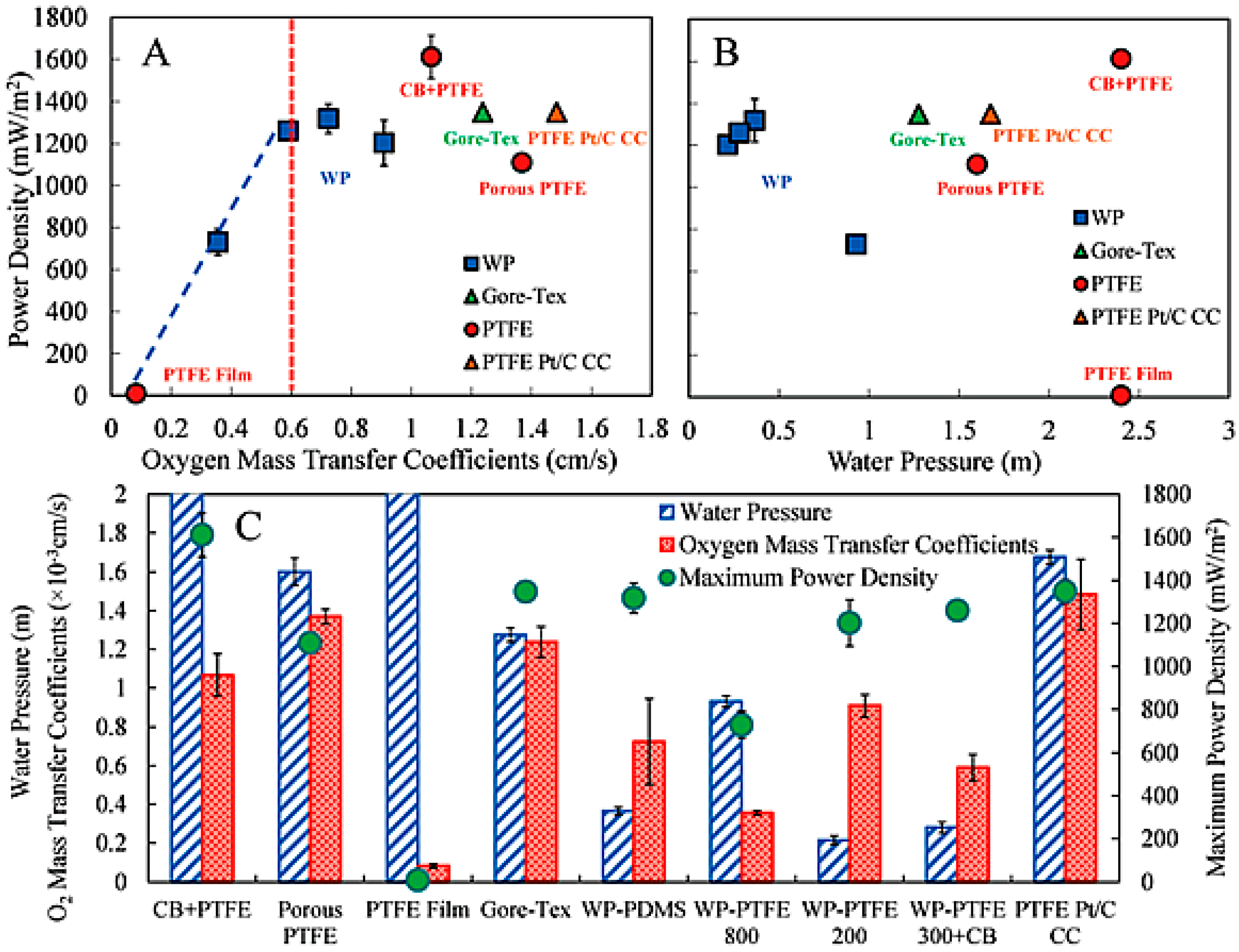
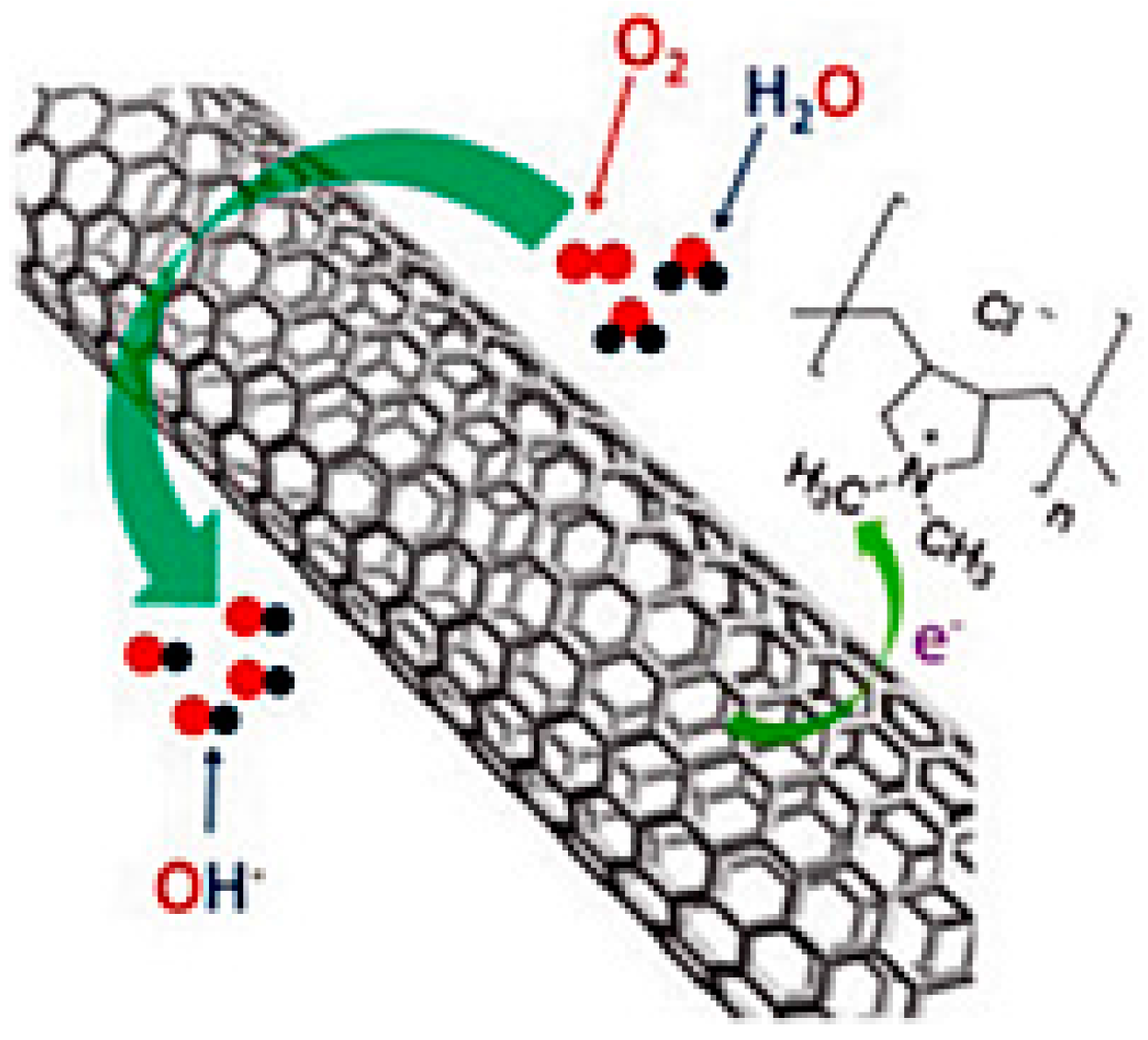
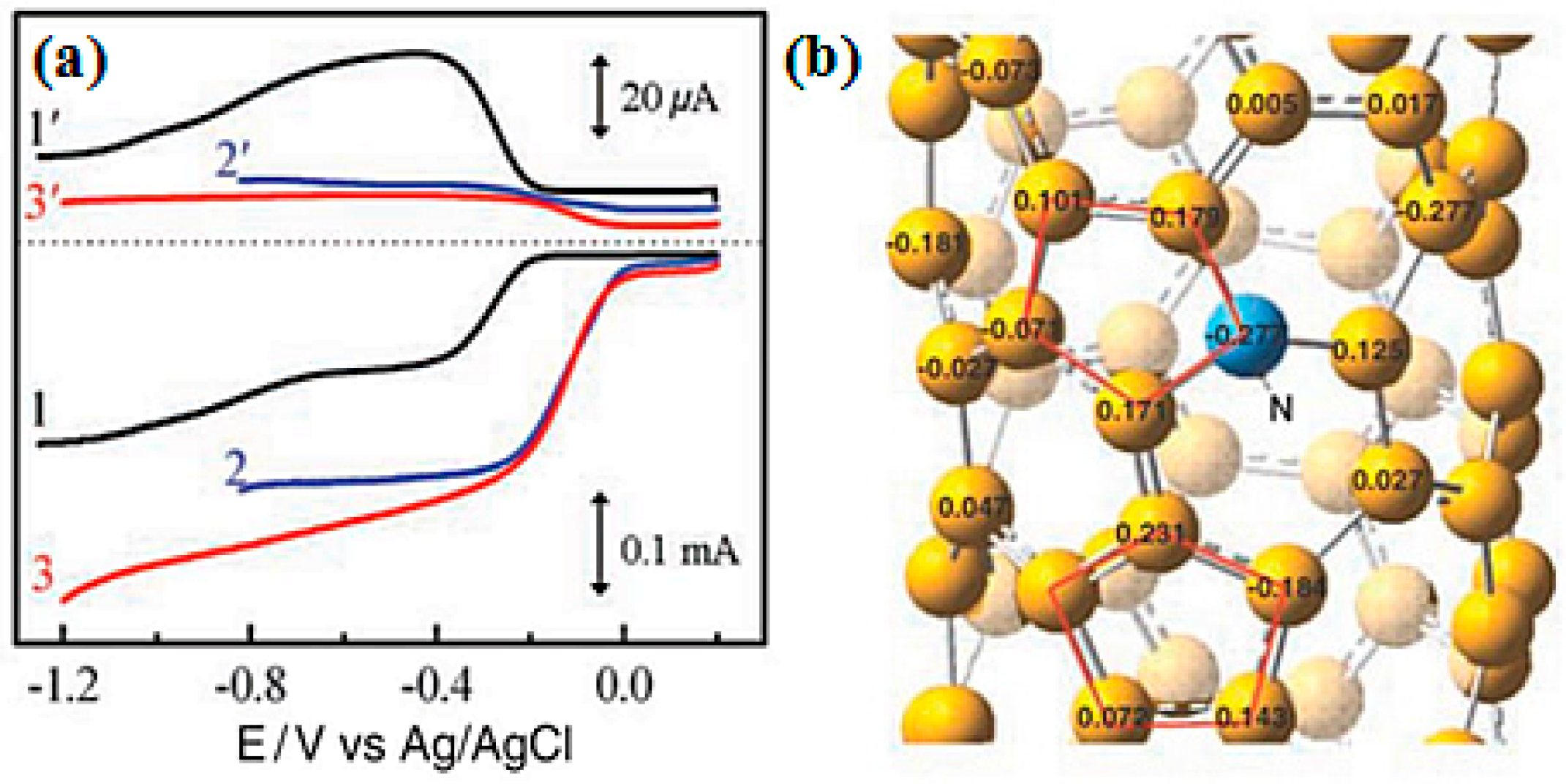
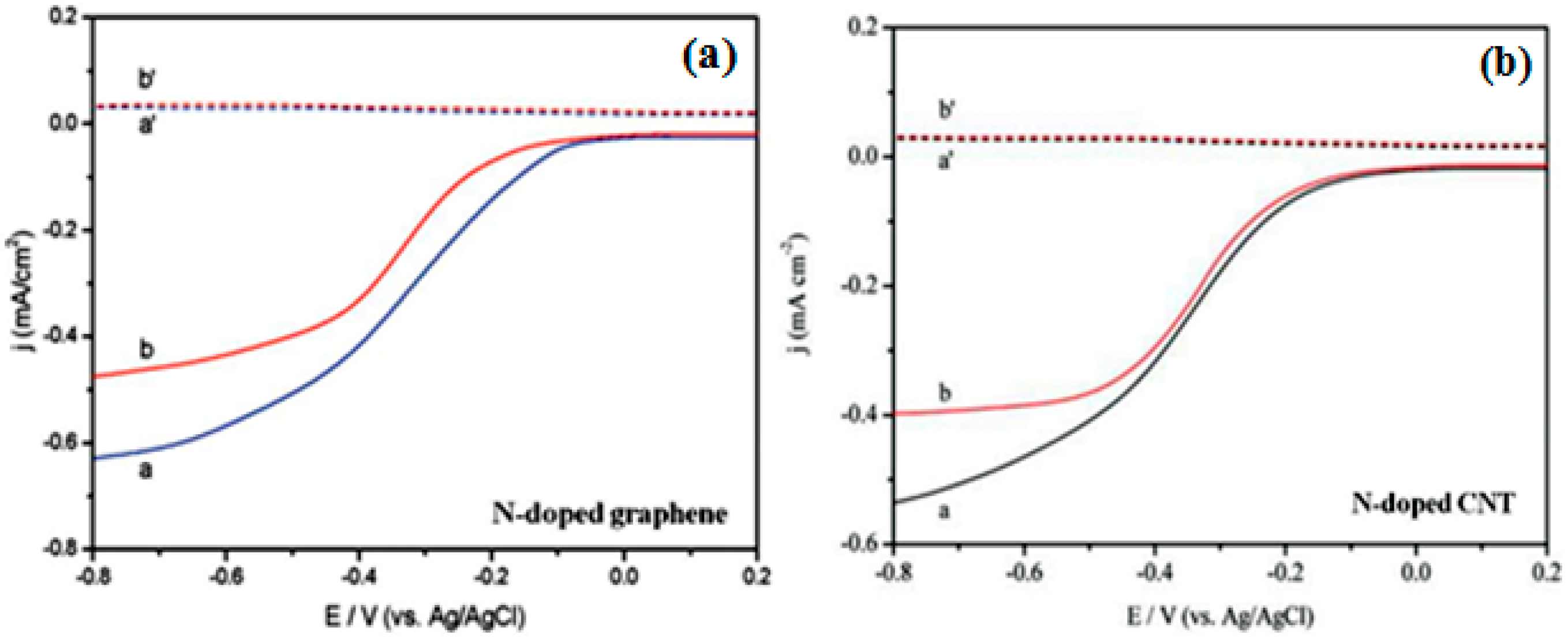
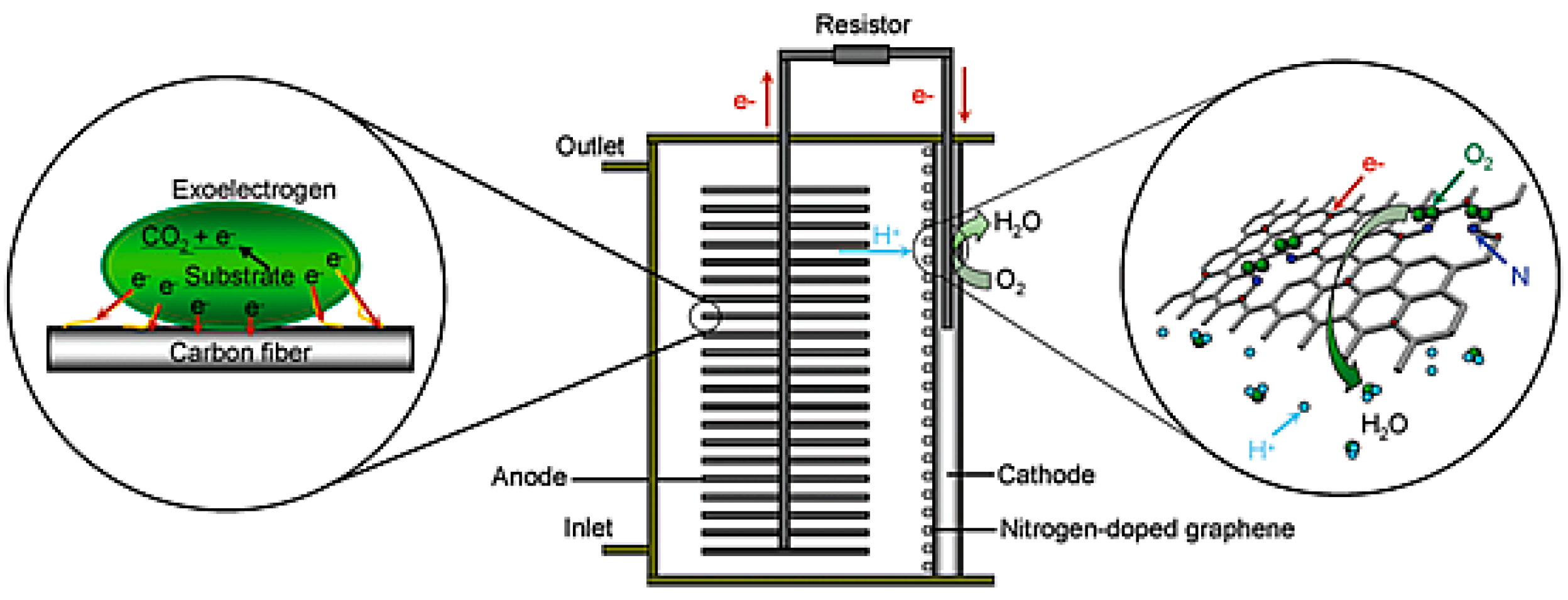
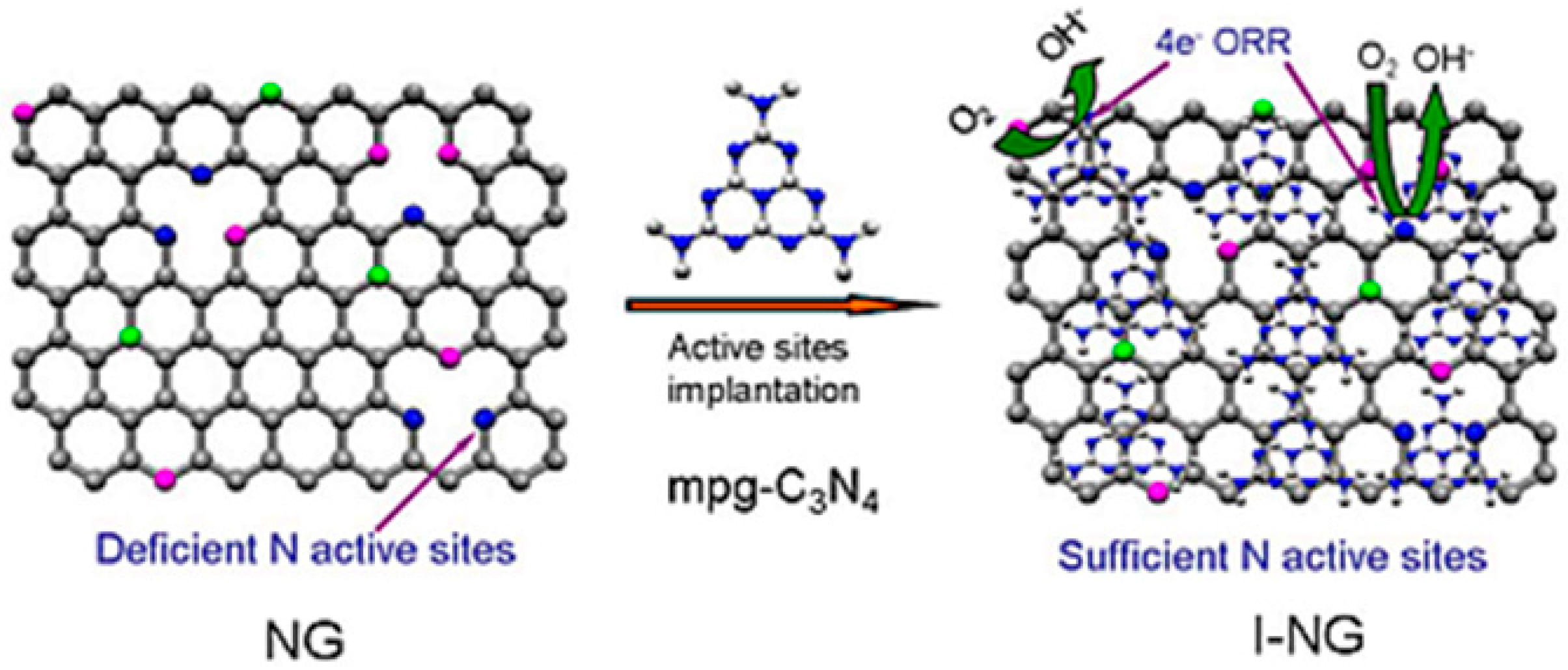
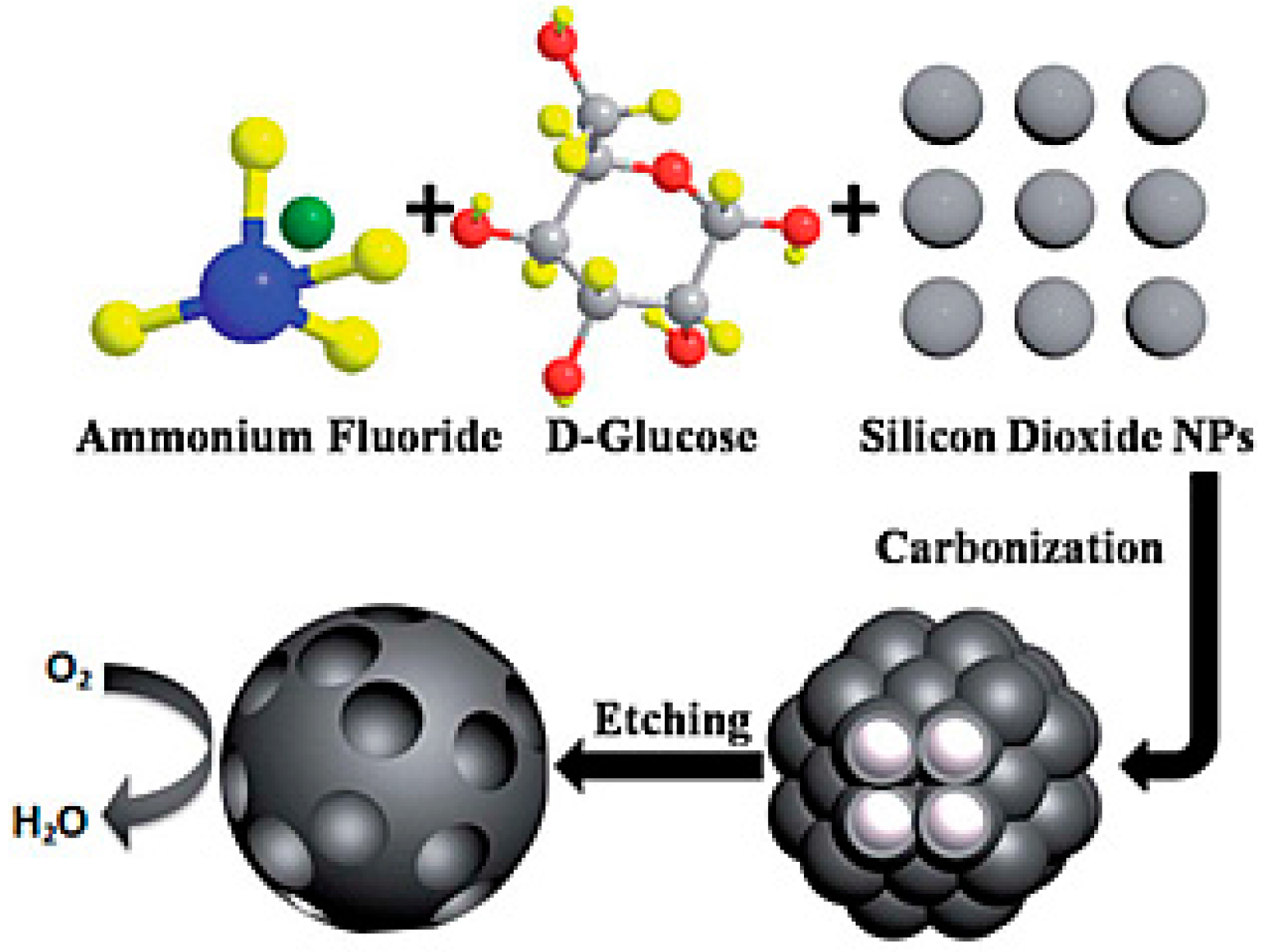
| Catalysts | Catalyst Support | Casting Method | Power Output (mW/m2) | Performance Comparison | MFC Type | Bacteria Culture | Anode | Ref. |
|---|---|---|---|---|---|---|---|---|
| FePc/CB | CP | Drop casting | 17.37 | 4.2 times vs. CP | Double chamber | Enterobacter cloacae | CP | [70] |
| FePc/CB (agitated) | CP | Drop casting | 140.30 | 13.7 times vs. FePc | Double chamber | Enterobacter cloacae | CB | [70] |
| FePc/CB | CP | Drop casting | 7.55 | 4.5 times vs. CP | Double chamber | Beer brewery wastewater | CP | [70] |
| FePc/CB (agitated) | CP | Drop casting | 38.34 | 7.8 times vs. FePc | Double chamber | Beer brewery wastewater | CB | [70] |
| CoNPc/CB | CP | Brush casting | 64.7 | 0.8 times vs. Pt/C 2.2 times vs. NPc/CB 6.9 times vs. CB | Double chamber | Anaerobic digester sludge | CP | [73] |
| ZrO2/CB | CC | Brush casting | 596 | 1.4 times vs. CB 0.6 times vs. Pt/C | Single chamber | domestic wastewater | GBr | [74] |
| CuPc/CB | CP | Brush casting | 118.2 | 3.1 times vs. CB 2.1 times vs. Pc/CB 1.2 times vs. Ni/CB 0.99 times vs. Pt/C | Double chamber | Palm oil effluent | CP | [79] |
| Nitric acid-treated CB | Membrane | Spray casting | 170 | 3.3 times vs. CB 0.8 times vs. Pc/CB | Single chamber | Anaerobic sludge | CF | [80] |
| 10% CB/AC | SS mesh | Spoon casting | 1560 | 1.2 times vs. AC 1.1 times vs. 2% CB/AC 1.0 times vs. 5% CB/AC 1.0 times vs. 15% CB/AC 1.1 times vs. Pt/C | Single chamber | Pre-acclimated | GBr | [43] |
| PANI nanofiber/CB | CC | Brush casting | 496 | 2.7 times vs. PANI nanofiber 0.8 times vs. Pt/C | Single chamber | Anaerobic sludge | CC | [81] |
| PPy/CB | CC | - | 401.8 | 4.4 times vs. CB 0.7 times vs. Pt/C | Single chamber | Activated sludge | CC | [15] |
| Catalyst | Catalyst Support | Casting Method | Power Output (mW/m2) | Performance Comparison | MFC Type | Bacteria Culture | Anode | Ref. |
|---|---|---|---|---|---|---|---|---|
| AC | SS mesh | Press | 892 | 0.9 times vs. Pt/C (initial) 1.2 times vs. Pt/C (4 months) | Single chamber | Domestic wastewater | CBr | [92] |
| AC | SS mesh | Rollingpress | 2348 | - | Single chamber | Pre-acclimated | CBr | [93] |
| AC (heat-treated) | SS mesh | Press | 1400 | 1.3 times vs. AC 1.1 times vs. AC/CB 1.1 times vs. Pt/C | Single chamber | Pre-acclimated | GBr | [67] |
| AC | SS mesh | Spatula | 1430 | 1.3 times vs. Pt/C | Single chamber | Pre-acclimated | GBr | [76] |
| AC | SS mesh (40 mesh) | Rolling press | 2151 | 1.5 times vs. AC on 80 Mesh SS | Single chamber | Domestic wastewater | CBr | [94] |
| Modified AC | SS mesh | Rolling | 892 | 1.3 times vs. AC | Single chamber | Domestic wastewater | CBr | [95] |
| Granular AC | - | - | 676 | 1.8 times vs. semi-coke 5.5 times vs. graphite 11.2 times vs. CF | Packed-bed | Pre-acclimated | GBr | [96] |
| AC (coal-based) | SS mesh | Press | 1620 | Similar with AC (peat) 2.6 times vs. AC (hardwood) 0.8 times vs. Pt/C | Single chamber | Pre-acclimated | CBr | [18] |
| AC (KOH-treated) | SS mesh | Rollingpress | 957 | 1.2 times vs. AC 1.8 times vs. AC (HNO3-treated) | Single chamber | Pre-acclimated | AC on SS mesh | [84] |
| AC (not sintered catalyst layer) | SS mesh | Rolling | 1086 | 1.3 times vs. AC (sintered) | Single chamber | Pre-acclimated | CM | [97] |
| AC | Nickel foam | - | 1190 | 0.9 times vs. Pt/C on CC | Single chamber | Pre-acclimated | CBr | [85] |
| Cathodes | Max. OCP (mV) | Max. Vol. Power Density (W/m3) | Max. Columbic Efficiency (%) | COD Removal Efficiency (%) | Internal Resistance (Ω) |
|---|---|---|---|---|---|
| Catalyst-free | 677 | 0.57 | 5.0 | 69.2 | 172 |
| MnO2-NTs/Vulcan XC | 754 | 2.2 | 8.4 | 78.7 | 108 |
| MnO2-NTs/MWCNTs | 793 | 3.94 | 11.0 | 82.9 | 97 |
| MnO2-NTs/graphene | 812 | 4.68 | 11.5 | 83.7 | 85 |
| Pt/C | 839 | 5.67 | 12.6 | 84.4 | 75 |
| Electrode | Pt (0.5 mg/cm2) Coating Method | Power Density (mW/m2) |
|---|---|---|
| CC | - | 151 |
| CC-Pt | 10% Pt/CB mixture (brush) | 1071 |
| CNT Mat | - | 329 |
| CNT Mat-Pt | 10% Pt/CB mixture (brush) | 1118 |
| Single-walled CNTs (SWCNTs) | 117 | |
| SWCNTs-Pt | Laboratory-synthesized SWCNTs; H2PtCl6 (Microwave) | 302 |
| SWCNTs-Pt | Commercial SWCNTs; H2PtCl6 (Microwave) | 522 |
| MWCNTs-Pt | Commercial MWCNTs; H2PtCl6 (Microwave) | 174 |
| Catalyst | Catalyst Support | Casting Method | Power Output (mW/m2) | Performance Comparison | MFC Type | Bacteria Culture | Anode | Ref. |
|---|---|---|---|---|---|---|---|---|
| r-Graphene oxide sheet | CC | - | 2.9 (W/m3) | 0.6 times vs. Pt/C | Double chamber | Anaerobic sludge | CBr | [55] |
| r-Graphene oxide sheet | CC | - | 2.5 (W/m3) | 8.3 times vs. CC | Double chamber | Anaerobic sludge | CBr | [55] |
| r-Graphene oxide particles | CC | - | 3.3 (W/m3) | 11.0 times vs. CC | Double chamber | Anaerobic sludge | CBr | [55] |
| Graphene/PANI | GF | In situ deposition | 99 | 116.5 times vs. GF | Sediment | Residual sludge | Graphite | [104] |
| Graphene/MnO2 | CP | Spray casting | 4.68 (W/m3) | 1.2 times vs. MWCNTs/MnO2 2.1 times vs. CB/MnO2 0.8 times vs. Pt/C | Single chamber | Anaerobic consortia | CC | [105] |
| Pt(15%)-Co/graphene | CC | - | 1378 | Almost similar with Pt/C (20%) | Single chamber | Domestic | CC | [13] |
| r-Graphene oxide /PEDOT/Fe3O4 | CC | Spray casting | 3525 | 8.2 times vs. CC 4.5 times vs. Fe3O4 2.2 times vs. r-Graphene oxide/Fe3O4 1.5 times vs. r-Graphene oxide/PEDOT | Single chamber | Anaerobic sludge | CC | [117] |
| r-Graphene oxide/SnO2 | SS grid/CF | - | 80 | 1.6 times vs. Pt/C | Single chamber | Seawater inoculums | CF | [118] |
| Graphene/MnO2 | SS net | - | 2084 | 6.2 times vs. non-catalyzed 1.4 times vs. MnO2 1.2 times vs. Pt/C | Single chamber | Anaerobic sludge | CF | [119] |
| CNTs mat | - | - | 329 | 2.2 times vs. CC | Single chamber | Exoelectrogenic bacteria | GBr | [107] |
| β-MnO2/CNT | CC | Spray casting | 97.8 | 4.4 times vs. α-MnO2/CNT 1.2 times vs. γ-MnO2/CNT 0.6 times vs. Pt/C | Single chamber | Domestic wastewater | CC | [120] |
| Pt-Ni-MWCNTs | CC | - | 1220 | 0.9 times vs. Pt/C | Single chamber | Predomesticated | CC | [121] |
| Pt/modified CNTs | Titanium mesh | Brush casting | 911.3 | 2.0 times vs. Pt/C | Single chamber | Local pond | CF | [122] |
| CuSe/CNTs | CC | Brush casting | 425.9 | 1.7 times vs. CNTs 1.6 times vs. CuSe 0.9 times vs. Pt/C | Single chamber | Activated sludge | CC | [114] |
| Dual layered CNTs | SS | Spray casting | 207 | 2.4 times vs. Single layer CNTs 1.4 times vs. Pt/Graphite cloth | Single chamber | Anaerobic sludge | Graphite fabric | [112] |
| MWCNTs | GF | Electrophoretic deposition | 214.7 | 1.6 times vs. GF | Sediment | - | GF | [110] |
| MWCNTs | SS net | Electrophoretic deposition | 31.6 | 3.2 times vs. SS net | Sediment | - | SS net | [123] |
| MnO2/CNT | CP | In situ synthesis | 210 | 2.3 times vs. MnO2/CNT (mechanical mixing) 0.9 times vs. Pt/C | Single chamber | Anaerobic sludge | GF | [124] |
| PEPU-SWCNTs | CC | Spray casting | 270.1 | 2.3 times vs. PDDA-CNT 0.9 times vs. Pt/C | Single chamber | Activated sludge | CC | [117] |
| Catalyst | Doping Agent/Method | Catalyst Support | Power Output (mW/m2) | Performance Comparison | MFC Type | Bacteria Culture | Anode | Ref. |
|---|---|---|---|---|---|---|---|---|
| N-CNFs (activated) | PPy | CP | 1377 | 1.5 times vs. N-CNFs 4.0 times vs. CP | Double chamber | Sewage wastewater | CG | [130] |
| N-carbon powder (pre-treated) | HNO3 treatment | CC | 934.7 | Almost similar with Pt/C | Single chamber | Domestic sewage | CBr | [131] |
| N-doped graphene | Cyanuric chloride | CC | 1350 | Almost similar to Pt/C | Single chamber | Suspended bacteria | CBr | [81] |
| N-carbon powder | HNO3 treatment | CC | 222.5 | 0.9 times vs. Pt/C 1.1 times vs. Pt/C (with Na2S) | Double chamber | Domestic wastewater | CC | [146] |
| N-graphene/C3N4 | NH4OH, cyanamide | CC | 1618 | 1.2 times vs. N-graphene 1.1 times vs. Pt/C | Single chamber | Suspended bacteria | CBr | [128] |
| Co3O4/N-graphene | NH4OH | ITO substrate | 1340 | 0.9 times vs. Pt/C | Double chamber | Shewanella oneidensis, MR-1 | CG | [147] |
| Fe-N-AC | Ethylenediamine | SS mesh | 2437 | 2.1 times vs. AC | Single chamber | Domestic wastewater | CF | [148] |
| Mesoporous N-carbon | Ethylenediamine | CC | 979 | 0.8 times vs. Pt/C | Single chamber | Domestic wastewater | GBr | [149] |
| N-CNT | Ethylenediamine | CC | 1600 | 1.1 times vs. Pt/C | Single chamber | Domestic wastewater | CBr | [54] |
| N-carbon | NH3 gas | SS mesh | 1041 | 2.5 times vs. undoped carbon 1.8 times vs. Pt/C | Single chamber | Domestic wastewater | GBr | [28] |
| Porous N-carbon nanosheets/graphene | PANI | SS net | 1159 | 1.8 times vs. N-carbon 1.3 times vs. Pt/C 18.1 times vs. without catalyst | Single chamber | Anaerobic sludge | CBr | [46] |
| N-graphene | NH4OH | CP | 776 | Slightly higher than Pt/C | Double chamber | Activated sludge | CC | [129] |
| Acid/base treated N-AC | Cyanamide | CC | 650 | 2.1 times vs. AC 1.6 times vs. N-AC 1.4 times vs. Pt/C 1.1 times vs. acid-treated N-AC | Double chamber | Digester effluent | CBr | [58] |
| N-/P-doped carbon | Ammonium phosphate | SS mesh | 2293 | 2.9 times vs. N-carbon 2.6 times vs. P-carbon 1.4 times vs. Pt/C | Single chamber | Domestic wastewater | GBr | [63] |
| N-/F-CB | NH3, PTFE | SS mesh | 672 | 1.4 times vs. undoped carbon 1.1 times vs. F-carbon 1.3 times vs. N-carbon 1.2 times vs. Pt/C | Single chamber | --- | CM | [142] |
| P-AC | H3PO4 | SS mesh | 1278 | 1.75 times vs. untreated AC 1.5 times vs. AC heated at 800 °C | Single chamber | Domestic wastewater | CF | [138] |
| N-/S-CNF | Spider silk | SS mesh | 1800 | 1.6 time vs. Pt/C | Single chamber | Domestic wastewater | GBr | [145] |
| N-/B-carbon nanoparticles | Polydopamine, Aminobenzene boronic acid | CC | 642 | 0.9 time vs. Pt/C 1.2 times vs. N-carbon nanoparticles 5.3 times vs. carbon nanoparticles | Single chamber | Anaerobic sludge | CC | [142] |
| P-AC | H3PO4 | SS mesh | 1096 | 1.5 times vs. AC | Single chamber | Domestic wastewater | CF | [139] |
| P-carbon | Cellulose phosphate | SS mesh | 1312 | 2.6 times vs. undoped carbon 1.1 times vs. Pt/C | Single chamber | Domestic wastewater | GBr | [140] |
| N-/S-carbon nanosheets | NH3, Diphenyl disulfide | CC | 1500 | 0.65 times vs. Pt/C | Double chamber | - | CBr | [66] |
| Si-/F-porous carbon | SiO2, Ammonium fluoride | SS mesh | 1026 | 1.1 times vs. Pt/C | Single chamber | Domestic wastewater | CM | [143] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawant, S.Y.; Han, T.H.; Cho, M.H. Metal-Free Carbon-Based Materials: Promising Electrocatalysts for Oxygen Reduction Reaction in Microbial Fuel Cells. Int. J. Mol. Sci. 2017, 18, 25. https://doi.org/10.3390/ijms18010025
Sawant SY, Han TH, Cho MH. Metal-Free Carbon-Based Materials: Promising Electrocatalysts for Oxygen Reduction Reaction in Microbial Fuel Cells. International Journal of Molecular Sciences. 2017; 18(1):25. https://doi.org/10.3390/ijms18010025
Chicago/Turabian StyleSawant, Sandesh Y., Thi Hiep Han, and Moo Hwan Cho. 2017. "Metal-Free Carbon-Based Materials: Promising Electrocatalysts for Oxygen Reduction Reaction in Microbial Fuel Cells" International Journal of Molecular Sciences 18, no. 1: 25. https://doi.org/10.3390/ijms18010025
APA StyleSawant, S. Y., Han, T. H., & Cho, M. H. (2017). Metal-Free Carbon-Based Materials: Promising Electrocatalysts for Oxygen Reduction Reaction in Microbial Fuel Cells. International Journal of Molecular Sciences, 18(1), 25. https://doi.org/10.3390/ijms18010025