Enantioselective Effects of Metalaxyl Enantiomers on Breast Cancer Cells Metabolic Profiling Using HPLC-QTOF-Based Metabolomics
Abstract
:1. Introduction
2. Results and Discussion
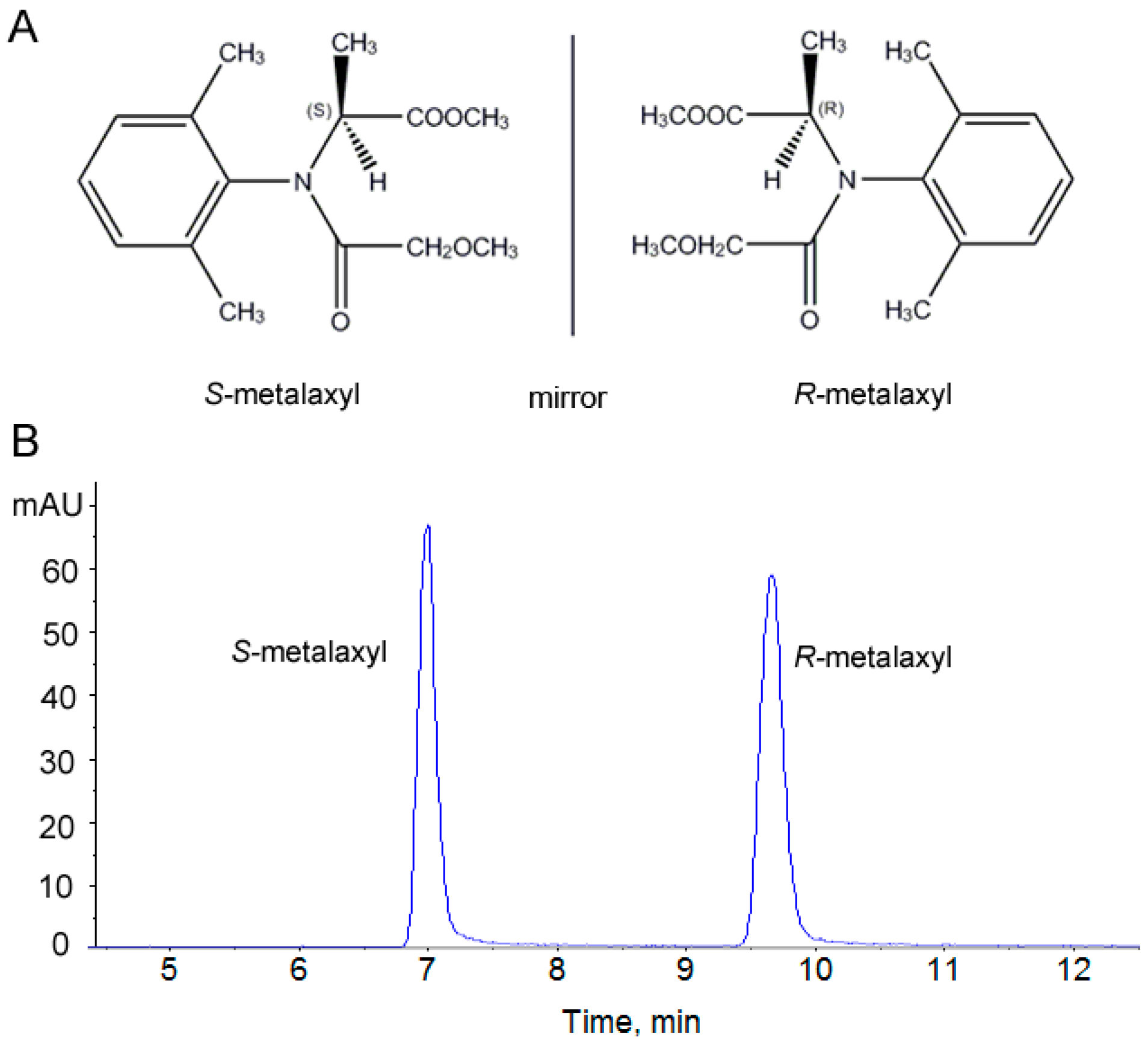
2.1. Assessment of the Stability and Reproducibility of the HPLC-QTOF Method
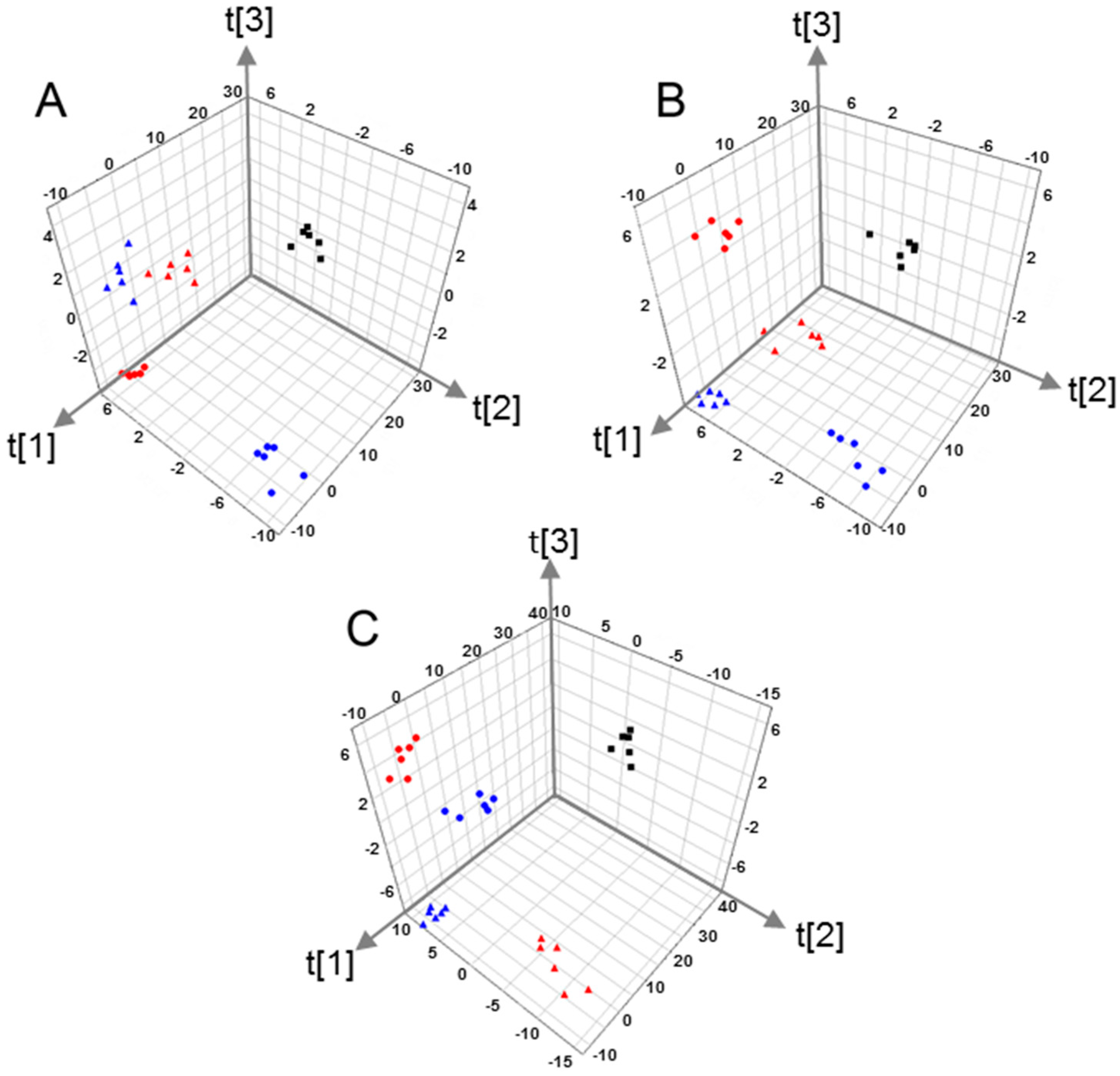
2.2. Multivariate Pattern Recognition Analysis
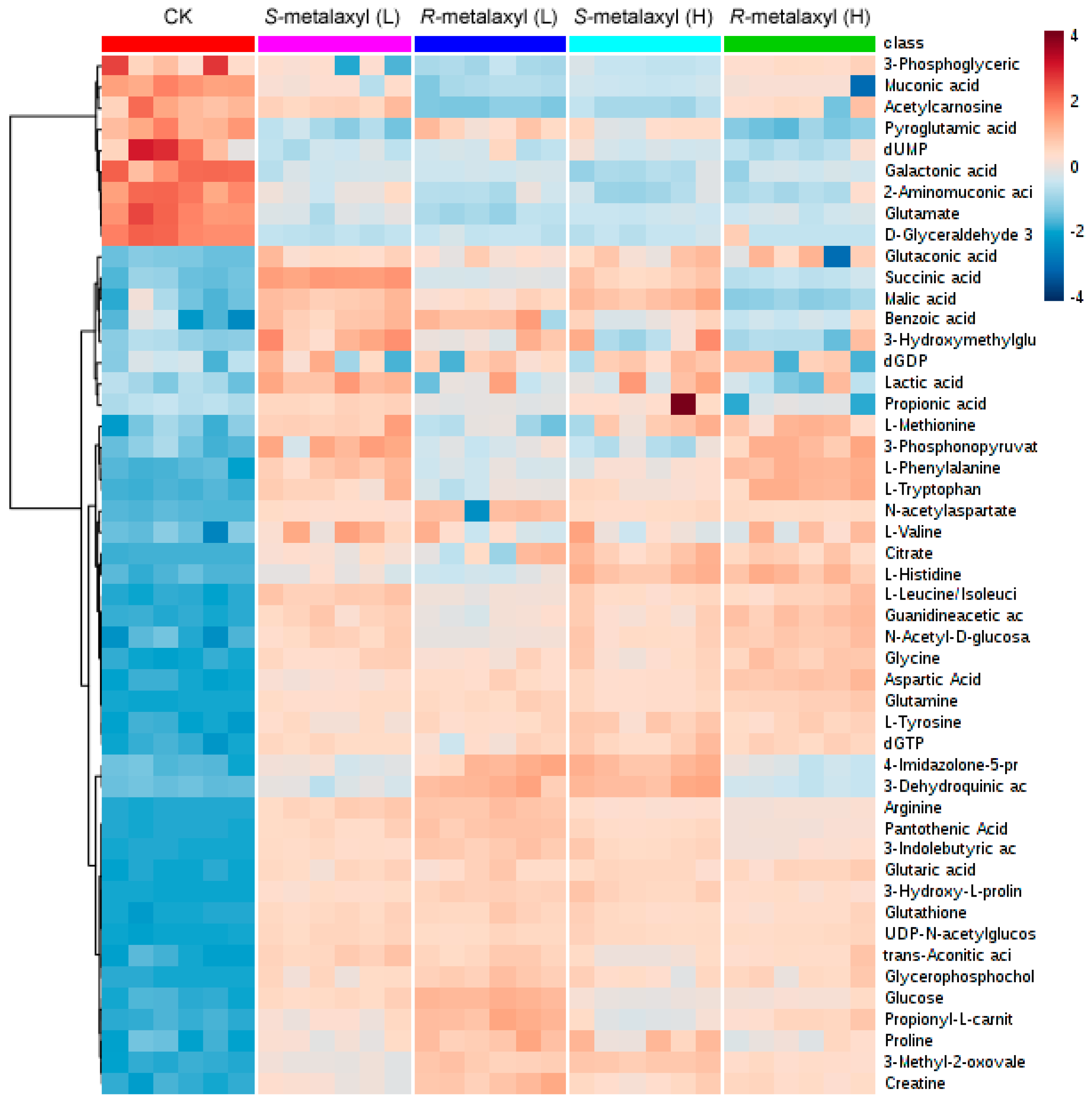
2.3. Metabolite Identification
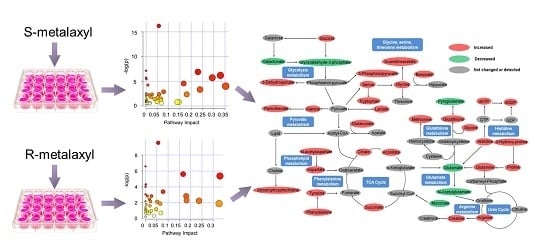
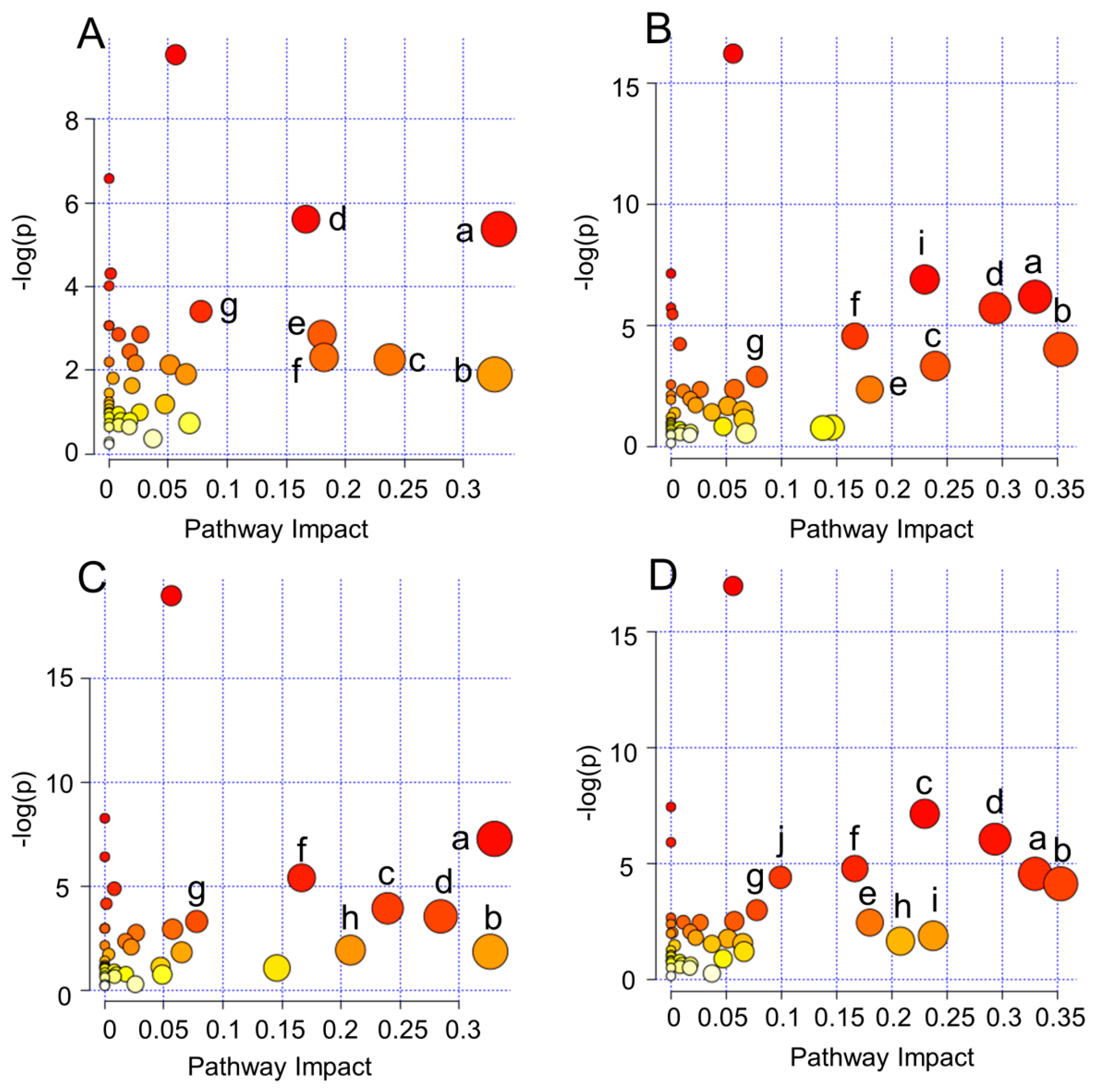
2.4. Biological Pathway Analysis
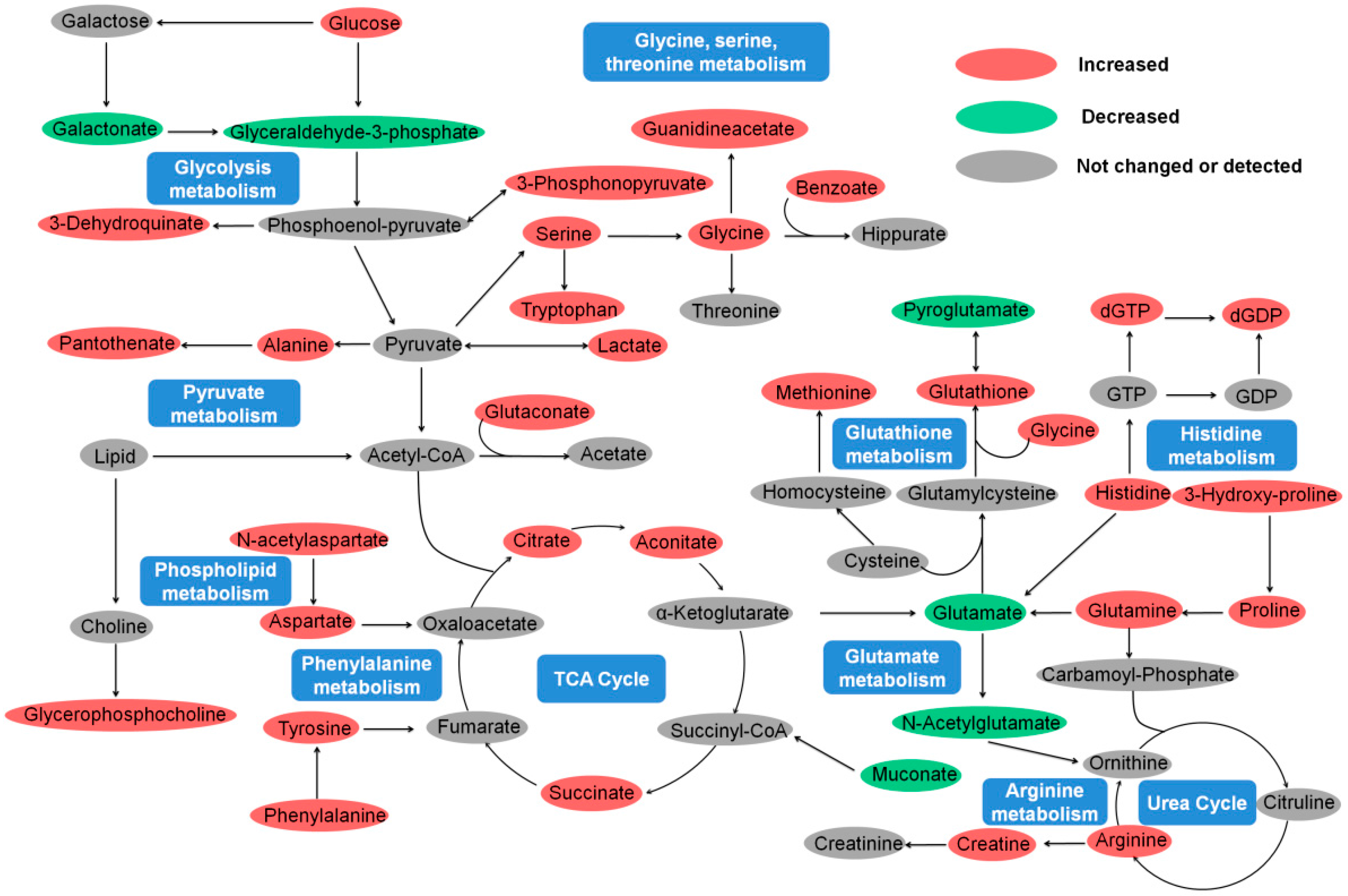
2.5. Amino Acid Metabolism
2.6. Energy Metabolism
2.7. Lipid Metabolism and Antioxidant Defense
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Cell Culture Procedures and Metabolite Extraction
3.3. HPLC-QTOF Analysis
3.4. Data Treatment
3.5. Biological Pathway Analysis
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Infante-Rivard, C.; Labuda, D.; Krajinovic, M.; Sinnett, D. Risk of childhood leukemia associated with exposure to pesticides and with gene polymorphisms. Epidemiology 1999, 10, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Alavanja, M.C.; Hoppin, J.A.; Kamel, F. Health effects of chronic pesticide exposure: Cancer and neurotoxicity. Annu. Rev. Public Health 2004, 25, 155–197. [Google Scholar] [CrossRef] [PubMed]
- Blair, A.; Freeman, L.B. Epidemiologic studies in agricultural populations: Observations and future directions. J. Agromed. 2009, 14, 125–131. [Google Scholar] [CrossRef] [PubMed]
- EPA (U.S. Environmental Protection Agency). Chemicals Evaluated for Carcinogenic Potential Office of Pesticide Programs; U.S. Environmental Protection Agency: Washington, DC, USA, 2015.
- Fryzek, J.P.; Garabrant, D.H.; Harlow, S.D.; Severson, R.K.; Gillespie, B.W.; Schenk, M.; Schottenfeld, D. A case-control study of self-reported exposures to pesticides and pancreas cancer in southeastern Michigan. Int. J. Cancer 1997, 72, 62–67. [Google Scholar] [CrossRef]
- Leet, T.; Acquavella, J.; Lynch, C.; Anne, M.; Weiss, N.S.; Vaughan, T.; Checkoway, H. Cancer incidence among alachlor manufacturing workers. Am. J. Ind. Med. 1996, 30, 300–306. [Google Scholar] [CrossRef]
- Undeger, U.; Basaran, N. Effects of pesticides on human peripheral lymphocytes in vitro: Induction of DNA damage. Arch. Toxicol. 2005, 79, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Hooghe, R.J.; Devos, S.; Hooghe-Peters, E.L. Effects of selected herbicides on cytokine production in vitro. Life Sci. 2000, 66, 2519–2525. [Google Scholar] [CrossRef]
- Corsini, E.; Codeca, I.; Mangiaratti, S.; Birindelli, S.; Minoia, C.; Turci, R.; Viviani, B.; Facchi, A.; Vitelli, N.; Lucchi, L.; et al. Immunomodulatory effects of the herbicide propanil on cytokine production in humans: In vivo and in vitro exposure. Toxicol. Appl. Pharmacol. 2007, 222, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Stiller-Winkler, R.; Hadnagy, W.; Leng, G.; Straube, E.; Idel, H. Immunological parameters in humans exposed to pesticides in the agricultural environment. Toxicol. Lett. 1999, 107, 219–224. [Google Scholar] [CrossRef]
- Buser, H.-R.; Muller, M.D.; Poiger, T.; Balmer, M.E. Environmental behavior of the chiral acetamide pesticide metalaxyl:enantioselective degradation and chiral stability in soil. Environ. Sci. Technol. 2001, 36, 221–226. [Google Scholar] [CrossRef]
- Buerge, I.J.; Poiger, T.; Muller, M.D.; Buser, H.R. Enantioselective degradation of metalaxyl in soils: Chiral preference changes with soil pH. Environ. Sci. Technol. 2003, 37, 2668–2674. [Google Scholar] [CrossRef] [PubMed]
- Lu, H. Stereoselectivity in drug metabolism. Expert Opin. Drug Metab. Toxicol. 2007, 3, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Hrelia, P.; Maffei, F.; Fimognari, C.; Vigagni, F.; Cantelli-Forti, G. Cytogenetic effects of Metalaxyl on human and animal chromosomes. Mutat. Res. 1996, 369, 81–86. [Google Scholar] [CrossRef]
- Paolini, M.; Mesirca, R.; Pozzetti, L.; Sapone, A.; Cantelli-Forti, G. Biomarkers of effect in evaluating metalaxyl cocarcinogenesis. Selective induction of murine CYP 3A isoform. Mutat. Res. 1996, 361, 157–164. [Google Scholar] [CrossRef]
- Sakr, S.A.; Badawy, G.M. Effect of ginger (Zingiber officinale R.) on metiram-inhibited spermatogenesis and induced apoptosis in albino mice. J. Appl. Pharm. Sci. 2011, 1, 131–136. [Google Scholar]
- DeSantis, C.; Ma, J.; Bryan, L.; Jemal, A. Breast cancer statistics, 2013. CA Cancer J. Clin. 2014, 64, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Frumkin, H.; Henley, S.J.; Savitz, D.A.; Thun, M.J. Organochlorines and breast cancer risk. CA Cancer J. Clin. 2002, 52, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Ewence, A.; Brescia, S.; Johnson, I.; Rumsby, P.C. An approach to the identification and regulation of endocrine disrupting pesticides. Food Chem. Toxicol. 2015, 78, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Marx-Stoelting, P.; Niemann, L.; Ritz, V.; Ulbrich, B.; Gall, A.; Hirsch-Ernst, K.I.; Pfeil, R.; Solecki, R. Assessment of three approaches for regulatory decision making on pesticides with endocrine disrupting properties. Regul. Toxicol. Pharmacol. 2014, 70, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Perocco, P.; Colacci, A.; Bonora, B.; Grilli, S. In vitro transforming effect of the fungicides metalaxyl and zineb. Teratog. Carcinog. Mutagen. 1995, 15, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Takeuchi, S.; Nagai, T. Endocrine-disrupting potential of pesticides via nuclear receptors and aryl hydrocarbon receptor. J. Health Sci. 2010, 56, 374–386. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Y.; Chen, S.; Nie, D. Regulation of drug resistance by human pregnane X receptor in breast cancer. Cancer Biol. Ther. 2009, 8, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Hao, F.; Huang, C.; Rantalainen, M.; Lei, H.; Tang, H.; Wang, Y. Systems responses of rats to mequindox revealed by metabolic and transcriptomic profiling. J. Proteome Res. 2012, 11, 4712–4721. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Lindon, J.C. Systems biology: Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Lefort, N.; Brown, A.; Lloyd, V.; Ouellette, R.; Touaibia, M.; Culf, A.S.; Cuperlovic-Culf, M. 1H NMR metabolomics analysis of the effect of dichloroacetate and allopurinol on breast cancers. J. Pharm. Biomed. Anal. 2014, 93, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Oakman, C.; Tenori, L.; Biganzoli, L.; Santarpia, L.; Cappadona, S.; Luchinat, C.; di Leo, A. Uncovering the metabolomic fingerprint of breast cancer. Int. J. Biochem. Cell Biol. 2011, 43, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- McCombie, G.; Browning, L.M.; Titman, C.M.; Song, M.; Shockcor, J.; Jebb, S.A.; Griffin, J.L. ω-3 oil intake during weight loss in obese women results in remodelling of plasma triglyceride and fatty acids. Metabolomics 2009, 5, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Marc Rhoads, J.; Wu, G. Glutamine, arginine, and leucine signaling in the intestine. Amino Acids 2009, 37, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Jobgen, W.S.; Fried, S.K.; Fu, W.J.; Meininger, C.J.; Wu, G. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J. Nutr. Biochem. 2006, 17, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Mannick, J.B. Regulation of apoptosis by protein S-nitrosylation. Amino Acids 2007, 32, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Nilsson, R.; Sharma, S.; Madhusudhan, N.; Kitami, T.; Souza, A.L.; Kafri, R.; Kirschner, M.W.; Clish, C.B.; Mootha, V.K. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science 2012, 336, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Meijer, A.J. Amino acids as regulators and components of nonproteinogenic pathways. J. Nutr. 2003, 133, 2057S–2062S. [Google Scholar] [PubMed]
- Xu, J.; Jiang, H.; Li, J.; Cheng, K.K.; Dong, J.; Chen, Z. 1H NMR-based metabolomics investigation of copper-laden rat: A model of Wilson’s disease. PLoS ONE 2015, 10, e0119654. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.A.; Mohamed, B.M.; El-Wakil, M.A.; Ibrahem, S.O. Impact of breast cancer and combination chemotherapy on oxidative stress, hepatic and cardiac markers. J. Breast Cancer 2012, 15, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, M.; da Costa, K.A.; Fischer, L.M.; Zeisel, S.H. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc. Natl. Acad. Sci. USA 2005, 102, 16025–16030. [Google Scholar] [CrossRef] [PubMed]
- Al Rajabi, A.; Castro, G.S.; da Silva, R.P.; Nelson, R.C.; Thiesen, A.; Vannucchi, H.; Vine, D.F.; Proctor, S.D.; Field, C.J.; Curtis, J.M.; et al. Choline supplementation protects against liver damage by normalizing cholesterol metabolism in Pemt/Ldlr knockout mice fed a high-fat diet. J. Nutr. 2014, 144, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Trousil, S.; Lee, P.; Pinato, D.J.; Ellis, J.K.; Dina, R.; Aboagye, E.O.; Keun, H.C.; Sharma, R. Alterations of choline phospholipid metabolism in endometrial cancer are caused by choline kinase α overexpression and a hyperactivated deacylation pathway. Cancer Res. 2014, 74, 6867–6877. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wu, Y.; Tang, Q.; Leng, Y.; Cai, W. The effects of choline on hepatic lipid metabolism, mitochondrial function and antioxidative status in human hepatic C3A cells exposed to excessive energy substrates. Nutrients 2014, 6, 2552–2571. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Agellon, L.B.; Vance, D.E. Phosphatidylcholine homeostasis and liver failure. J. Biol. Chem. 2005, 280, 37798–37802. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Nutritional genomics: Defining the dietary requirement and effects of choline. J. Nutr. 2011, 141, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Xu, Y.; Lei, H.; Zhao, Y.; Li, H.; Lin, X.; Chen, G.; Tang, H. Metabonomic analysis reveals efficient ameliorating effects of acupoint stimulations on the menopause-caused alterations in mammalian metabolism. Sci. Rep. 2014, 4, 3641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hatzakis, E.; Nichols, R.G.; Hao, R.; Correll, J.; Smith, P.B.; Chiaro, C.R.; Perdew, G.H.; Patterson, A.D. Metabolomics reveals that aryl hydrocarbon receptor activation by environmental chemicals induces systemic metabolic dysfunction in mice. Environ. Sci. Technol. 2015, 49, 8067–8077. [Google Scholar] [CrossRef] [PubMed]
- Bollard, M.E.; Contel, N.R.; Ebbels, T.M.; Smith, L.; Beckonert, O.; Cantor, G.H.; Lehman-McKeeman, L.; Holmes, E.C.; Lindon, J.C.; Nicholson, J.K.; et al. NMR-based metabolic profiling identifies biomarkers of liver regeneration following partial hepatectomy in the rat. J. Proteome Res. 2010, 9, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ye, Y.; An, Y.; Tian, Y.; Wang, Y.; Tang, H. Systems responses of rats to aflatoxin B1 exposure revealed with metabonomic changes in multiple biological matrices. J. Proteome Res. 2011, 10, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Shanware, N.P.; Mullen, A.R.; DeBerardinis, R.J.; Abraham, R.T. Glutamine: Pleiotropic roles in tumor growth and stress resistance. J. Mol. Med. 2011, 89, 229–236. [Google Scholar] [CrossRef] [PubMed]

| No. | Mode | Compounds | Mass | S-Metalaxyl L vs. C | S-Metalaxyl H vs. C | R-Metalaxyl L vs. C | R-Metalaxyl H vs. C | Class | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VIP a | FC b | Trend c | VIP | FC | Trend | VIP | FC | Trend | VIP | FC | Trend | |||||
| 1 | Negative | Lactate | 72.0213 | 1.14 | 3.55 | ↑ | <1 | 2.80 | ↑ | <1 | 2.28 | ↑ | <1 | 1.66 | ↑ | Hydroxy acids and derivatives |
| 2 | Negative | Propionate | 74.0368 | 1.17 | 1.92 | ↑ | <1 | 2.22 | ↑ | 1.13 | 1.42 | ↑ | 1.09 | 1.38 | ↑ | Carboxylic acids and derivatives |
| 3 | Negative | Glycine | 75.0322 | 1.18 | 3.64 | ↑ | 1.16 | 3.55 | ↑ | 1.17 | 3.44 | ↑ | 1.19 | 3.91 | ↑ | Carboxylic acids and derivatives |
| 4 | Negative | Alanine | 89.0474 | 1.17 | 2.19 | ↑ | 1.16 | 2.53 | ↑ | 1.17 | 2.30 | ↑ | 1.19 | 2.72 | ↑ | Carboxylic acids and derivatives |
| 5 | Negative | Serine | 105.0420 | 1.15 | 1.62 | ↑ | 1.13 | 1.62 | ↑ | 1.11 | 1.49 | ↑ | 1.17 | 1.73 | ↑ | Carboxylic acids and derivatives |
| 6 | Negative | Proline | 115.0624 | 1.23 | 1.21 | ↑ | 1.57 | 1.26 | ↑ | <1 | 1.29 | ↑ | 1.06 | 1.20 | ↑ | Carboxylic acids and derivatives |
| 7 | Negative | Guanidineacetate | 117.0419 | 1.18 | 2.55 | ↑ | 1.16 | 2.45 | ↑ | 1.18 | 2.22 | ↑ | 1.19 | 2.75 | ↑ | Carboxylic acids and derivatives |
| 8 | Negative | Succinate | 118.0259 | 1.18 | 1.85 | ↑ | 1.15 | 1.60 | ↑ | 1.12 | 1.34 | ↑ | 1.08 | 1.24 | ↑ | Carboxylic acids and derivatives |
| 9 | Negative | Glutaconate | 130.0256 | 1.16 | 2.11 | ↑ | 1.12 | 2.12 | ↑ | 1.15 | 1.96 | ↑ | 1.12 | 2.17 | ↑ | Carboxylic acids and derivatives |
| 10 | Negative | 3-Methyl-2-oxovalerate | 130.0623 | <1 | 3.10 | ↑ | 1.03 | 4.01 | ↑ | <1 | 3.93 | ↑ | <1 | 3.53 | ↑ | Keto acids and derivatives |
| 11 | Negative | Creatine | 131.0582 | 1.17 | 2.76 | ↑ | 1.16 | 3.03 | ↑ | 1.17 | 3.45 | ↑ | 1.19 | 3.05 | ↑ | Carboxylic acids and derivatives |
| 12 | Negative | Aspartate | 133.0371 | 1.21 | 2.14 | ↑ | 1.23 | 2.24 | ↑ | <1 | 2.25 | ↑ | <1 | 2.47 | ↑ | Carboxylic acids and derivatives |
| 13 | Negative | Muconate | 142.0237 | 3.50 | 0.82 | ↓ | 4.12 | 0.75 | ↓ | 4.9 | 0.70 | ↓ | 4.1 | 0.76 | ↓ | Fatty acids and conjugates |
| 14 | Negative | Glutamine | 146.0685 | 1.66 | 4.84 | ↑ | 1.62 | 4.87 | ↑ | <1 | 5.10 | ↑ | <1 | 5.34 | ↑ | Carboxylic acids and derivatives |
| 15 | Negative | Glutamate | 147.0524 | 1.14 | 0.67 | ↓ | 1.14 | 0.65 | ↓ | 1.15 | 0.59 | ↓ | 1.16 | 0.66 | ↓ | Carboxylic acids and derivatives |
| 16 | Negative | Methionine | 149.0503 | 1.12 | 2.52 | ↑ | 1.01 | 2.32 | ↑ | <1 | 1.56 | ↑ | 1.12 | 2.54 | ↑ | Carboxylic acids and derivatives |
| 17 | Negative | 4-Imidazolone-5-propionate | 156.0523 | 1.15 | 3.19 | ↑ | 1.16 | 4.79 | ↑ | 1.14 | 4.65 | ↑ | 1.14 | 2.84 | ↑ | Imidazoles |
| 18 | Negative | 2-Aminomuconate | 157.0353 | 1.12 | 0.58 | ↓ | 1.13 | 0.42 | ↓ | 1.14 | 0.46 | ↓ | 1.14 | 0.44 | ↓ | Carboxylic acids and derivatives |
| 19 | Negative | Benzoate | 157.9976 | 4.06 | 1.81 | ↑ | 3.71 | 1.72 | ↑ | 3.65 | 1.80 | ↑ | 3.39 | 1.71 | ↑ | Benzene and substituted derivatives |
| 20 | Negative | 3-Phosphonopyruvate | 167.9816 | 1.10 | 1.88 | ↑ | <1 | 1.33 | ↑ | 1.06 | 1.42 | ↑ | 1.05 | 1.87 | ↑ | Organic phosphoric acids and derivatives |
| 21 | Negative | Glyceraldehyde-3-phosphate | 169.9970 | 1.14 | 0.05 | ↓ | 1.13 | 0.08 | ↓ | 1.15 | 0.09 | ↓ | 1.13 | 0.14 | ↓ | Carbohydrates and carbohydrate conjugates |
| 22 | Negative | Aconitate | 174.0157 | 1.94 | 1.93 | ↑ | 1.25 | 1.77 | ↑ | <1 | 1.91 | ↑ | <1 | 1.86 | ↑ | Carboxylic acids and derivatives |
| 23 | Negative | N-acetylaspartate | 175.0478 | 2.36 | 3.78 | ↑ | 2.35 | 3.93 | ↑ | 1.28 | 4.62 | ↑ | 1.39 | 3.94 | ↑ | Carboxylic acids and derivatives |
| 24 | Negative | Glucose | 180.0629 | 1.98 | 2.15 | ↑ | 1.71 | 1.92 | ↑ | 1.11 | 2.45 | ↑ | <1 | 2.01 | ↑ | Carbohydrates and carbohydrate conjugates |
| 25 | Negative | Tyrosine | 181.0728 | 1.18 | 2.68 | ↑ | 1.16 | 2.97 | ↑ | 1.17 | 2.80 | ↑ | 1.18 | 2.86 | ↑ | Phenylpropanoic acids |
| 26 | Negative | N-Acetyl-l-glutamate | 189.0632 | 1.76 | 0.11 | ↓ | 1.71 | 0.13 | ↓ | <1 | 0.09 | ↓ | <1 | 0.16 | ↓ | Carboxylic acids and derivatives |
| 27 | Negative | 3-Dehydroquinate | 190.0404 | <1 | 2.64 | ↑ | 1.22 | 4.38 | ↑ | <1 | 4.33 | ↑ | <1 | 2.37 | ↑ | Alcohols and polyols |
| 28 | Negative | Citrate | 192.0268 | 3.26 | 7.09 | ↑ | 3.56 | 8.97 | ↑ | 2.31 | 6.23 | ↑ | 2.98 | 8.37 | ↑ | Carboxylic acids and derivatives |
| 29 | Negative | Galactonate | 196.0571 | 1.14 | 0.24 | ↓ | 1.12 | 0.14 | ↓ | 1.13 | 0.24 | ↓ | 1.08 | 0.25 | ↓ | Hydroxy acids and derivatives |
| 30 | Negative | Pantothenate | 219.1099 | 2.04 | 11.52 | ↑ | 1.97 | 11.39 | ↑ | 1.07 | 13.02 | ↑ | <1 | 10.04 | ↑ | Carboxylic acids and derivatives |
| 31 | Negative | N-Acetyl-d-glucosamine | 257.0662 | 1.07 | 2.29 | ↑ | 1.03 | 2.27 | ↑ | <1 | 2.01 | ↑ | <1 | 2.41 | ↑ | Carbohydrates and carbohydrate conjugates |
| 32 | Negative | Acetylcarnosine | 306.0308 | <1 | 0.95 | ↓ | 1.66 | 0.82 | ↓ | <1 | 0.77 | ↓ | <1 | 0.91 | ↓ | Carboxylic acids and derivatives |
| 33 | Negative | dUMP | 344.0442 | 1.24 | 0.73 | ↓ | 1.1 | 0.76 | ↓ | <1 | 0.76 | ↓ | <1 | 0.72 | ↓ | Pyrimidine nucleotides |
| 34 | Negative | dGDP | 427.0289 | 1.12 | 3.95 | ↑ | 1.11 | 4.07 | ↑ | <1 | 4.16 | ↑ | <1 | 3.91 | ↑ | Purine nucleotides |
| 35 | Negative | dGTP | 506.9954 | 1.95 | 2.51 | ↑ | 1.95 | 2.60 | ↑ | <1 | 2.37 | ↑ | <1 | 2.56 | ↑ | Purine nucleotides |
| 36 | Positive | Valine | 117.0779 | 1.27 | 1.57 | ↑ | 1.15 | 1.43 | ↑ | 1.23 | 1.41 | ↑ | 1.06 | 1.50 | ↑ | Carboxylic acids and derivatives |
| 37 | Positive | Pyroglutamate | 129.0416 | 1.08 | 0.64 | ↓ | <1 | 0.81 | ↓ | <1 | 0.87 | ↓ | 1.11 | 0.56 | ↓ | Carboxylic acids and derivatives |
| 38 | Positive | 3-Hydroxy-l-proline | 131.0677 | 1.28 | 7.63 | ↑ | 1.41 | 8.39 | ↑ | 1.41 | 8.44 | ↑ | 1.26 | 7.58 | ↑ | Carboxylic acids and derivatives |
| 39 | Positive | Leucine/Isoleucine | 131.0932 | 1.82 | 2.98 | ↑ | 1.72 | 2.78 | ↑ | 1.33 | 2.48 | ↑ | 1.81 | 2.87 | ↑ | Carboxylic acids and derivatives |
| 40 | Positive | Glutarate | 132.0521 | 1.07 | 3.59 | ↑ | 1.07 | 3.61 | ↑ | 1.04 | 3.60 | ↑ | 1.11 | 3.68 | ↑ | Carboxylic acids and derivatives |
| 41 | Positive | Histidine | 155.0674 | <1 | 3.47 | ↑ | 1.04 | 4.96 | ↑ | <1 | 3.07 | ↑ | 1.06 | 4.94 | ↑ | Carboxylic acids and derivatives |
| 42 | Positive | Phenylalanine | 165.0773 | 1.43 | 2.05 | ↑ | 1.23 | 1.77 | ↑ | 1.07 | 1.61 | ↑ | 1.56 | 2.18 | ↑ | Phenylpropanoic acids |
| 43 | Positive | Arginine | 174.1099 | 2.13 | 6.52 | ↑ | 1.86 | 5.83 | ↑ | 2.3 | 6.99 | ↑ | 1.75 | 5.55 | ↑ | Carboxylic acids and derivatives |
| 44 | Positive | 3-Indolebutyrate | 203.1137 | 1.75 | 5.98 | ↑ | 1.79 | 6.25 | ↑ | 1.83 | 6.68 | ↑ | 1.7 | 5.51 | ↑ | Indoles and derivatives |
| 45 | Positive | Tryptophan | 204.0874 | 1.07 | 2.41 | ↑ | <1 | 2.23 | ↑ | <1 | 1.91 | ↑ | 1.2 | 2.68 | ↑ | Indoles and derivatives |
| 46 | Positive | Propionyl-l-carnitine | 217.1288 | <1 | 1.50 | ↑ | <1 | 1.44 | ↑ | 1.1 | 1.72 | ↑ | 1.02 | 1.57 | ↑ | Fatty acid esters |
| 47 | Positive | Glycerophosphocholine | 257.1003 | 1.98 | 10.24 | ↑ | 1.98 | 10.24 | ↑ | 2.29 | 11.71 | ↑ | 2.08 | 10.77 | ↑ | Glycerophospholipids |
| 48 | Positive | Glutathione | 307.0812 | 3.54 | 1.98 | ↑ | 3.49 | 1.97 | ↑ | 3.63 | 2.01 | ↑ | 3.36 | 1.94 | ↑ | Carboxylic acids and derivatives |
| 49 | Positive | UDP-N-acetylglucosamine | 607.0766 | 1.25 | 4.12 | ↑ | 1.23 | 4.07 | ↑ | 1.21 | 4.03 | ↑ | 1.21 | 4.04 | ↑ | Pyrimidine nucleotides |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Zhu, W.; Wang, D.; Yan, J.; Wang, Y.; He, L. Enantioselective Effects of Metalaxyl Enantiomers on Breast Cancer Cells Metabolic Profiling Using HPLC-QTOF-Based Metabolomics. Int. J. Mol. Sci. 2017, 18, 142. https://doi.org/10.3390/ijms18010142
Zhang P, Zhu W, Wang D, Yan J, Wang Y, He L. Enantioselective Effects of Metalaxyl Enantiomers on Breast Cancer Cells Metabolic Profiling Using HPLC-QTOF-Based Metabolomics. International Journal of Molecular Sciences. 2017; 18(1):142. https://doi.org/10.3390/ijms18010142
Chicago/Turabian StyleZhang, Ping, Wentao Zhu, Dezhen Wang, Jin Yan, Yao Wang, and Lin He. 2017. "Enantioselective Effects of Metalaxyl Enantiomers on Breast Cancer Cells Metabolic Profiling Using HPLC-QTOF-Based Metabolomics" International Journal of Molecular Sciences 18, no. 1: 142. https://doi.org/10.3390/ijms18010142
APA StyleZhang, P., Zhu, W., Wang, D., Yan, J., Wang, Y., & He, L. (2017). Enantioselective Effects of Metalaxyl Enantiomers on Breast Cancer Cells Metabolic Profiling Using HPLC-QTOF-Based Metabolomics. International Journal of Molecular Sciences, 18(1), 142. https://doi.org/10.3390/ijms18010142