Susceptibility and Immune Defence Mechanisms of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) against Entomopathogenic Fungal Infections
Abstract
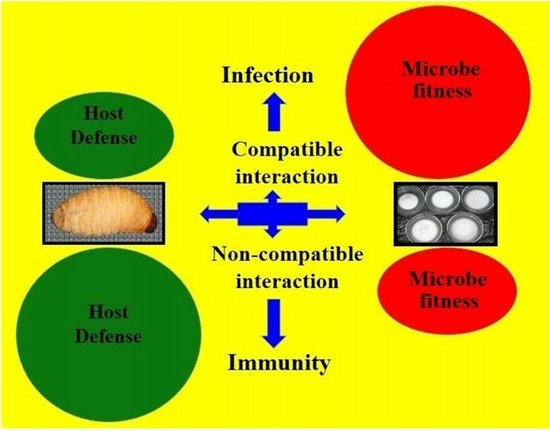
:1. Introduction
2. Results
2.1. Conidial Virulence-Related Traits Evaluation of the Entomopathogenic Fungal Isolates
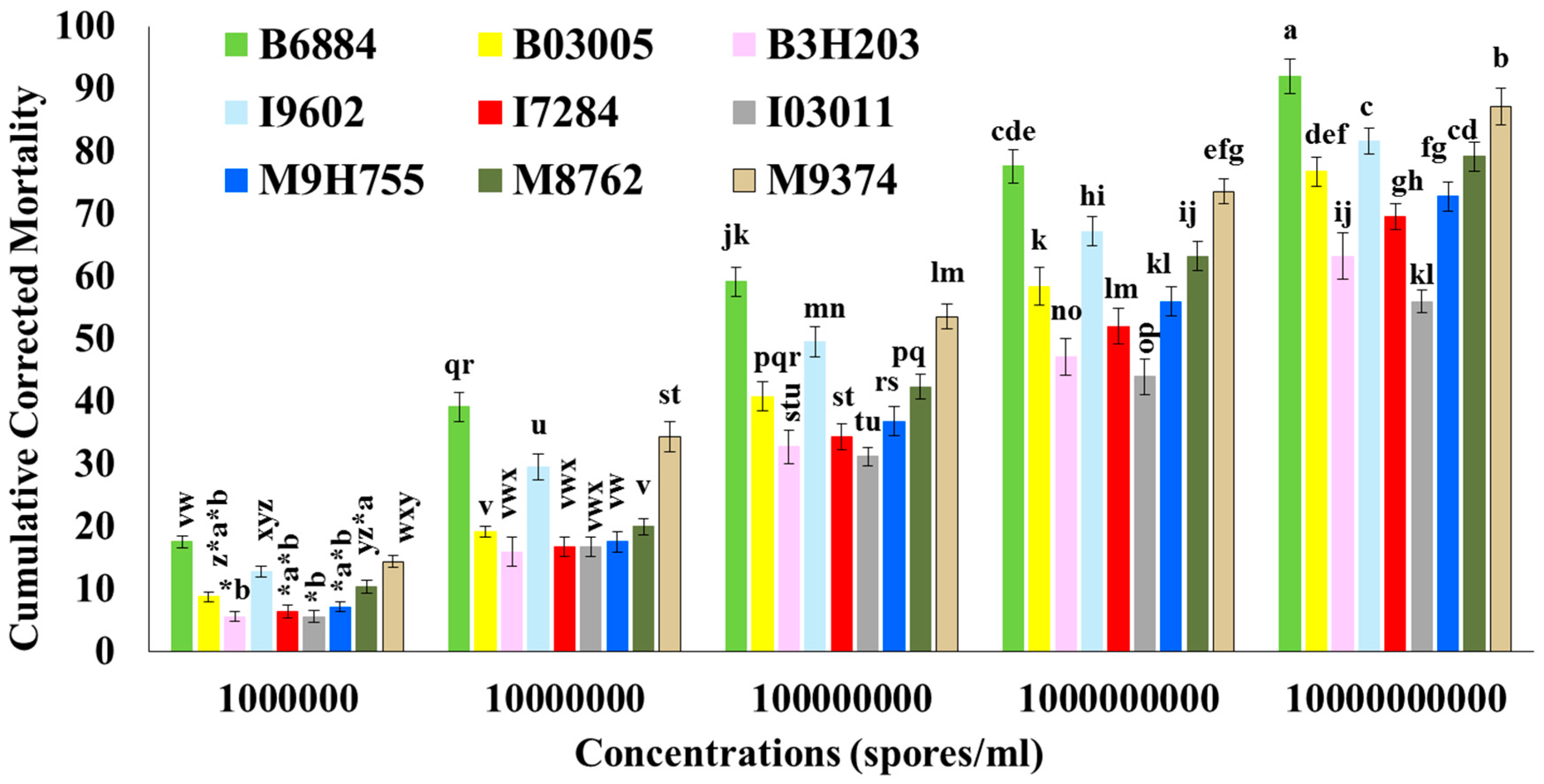
2.2. Mortality of R. ferrugineus
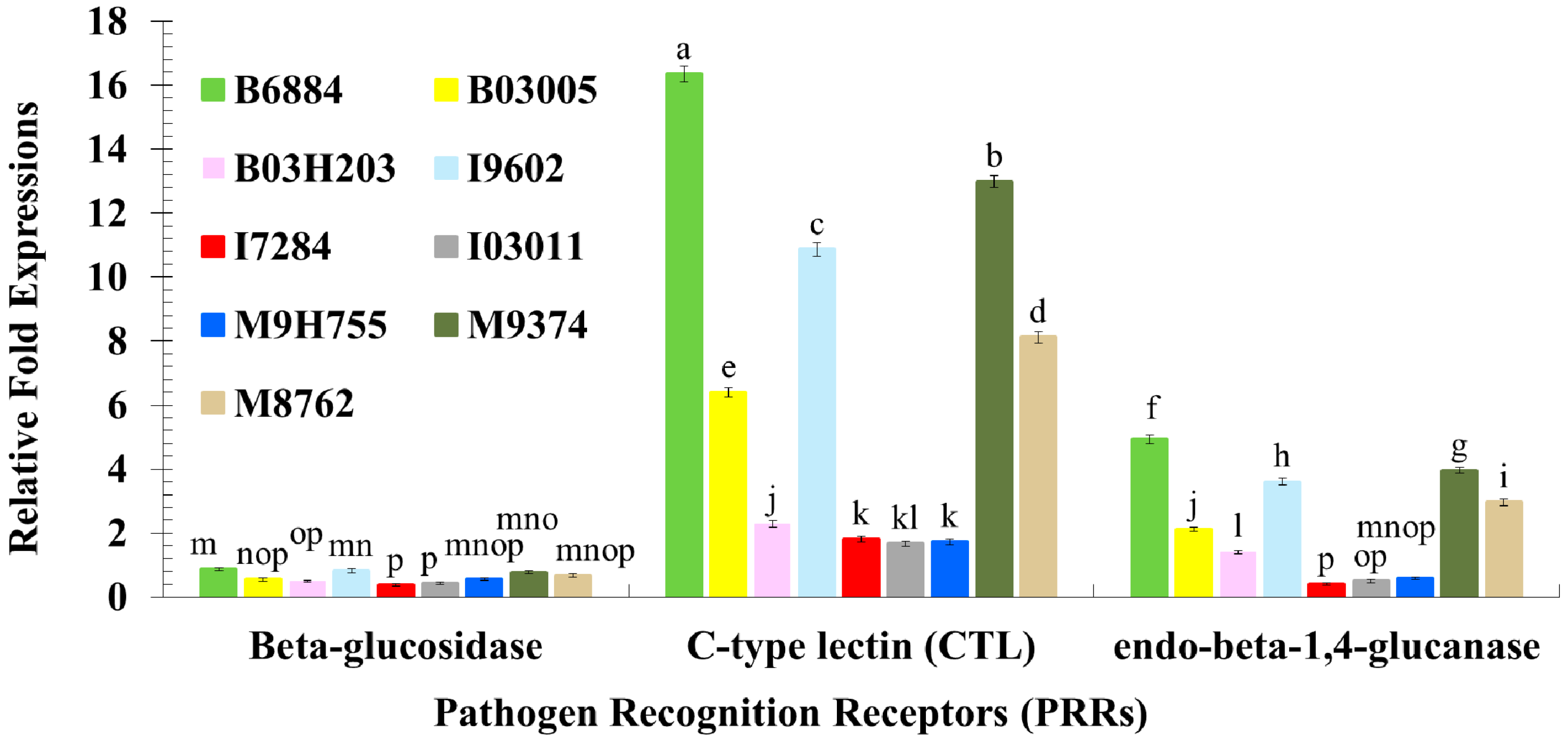
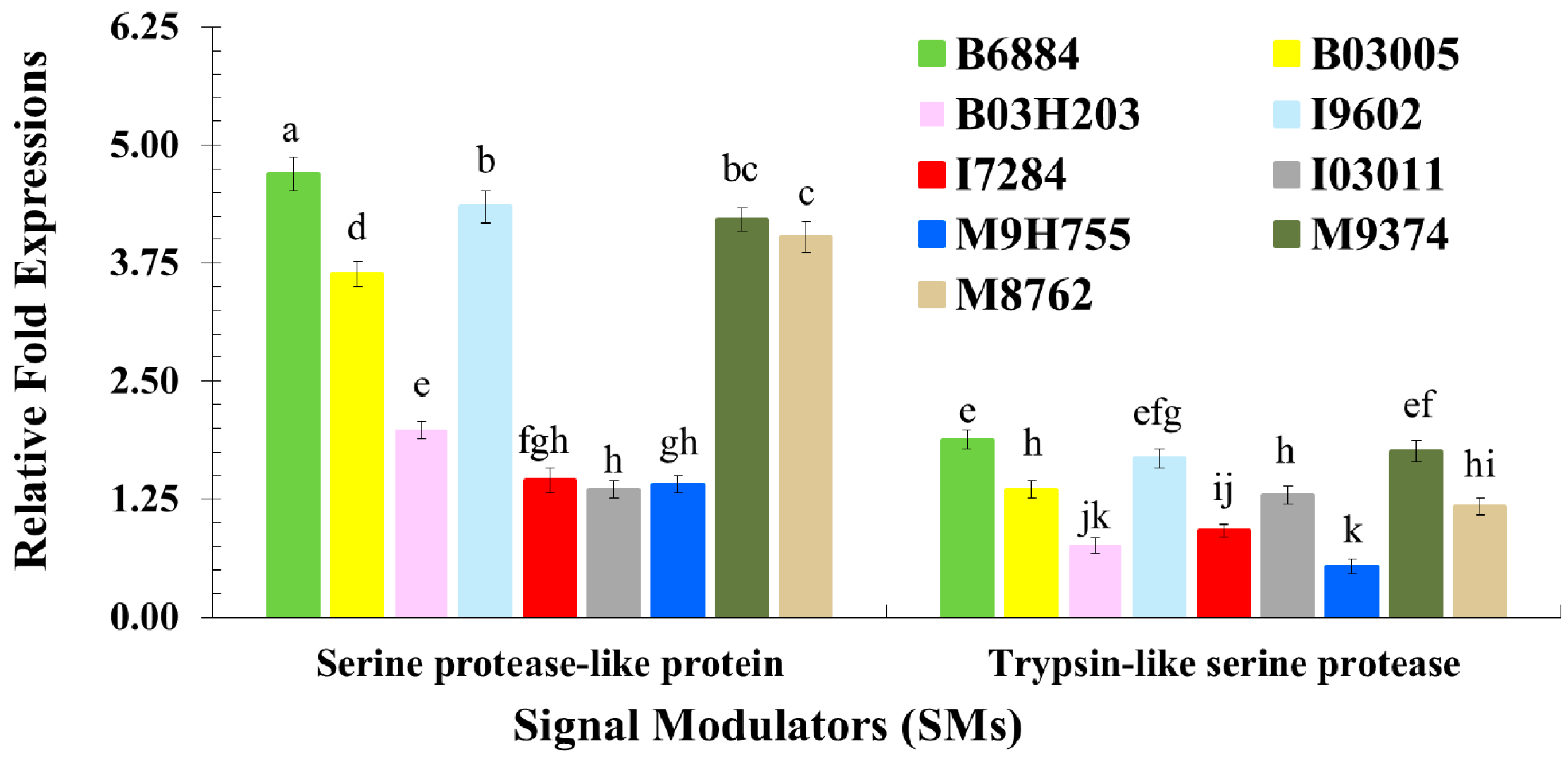
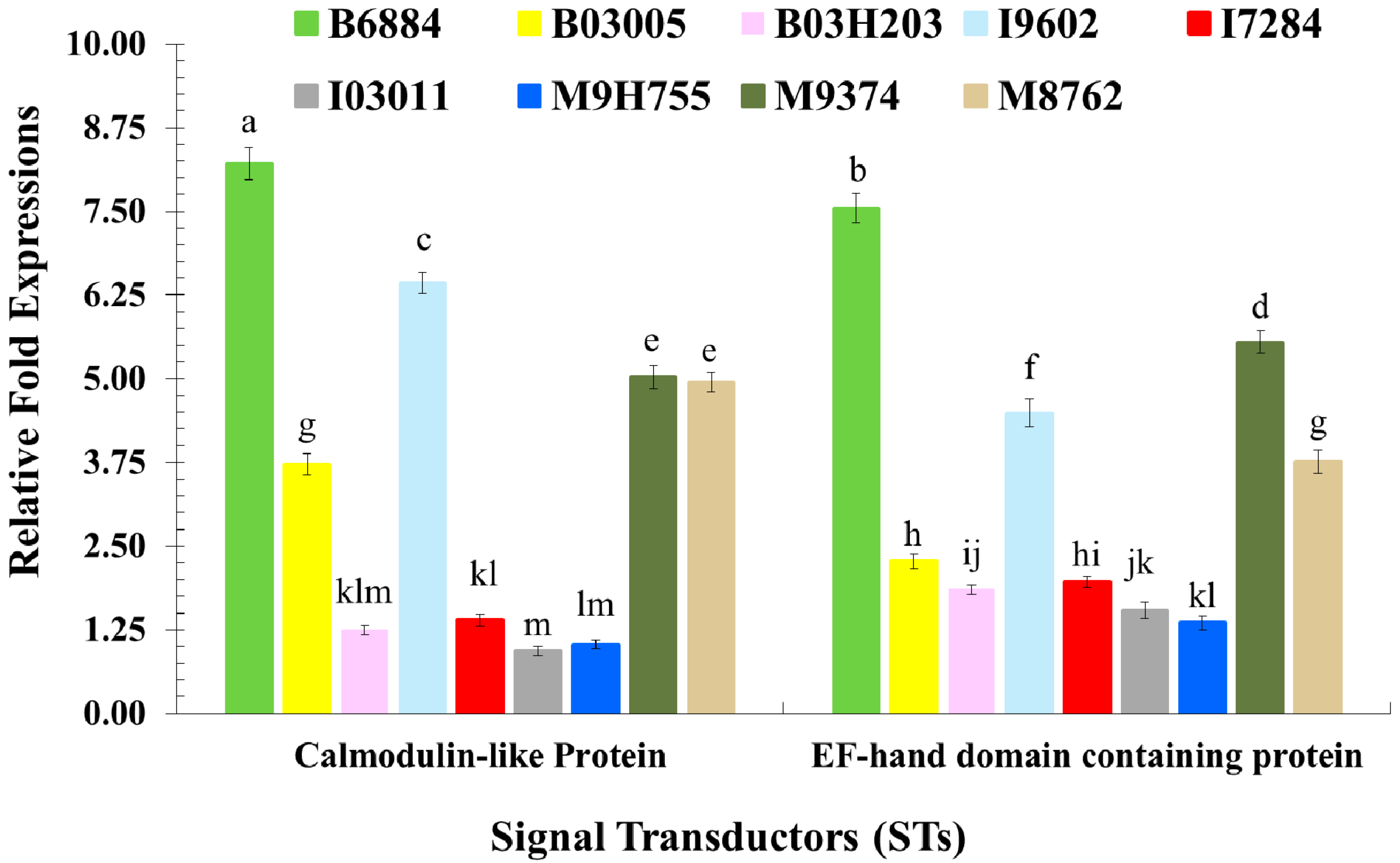
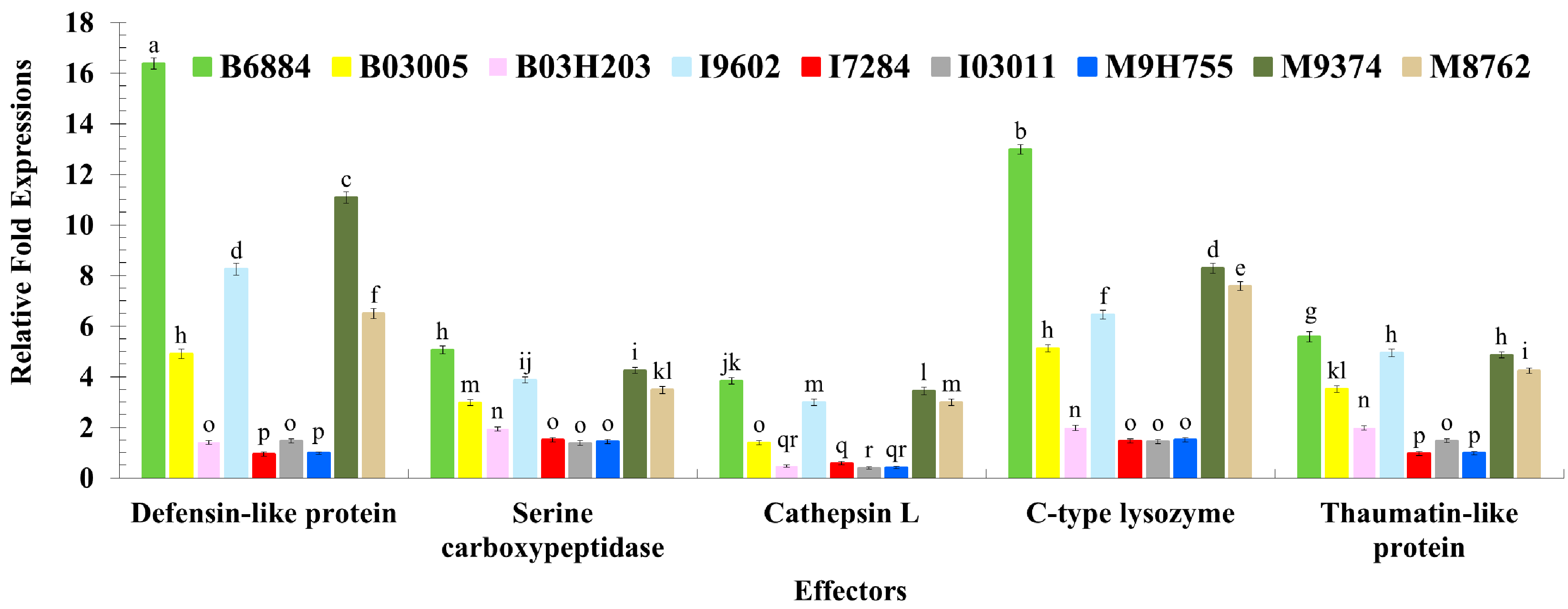
2.3. Quantification of Host Immune Defence-Related Genes Using Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.4. Impact of Entomopathogenic Fungi on the Growth of R. ferrugineus
3. Discussion
4. Materials and Methods
4.1. Entomopathogenic Fungi
4.2. Rearing of Red Palm Weevils
4.3. Virulence Evaluation of the Isolates of Entomopathogenic Fungi
4.3.1. Conidial Germination
4.3.2. Conidial Hydrophobicity
4.3.3. Mortality
4.4. Evaluation of the Host Immune Defence Mechanism against Entomopathogenic Fungi through the Quantification of Immune-Related Genes Using qRT-PCR
4.5. Impact of Entomopathogenic Fungi on the Growth of R. ferrugineus
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Jabr, A.M.; Al-Ayied, H.Y. Managing invasive populations of red palm weevil: A worldwide perspective. J. Food Agric. Environ. 2013, 11, 456–463. [Google Scholar]
- Rasool, K.; Khan, M.; Aldawood, A.; Tufail, M.; Mukhtar, M.; Takeda, M. Identification of proteins modulated in the date palm stem infested with red palm weevil (Rhynchophorus ferrugineus Oliv.) using two dimensional differential gel spectrometry. Int. J. Mol. Sci. 2015, 16, 19326–19346. [Google Scholar] [CrossRef] [PubMed]
- Francesca, N.; Alfonzo, A.; Verde, G.L.; Settanni, L.; Sinacori, M.; Lucido, P.; Moschetti, G. Biological activity of Bacillus spp. evaluated on eggs and larvae of red palm weevil Rhynchophorus ferrugineus. Ann. Microbiol. 2015, 65, 477–485. [Google Scholar] [CrossRef]
- Güerri-Agulló, B.; López-Follana, R.; Asensio, L.; Barranco, P.; Lopez-Llorca, L.V. Use of a solid formulation of Beauveria bassiana for biocontrol of the red palm weevil (Rhynchophorus ferrugineus) (Coleoptera: Dryophthoridae) under field conditions in SE Spain. Fla. Entomol. 2011, 94, 737–747. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H.; Ahmed, S.; Al-Jabr, A.M. Effect of Beauveria bassiana infection on the feeding performance and antioxidant defence of red palm weevil, Rhynchophorus ferrugineus. BioControl 2015, 60, 849–859. [Google Scholar] [CrossRef]
- Lo Verde, G.; Torta, L.; Mondello, V.; Caldarella, C.G.; Burruano, S.; Caleca, V. Pathogenicity bioassays of isolates of Beauveria bassiana on Rhynchophorus ferrugineus. Pest Manag. Sci. 2015, 71, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Manachini, B.; Schillaci, D.; Arizza, V. Biological responses of Rhynchophorus ferrugineus (Coleoptera: Curculionidae) to Steinernema carpocapsae (Nematoda: Steinernematidae). J. Econ. Entomol. 2013, 106, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.; Toma, R.; Sgrillo, R.; Delabie, J. Natural efficiency of parasitism by Billaea rhynchophorae (Blanchard) (Diptera: Tachinidae) for the control of Rhynchophorus palmarum (L.) (Coleoptera: Curculionidae). Neotrop. Entomol. 2006, 35, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Tian, M.Y.; He, Y.R.; Ahmed, S. Entomopathogenic fungi disturbed the larval growth and feeding performance of Ocinara varians (Lepidoptera: Bombycidae) larvae. Insect Sci. 2009, 16, 511–517. [Google Scholar] [CrossRef]
- Wang, C.; St Leger, R.J. The MAD1 adhesin of Metarhizium anisopliae links adhesion with blastospore production and virulence to insects, and the MAD2 adhesin enables attachment to plants. Eukaryot. Cell 2007, 6, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Hergannan, J.A.; Rechhart, J.V. Drosophila immunity. Trends Cell Biol. 1997, 7, 309–316. [Google Scholar] [CrossRef]
- Gillespie, J.P.; Bateman, R.; Charnley, A.K. Role of cuticle-degrading proteases in the virulence of Metarhizium spp. for the desert locust, Schistocerca gregaria. J. Invertebr. Pathol. 1998, 71, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Tiago, P.V.; Fungaro, M.H.P.; Furlaneto, M.C. Cuticle-degrading proteases from the entomopathogen Metarhizium flavoviride and their distribution in secreted and intracellular fractions. Lett. Appl. Microbiol. 2002, 34, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Li, Y.F.; Cheng, Y.; Liu, Y.; Chen, C.C.; Wen, S.Y. Immune-related transcriptome of Coptotermes formosanus Shiraki workers: The defense mechanism. PLoS ONE 2013, 8, e69543. [Google Scholar] [CrossRef] [PubMed]
- Mastore, M.; Arizza, V.; Manachini, B.; Brivio, M.F. Modulation of immune responses of Rhynchophorus ferrugineus (Insecta: Coleoptera) induced by the entomopathogenic nematode Steinernema carpocapsae (Nematoda: Rhabditida). Insect Sci. 2015, 22, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Manachini, B.; Arizza, V.; Parrinello, D.; Parrinello, N. Hemocytes of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) and their response to Saccharomyces cerevisiae and Bacillus thuringiensis. J. Invertebr. Pathol. 2011, 106, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.H.; Lin, Y.T.; Hou, Y.M. Mother-derived trans-generational immune priming in the red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera, Dryophthoridae). Bull. Entomol. Res. 2014, 104, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Mastore, M.; Rossetti, S.B.; Giovannardi, S.; Scari, G.; Brivio, M.F. Inducible factors with antimicrobial activity after immune challenge in the haemolymph of red palm weevil (Insecta). Innate Immun. 2015, 21, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H.; Al-Jabr, A. Mycoinsecticides: Potential and future perspective. Recent Pat. Food Nutr. Agric. 2014, 6, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Sevim, A.; Donzelli, B.G.G.; Wu, D.; Demirbag, Z.; Gibson, D.M.; Turgeon, B.G. Hydrophobin genes of the entomopathogenic fungus, Metarhizium brunneum, are differentially expressed and corresponding mutants are decreased in virulence. Curr. Genet. 2012, 58, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.M.; Kirkland, B.H.; Holder, D.J.; Keyhani, N.O. Phage display cDNA cloning and expression analysis of hydrophobins from the entomopathogenic fungus Beauveria (Cordyceps) bassiana. Microbiology 2007, 153, 3438–3447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xia, Y.X.; Kim, B.; Keyhani, N.O. Two hydrophobins are involved in fungal spore coat rodlet layer assembly and each play distinct roles in surface interactions, development and pathogenesis in the entomopathogenic fungus, Beauveria bassiana. Mol. Microbiol. 2011, 80, 811–826. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Ruan, L.; Tian, M.; He, Y. Pathogenic effect of Metarhizium anisopliae on the larval growth and development of Ocinara varians Walker (Lepidoptera: Bombycidae). Pak. Entomol. 2009, 31, 116–121. [Google Scholar]
- Martinez, D.S.T.; Freire, M.D.G.M.; Mazzafera, P.; Araujo-Júnior, R.T.; Bueno, R.D.; Macedo, M.L.R. Insecticidal effect of labramin, a lectin-like protein isolated from seeds of the beach apricot tree, Labramia bojeri, on the Mediterranean flour moth, Ephestia kuehniella. J. Insect Sci. 2012, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Tian, M.Y.; He, Y.R.; Ruan, L.; Ahmed, S. In vitro and in vivo culturing impacts on the virulence characteristics of serially passed entomopathogenic fungi. J. Food Agric. Environ. 2010, 8, 481–487. [Google Scholar]
- SAS Institute. SAS User’s Guide: Statistics; SAS Institute: Cary, NC, USA, 2000. [Google Scholar]
- Girardin, H.; Paris, S.; Rault, J.; Bellon-Fontaine, M.N.; Latgé, J.P. The role of the rodlet structure on the physicochemical properties of Aspergillus conidia. Lett. Appl. Microbiol. 1999, 29, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Analytical Software. Statistix Statistix, version 8.1; Analytical Software: Tallahassee, FL, USA, 2003. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.M.; Thaler, J.S.; Scott, J.G. Response of a generalist herbivore Trichoplusia ni to jasmonate-mediated induced defense in tomato. J. Chem. Ecol. 2010, 36, 490–499. [Google Scholar] [CrossRef] [PubMed]
| Fungal Species | Percent Conidial Germination | Relative Conidial Hydrophobicity | LC50 (Spores/mL) |
|---|---|---|---|
| B. bassiana 6884 | 98.40 ± 0.51 a | 94.80 ± 1.88 a | 4.59 × 107 f |
| B. bassiana 03005 | 97.60 ± 0.51 a | 79.80 ± 1.77 c | 4.36 × 108 cde |
| B. bassiana 3H203 | 68.80 ± 1.93 b | 67.80 ± 2.08 ef | 1.85 × 109 b |
| I. fumosorosea 9602 | 98.20 ± 0.37 a | 88.80 ± 2.52 ab | 1.53 × 108 ef |
| I. fumosorosea 7284 | 69.20 ± 2.08 b | 72.20 ± 2.29 de | 9.88 × 108 bc |
| I. fumosorosea 03011 | 63.60 ± 3.09 c | 65.60 ± 2.87 f | 3.29 × 109 a |
| M. anisopliae 9H755 | 96.00 ± 1.14 a | 75.60 ± 2.04 cd | 7.22 × 108 cd |
| M. anisopliae 8762 | 97.20 ± 1.02 a | 86.40 ± 2.11 b | 3.08 × 108 def |
| M. anisopliae 9374 | 97.60 ± 0.93 a | 91.20 ± 2.29 ab | 8.20 × 107 ef |
| Treatments | LT50 (Days) | Corrected Cumulative Percent Mortality at Different Time Intervals | ||
|---|---|---|---|---|
| 4th Day | 8th Day | 12th Day | ||
| B. bassiana 6884 | 3.96 ± 0.20 e | 44.80 ± 2.33 de | 91.20 ± 2.33 a | 100.00 ± 0.00 a |
| B. bassiana 03005 | 8.03 ± 0.31 c | 17.60 ± 1.60 h | 40.00 ± 2.83 e | 74.40 ± 3.25 b |
| B. bassiana 3H203 | 11.37 ± 0.28 a | 6.40 ± 0.98 i | 19.20 ± 1.50 fgh | 51.20 ± 1.96 cd |
| I. fumosorosea 9602 | 6.59 ± 0.28 d | 22.40 ± 2.04 fg | 49.60 ± 2.40 cd | 96.80 ± 1.50 a |
| I. fumosorosea 7284 | 11.21 ± 0.40 a | 7.20 ± 0.80 i | 20.00 ± 1.79 fgh | 52.80 ± 2.33 c |
| I. fumosorosea 03011 | 11.51 ± 0.38 a | 5.60 ± 0.98 i | 19.20 ± 2.33 fgh | 50.40 ± 2.04 cd |
| M. anisopliae 9H755 | 10.14 ± 0.33 b | 6.40 ± 0.98 i | 23.20 ± 1.50 f | 56.00 ± 2.53 c |
| M. anisopliae 8762 | 7.57 ± 0.24 c | 18.40 ± 1.60 gh | 41.60 ± 2.04 e | 76.80 ± 2.33 b |
| M. anisopliae 9374 | 6.43 ± 0.27 d | 23.20 ± 1.50 f | 52.80 ± 3.20 c | 96.80 ± 1.50 a |
| Treatments | Percent Inhibition Compared to Control | Percent Increase Compared to Control | |
|---|---|---|---|
| ECI | ECD | AD | |
| B. bassiana 6884 | 46.41 ± 0.49 a | 62.46 ± 0.45 a | 42.78 ± 0.83 a |
| B. bassiana 03005 | 14.88 ± 2.09 e | 21.85 ± 2.50 e | 9.02 ± 1.01 d |
| B. bassiana 3H203 | 2.67 ± 0.74 g | 4.38 ± 1.01 gh | 1.80 ± 0.41 ef |
| I. fumosorosea 9602 | 29.25 ± 0.99 c | 41.25 ± 0.79 c | 20.43 ± 0.67 c |
| I. fumosorosea 7284 | 4.22 ± 0. 45 g | 6.48 ± 0.88 g | 2.22 ± 0.62 ef |
| I. fumosorosea 03011 | 0.29 ± 0.03 g | 0.48 ± 0.05 h | 0.20 ± 0.02 f |
| M. anisopliae 9H755 | 8.74 ± 0.60 f | 11.83 ± 1.25 f | 3.56 ± 0.94 e |
| M. anisopliae 8762 | 20.00 ± 1.52 d | 33.07 ± 1.22 d | 19.54 ± 0.53 c |
| M. anisopliae 9374 | 36.59 ± 2.99 b | 50.90 ± 2.80 b | 29.49 ± 1.57 b |
| Fungal Species | Isolate | Host | Origin | Date |
|---|---|---|---|---|
| B. bassiana | B6884 | Otiorhynchus ligustici | Hungary | 2001 |
| B. bassiana | B03005 | Coptotermes formosanus | China | 2003 |
| B. bassiana | B03H203 | Rhynchophorus ferrugineus | Saudi Arabia | 2012 |
| Isaria fumosorosea | I9602 | Coleoptera | China | 2010 |
| Isaria fumosorosea | I7284 | Hypothenemus hampei | Mexico | 2004 |
| Isaria fumosorosea | I03011 | Coptotermes formosanus | China | 2003 |
| Metarhizium anisopliae | M9374 | Listronotus maculicollis | USA | 2005 |
| Metarhizium anisopliae | M9H755 | Rhynchophorus ferrugineus | Saudi Arabia | 2012 |
| Metarhizium anisopliae | M8762 | Rhabdoscelus obscurus | Australia | 1996 |
| No. | Ingredients | Quantity |
|---|---|---|
| 1 | Wheat flour | 45 g/L |
| 2 | Corn flour | 45 g/L |
| 3 | Yeast | 45 g/L |
| 4 | Sorbic acid (Sigma Aldrich, London, UK) | 1.6 g/L |
| 5 | l-Ascorbic acid (Sigma Aldrich, London, UK) | 4 g/L |
| 6 | Pharmaton (SITCO Pharma, Riyad, KSA) | 2 Capsules/L |
| 7 | Tetracycline | 500 mg/L |
| 8 | Agar (Sigma Aldrich, London, UK) | 17.5 g/L |
| 9 | Distilled water | 1 L |
| Target Gene | Accession No. | Amplicon Size | Functional Categories | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|---|---|---|
| Beta-glucosidase | KT223628 | 105 bp | Pathogen Recognition Receptor | TATGGCATGGGCCTTGACTG | GGTGTTCTCGGTCTCTCTGG |
| C-type lectin (CTL) | KT223638 | 81 bp | Pathogen Recognition Receptor | TGGTACTCCACGCCATCAAC | ATCAGCTACCCACTTTCCGC |
| endo-beta-1,4-glucanase | KT223630 | 110 bp | Pathogen Recognition Receptor | AGTGACACCTTGGCTTACGG | TTGCCGTTGAGAGCGTTTTG |
| Serine protease-like protein | KT223631 | 76 bp | Signal Modulation | TTTGTCTGACCGCACCAAGT | TACCGAGCACCATCCACAAC |
| Trypsin-like serine protease | KT223633 | 113 bp | Signal Modulation | ACAGCTCGGACCAACATGAG | GAAAGAGCTGGGAAGGGTCC |
| Calmodulin-like Protein | KT223632 | 113 bp | Signal Transduction | GTATCACCACCACCGAGCAA | AACCATGAACTTAGCGGCGA |
| EF-hand domain containing protein | KT223636 | 88 bp | Signal Transduction | CCAACTGATGGACCACGACA | CTCGTTGGCGATCTTACCGA |
| Defensin-like protein | KT223639 | 86 bp | Effector | AGGCTGCAGCTATCAAGGAA | AGTGGTGCCTCCATTGTGAC |
| Serine carboxypeptidase | KT223634 | 108 bp | Effector | CCGAGGAGTACAAAACGGCT | CAGCGTTCCGAACCAGTAGT |
| Cathepsin L. | KT223635 | 82 bp | Effector | GCCCCTACTCCTTGAACCAC | CCACCCCAGGAGTTCTTGAC |
| C-type lysozyme | KT223629 | 117 bp | Effector | TAGCACACCAGGCAAAGGTT | TTCGTTGATCCCTTGGCAGT |
| Thaumatin-like protein | KT223637 | 70 bp | Effector | TCGGAGATGTGGTAGCTTGC | TCCACTACAGCCAGAGGACA |
| beta-Actin | KM438516 | 129 bp | House-keeping gene | AAAGGTTCCGTTGCCCTGAA | TGGCGTACAAGTCCTTCCTG |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H.; AlJabr, A.M. Susceptibility and Immune Defence Mechanisms of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) against Entomopathogenic Fungal Infections. Int. J. Mol. Sci. 2016, 17, 1518. https://doi.org/10.3390/ijms17091518
Hussain A, Rizwan-ul-Haq M, Al-Ayedh H, AlJabr AM. Susceptibility and Immune Defence Mechanisms of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) against Entomopathogenic Fungal Infections. International Journal of Molecular Sciences. 2016; 17(9):1518. https://doi.org/10.3390/ijms17091518
Chicago/Turabian StyleHussain, Abid, Muhammad Rizwan-ul-Haq, Hassan Al-Ayedh, and Ahmed Mohammed AlJabr. 2016. "Susceptibility and Immune Defence Mechanisms of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) against Entomopathogenic Fungal Infections" International Journal of Molecular Sciences 17, no. 9: 1518. https://doi.org/10.3390/ijms17091518
APA StyleHussain, A., Rizwan-ul-Haq, M., Al-Ayedh, H., & AlJabr, A. M. (2016). Susceptibility and Immune Defence Mechanisms of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) against Entomopathogenic Fungal Infections. International Journal of Molecular Sciences, 17(9), 1518. https://doi.org/10.3390/ijms17091518