Abstract
Nicotiana alata defensins 1 and 2 (NaD1 and NaD2) are plant defensins from the ornamental tobacco that have antifungal activity against a variety of fungal pathogens. Some plant defensins interact with fungal cell wall O-glycosylated proteins. Therefore, we investigated if this was the case for NaD1 and NaD2, by assessing the sensitivity of the three Aspergillus nidulans (An) O-mannosyltransferase (pmt) knockout (KO) mutants (An∆pmtA, An∆pmtB, and An∆pmtC). An∆pmtA was resistant to both defensins, while An∆pmtC was resistant to NaD2 only, suggesting NaD1 and NaD2 are unlikely to have a general interaction with O-linked side chains. Further evidence of this difference in the antifungal mechanism was provided by the dissimilarity of the NaD1 and NaD2 sensitivities of the Fusarium oxysporum f. sp. lycopersici (Fol) signalling knockout mutants from the cell wall integrity (CWI) and high osmolarity glycerol (HOG) mitogen-activated protein kinase (MAPK) pathways. HOG pathway mutants were sensitive to both NaD1 and NaD2, while CWI pathway mutants only displayed sensitivity to NaD2.
1. Background
Plant defensins are small, basic, cysteine-rich proteins that are often produced as a first line of defense against fungal attack [1,2,3]. Both NaD1 and NaD2 from the ornamental tobacco (Nicotiana alata) have antifungal activity, although NaD1 is more active on most fungal species [2,4]. More is known about the mechanism of action of NaD1, compared to NaD2. The antifungal activity of NaD1 involves a multi-step mode of action; whereby NaD1 interacts specifically with the fungal cell wall and ultimately enters the cytoplasm [5], resulting in the disruption of the plasma membrane and the production of reactive oxygen species [6]. NaD2 has also been shown to permeabilise the plasma membrane of Puccinia spp. [4].
NaD1 and NaD2 share only 40% sequence identity and 51% sequence similarity and have different mechanisms of antifungal activity to each other [4,7]. One obvious difference is that the two defensins interact with different phospholipids in bilayers; NaD1 with phosphatidylinositol 4,5 bisphosphate (PI(4,5)P2) [8,9] and NaD2 with phosphatidic acid (PA) [7]. To reach the fungal plasma membrane, NaD1 and NaD2 must first pass through the wall. However, it is not known whether both NaD1 and NaD2 interact with the same components of the cell wall or induce the same cellular response pathways in fungi.
The glycoprotein layer of the fungal cell wall has been implicated in the antifungal activity of NaD1, because proteinase K treated fungi are resistant to NaD1 [5]. These cell wall glycoproteins are often heavily glycosylated with both N- and O-linked side chains. A genetic screen of Candida albicans revealed that mutations in the α-1,6-mannosyltransferase complex, which is involved in the O-mannosylation of cell wall proteins, resulted in resistance to dermaseptin S3 and the plant defensin Pharbitis nil antimicrobial peptide 1 (Pn-AMP1) [10,11]. We have used a similar approach to examine whether O-linked side chains are involved in the antifungal activity of NaD1 and NaD2, by assessing the sensitivity of Aspergillus nidulans O-mannosyltransferase knockout strains. O-mannosyltransferase catalyze the initial transfer of mannose onto the serine or threonine to start the O-linked side chain of the protein. A. nidulans is ideal for this work as it possesses a single gene for each of the O-mannosyltransferase sub-families (pmtA, pmtB and pmtC) and mutants are viable [12]. These pmt mutants have different phenotypes suggesting that the enzymes have distinct protein substrates [13,14].
Cell wall glycoproteins have roles in a variety of cellular functions including signaling, protection, and coping with cell stress [15]. The fungal response to antifungal peptides is often sensed and initiated through these glycoproteins, leading to the activation of the mitogen-activated cell wall integrity (CWI) pathway or the high osmolarity glycerol (HOG) pathway [16,17,18,19]. Studying the pathways activated by antifungal peptides gives an insight into their mechanism of action. For example, a recent study in C. albicans revealed that the HOG signalling pathway plays a role in the cellular tolerance and response to NaD1 as exposure to NaD1 leads to oxidative stress through the production of reactive oxygen species [6]. In contrast the plant defensins Raphanus sativus antifungal peptide 2 (RsAFP2), and Medicago sativa defensin 1 (MsDef1), activate the CWI pathway in C. albicans and Fusarium graminearum respectively [20,21], possibly due to their interaction with the cell wall sphingolipid, glucosylceramide [20]. However, it is not known what pathways are activated in response to NaD2. Therefore, the activation of cell stress responses to NaD1 and NaD2 was also investigated by testing the sensitivity of F. oxysporum f. sp. lycopersicum (Fol) KO mutants of the CWI and HOG pathways.
2. Results
2.1. Impact of Plant Defensins, NaD1 and NaD2, on the Growth of Aspergillus nidulans (A. nidulans) O-Mannosyltransferase Knockout (KO) Mutants
Disruption of the main O-mannosyltransferase in A. nidulans, An∆pmtA, led to enhanced resistance to both NaD1 and NaD2, compared to the WT, An strain A850 (Figure 1). A limited amount of growth of the O-mannosyltransferase C mutant, An∆pmtC, was observed in the presence of NaD2, but not with NaD1. In all experiments, the sensitivity of the O-mannosyltransferase B KO mutant, An∆pmtB, closely resembled that of the WT. All knockout mutants grew as per the wild type strain in the absence of defensins (Figure 1).
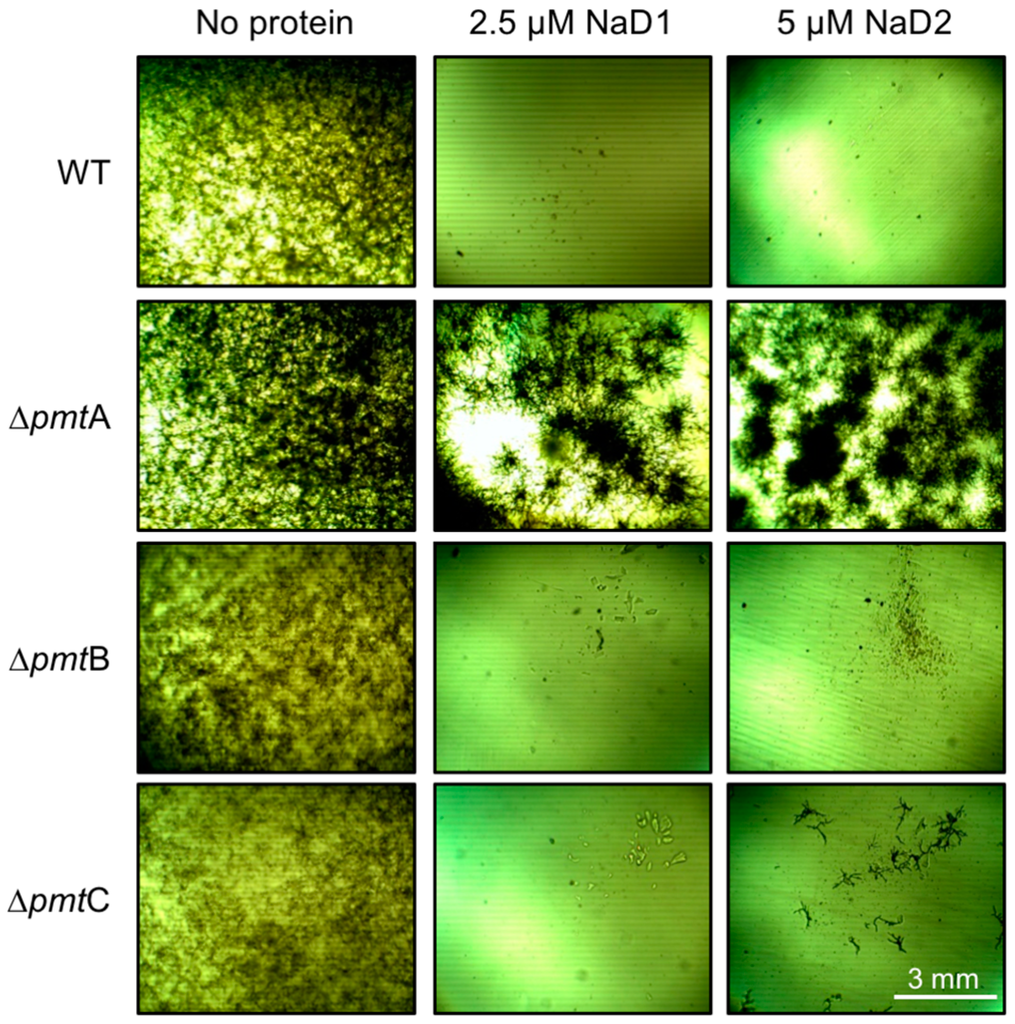
Figure 1.
Growth of the A. nidulans (An) O-mannosyltransferase (pmt A, B or C) knockout mutants (An∆pmtA, An∆pmtB or An∆pmtC) in the presence/absence of plant defensins NaD1 (2.5 µM) and NaD2 (5 µM). Images are representative of three experiments.
2.2. Defensin Growth Inhibition of the Fusarium oxyspoprum f. sp. lycopersici Mitogen-Activated Protein Kinase (MAPK) Pathway KO Mutants
The sensitivity of F. oxysporum f. sp. lycopersici (Fol) knockout mutants of cellular response pathway genes were assessed to determine the potential involvement of mitogen-activated protein kinase (MAPK) cascades in enhancing tolerance to NaD1 and NaD2. Mutants lacking components of the HOG pathway, specifically the Fusarium histidine kinase 1 (Fhk1) and the MAPK Hog1, were both hypersensitive to NaD1, with a 2-fold decrease in IC50 compared to WT (Figure 2). In addition, the KO strains in the Fusarium MAPK 1 (FolΔfmk1) and the transmembrane mucin Msb2 (FolΔmsb2) were also significantly hypersensitive to NaD1. Similarly, the hog1 and msb2 KO mutants were more sensitive to NaD2. However, the mutant (FolΔmpk1) lacking the stress response MAPK, Mpk1, exhibited a 4-fold decrease in IC50 compared to WT nd hence was more sensitive to NaD2 than NaD1 and conversely the FolΔfmk1 and FolΔfhk1 mutants were more sensitive to NaD1 than WT and not sensitive to NaD2. The complemented strain, FolΔmsb2 + Msb2, had a similar IC50 to that of the WT for both defensins.
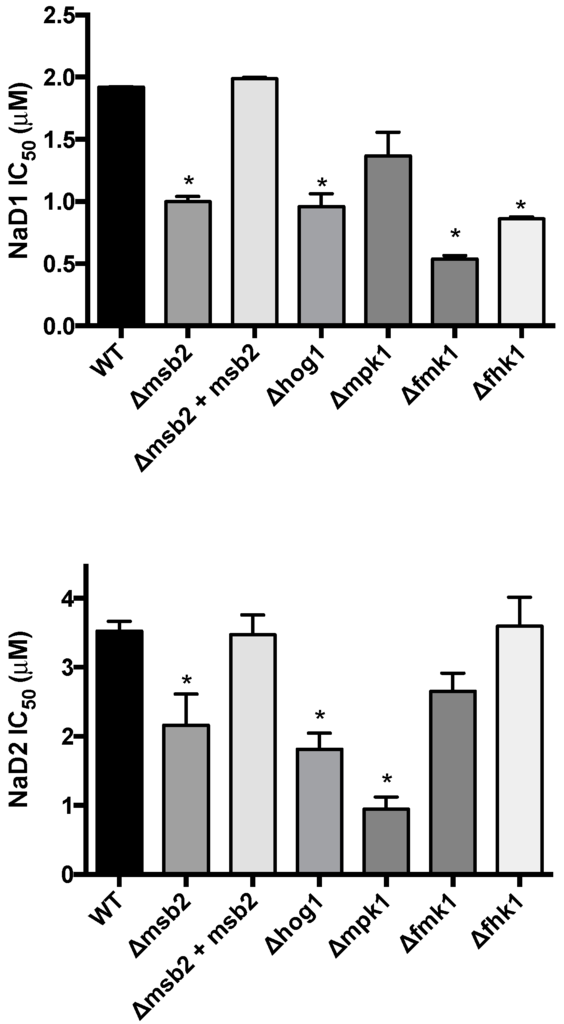
Figure 2.
Concentrations of the plant defensins, NaD1 and NaD2, required to inhibit 50% growth (IC50) of Fusarium oxysporum f. sp. lycopersici knock out mutants of the mitogen-activated protein kinase (MAPK) pathways. Error bars are SEM, NaD1 n = 3, NaD2 n = 6. * indicates data that is significantly different (p < 0.05) to WT.
3. Discussion
The plant defensins NaD1 and NaD2 have antifungal activity against a wide range of agronomically and medically important microbes, including Fusarium and Aspergillus species [4,22]. Understanding the mechanism of action of these defensins will therefore help to develop these antifungal peptides for agricultural and pharmaceutical use. Combining molecules with multiple mechanisms of action into one treatment has been suggested as a way to reduce the occurrence of resistance. NaD1 and NaD2 differ markedly in their amino acid sequence, especially in their γ-core [4]. The presence of different sequences in this area gives rise to different lipid specificity and antifungal mechanisms [23,24,25,26]. We therefore investigated the N. alata defensins to examine their differences in activity, to determine if the ornamental tobacco produces defensins with multiple mechanisms of action. This study was aimed at testing the hypothesis that NaD1 and NaD2 are likely to interact with distinct components of the fungal cell wall, and induce different cellular stress response pathways.
We tested the sensitivity of A. nidulans O-mannosyltransferases (pmtA, pmtB and pmtC) KO mutants in response to NaD1 and NaD2 to determine if O-linked side chains were involved in their antifungal activity. Assessing the sensitivity of the A. nidulans protein O-mannosyltransferase (PMT) mutants to both defensins revealed that only the deletion of the pmtA gene resulted in a mutant (An∆pmtA) with a resistant phenotype to both NaD1 and NaD2, compared to WT. No resistance to NaD1 and NaD2 was observed with the An∆pmtB mutant. Both defensins are therefore unlikely to have a general interaction with cell wall O-linked sided chains. This is in contrast to observations with the antifungal peptide dermaseptin S3 and the plant defensin Pn-AMP1, where O-linked side chains on glycoproteins have been implicated in their mechanism of action from mutant screening [10,11]. The mutant An∆pmtC was mildly resistant to NaD2 only, suggesting that the glycoprotein targets for NaD1 and NaD2 may be different. The selective resistance of just An∆pmtA to both defensins may be explained by examining the proteins that receive their O-linked side chains from PmtA. The O-mannosyltransferases in A. nidulans are believed to have overlapping and distinct specificities for protein substrates due to the distinct phenotypes of the mutants and their different sensitivities to antifungal agents [12,13]. The phenotype of An∆pmtB mutant suggests involvement in polarity maintenance, while the An∆pmtC phenotype suggests involvement in cell wall integrity [13]. The mannosyltransferases responsible for glycosylation of individual proteins are only known for a couple of proteins. For example, MsbA (an orthologue of Msb2 from C. albicans and Fol) is differentially glycosylated by PmtA and PmtB [27]. Msb2 is an osmosensor in the HOG pathway, and the HOG pathway has been implicated in the fungal response to NaD1, as deletion of the hog1 gene generates sensitivity to NaD1 in C. albicans [6]. Another protein mannosylated by PmtA is WscA (an orthologue of Wsc1 in Saccharomyces cerevisiae) [13], which has a role in cell wall integrity under changes in osmotic and pH conditions [28]. The observed resistance phenotype of An∆pmtA to NaD1 and NaD2 may be due to the lack of mannosylation of the membrane mucin MsbA, and/or WscA, which leads to the defect in the signalling of the HOG and CWI pathways, rather than a direct interaction of the defensins with these receptors. However, further experiments are required to confirm this hypothesis.
The involvement of the HOG pathway in the activity of both NaD1 and NaD2 was supported as deletion of either Msb2 or Hog1 led to enhanced sensitivity to both defensins. Loss of the HOG pathway components would create a defect in the downstream signalling, resulting in a fungus that is more sensitive to defensin attack. This suggests that the HOG pathway is involved in tolerance of Fol to these two defensins. Therefore, the HOG pathway is not just activated in response to NaD1 in the human pathogen C. albicans [6], but also in the agronomically important pathogen, Fol. Modulation of the HOG pathway in combination with either NaD1 or NaD2 could be a promising strategy for increasing the activity of these defensins against pathogenic fungi. This has been suggested previously as a combinational strategy against human pathogens for the human antimicrobial peptides; histatin 5 and β-defensins 2 and 3, which also activate the HOG pathway [29,30]. Here we show that this could be extended to strategies to combat plant pathogens.
MAPK pathways, such as CWI pathway or the HOG pathway are responsible for the quick transduction of signals in response to environmental stresses, such as those caused by the antifungal defensins. The pathway activated depends upon the stimulus, and therefore can give insights into the mechanism of action of an antifungal peptide. For example, the fungal cell responds to some plant defensins by activating the CWI pathway, and these defensins have interactions with the cell wall as part of their activity. These include Pn-AMP1 which interacts with O-linked side chains resulting in activation of CWI pathway [10]; and MsDef1 and RsAFP2, which interact with glucosylceramide in the cell wall, causing CWI pathway activation [20,21]. In this present study, we observed that deletion of the CWI MAPK Mpk1 lead to increased sensitivity to NaD2, but not to NaD1. It would therefore be interesting to investigate the direct interaction of NaD2 with the cell wall beyond the O-linked side chains to determine why NaD2 is activating the CWI pathway. Further difference in cell response pathways was also observed between NaD1 and NaD2 when the deletion of the histidine kinase Fhk1 and the pathogenicity MAPK Fmk1 lead to sensitivity to only NaD1. Therefore, the cell stress pathways activated by NaD1 and NaD2 in Fol appear to be different, providing further evidence that the two defensins have distinct mechanisms of action.
Taken together, sequence variations between NaD1 and NaD2 are likely to explain the differences in fungal mutant sensitivities observed. The HOG pathway appears to be involved in the stress response to both NaD1 and NaD2, however it remains to be determined if NaD2, like NaD1, activates this pathway through reactive oxygen species or, instead, through osmotic stress as occurs with the activity of histatin 5. To study differences in the mode of action of defensins in more detail, we are attempting to identify targets by screening yeast deletion libraries to identify genes that confer resistance or sensitivity to different defensins [31]. We are also determining whether fungal pathogens respond differently to different defensins by examining changes in the transcriptome of various fungal pathogens after exposure to sub-lethal amounts of defensins. In summary, NaD1 and NaD2 have evolved as part of the arsenal of innate immunity molecules that protect the ornamental tobacco against fungal disease. Their overlapping, as well as distinct modes of action are likely to provide activity against a broader range of fungal pathogens and make it more difficult for pathogens to become resistant to these defensins.
4. Methods
4.1. Fungal Strains and Media
The A. nidulans (An) and F. oxysporum f. sp. lycopersici (Fol) knockout (KO) mutants used in this study are listed in Table 1. Complete Medium (CM) was prepared essentially as described by Kriangkripipat and Momany [32]. In all cases the A. nidulans isolates were grown on minimal media that contained arginine; arginine and tryptophan; or arginine, tryptophan and methionine (depending on the specific mutant); and the pH was increased to 6.5 by addition of 1 M NaOH. For sporulation, A. nidulans strains were grown at 26 °C on CM media containing 1.5% agar and 1 M sorbitol as an osmotic stabilizer.

Table 1.
List of fungal strains used in this study.
4.2. Protein Source
NaD1 and NaD2 were extracted from the flowers of ornamental tobacco as described in van der Weerden, Lay and Anderson [22].
4.3. Fungal Growth Assays
Fungal growth assays were performed in microtitre plates and the concentrations of defensins required to inhibit fungal growth by 50% (IC50) were calculated as described in van der Weerden, Lay and Anderson [22]. IC50 values were plotted using Graphpad Prism v6 (GraphPad Software, La Jolla, CA, USA) and t-tests with Welch′s corrections were performed to determine significance of the growth data of each mutant compared to WT. Calculation of the IC50 was not possible for the A. nidulans mutants due to uneven growth. Instead the growth of the A. nidulans mutants in the presence of plant defensins was assessed visually by capturing images using an Olympic microscope at 10× magnification. The growth assays were conducted in the dark at 25 °C for 48 h.
Acknowledgments
The authors thank Michelle Momany for the provision of Aspergillus nidulans O-mannosyltransferase mutants. The authors also thank the Anderson Lab, for the extraction of NaD1 and NaD2 for use in all experiments undertaken in this study. The authors also thank Mark Bleackley for constructive discussion and providing valuable advice on the final manuscript. We also thank the Australian Research Council for funding this work (Australian Research Council grant, DP120102694 to Marilyn Anderson and Kim Plummer).
Author Contributions
Peter M. Dracatos wrote the manuscript, designed and performed the experimental work. Jennifer Payne assisted with data analysis, drafting of the manuscript and composition of figures. Antonio Di Pietro provided fungal strains for the experimental analysis. Marilyn A. Anderson provided the defensins for all experiments, conceptualized the project and assisted in the final drafting of the manuscript. Kim M. Plummer co-conceptualized the project, interpretation of results, and assisted in final drafting and editing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lay, F.T.; Anderson, M.A. Defensins-components of the innate immune system in plants. Curr. Protein Pept. Sci. 2005, 6, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Lay, F.T.; Schirra, H.J.; Scanlon, M.J.; Anderson, M.A.; Craik, D.J. The three-dimensional solution structure of NaD1, a new floral defensin from Nicotiana alata and its application to a homology model of the crop defense protein alfAFP. J. Mol. Biol. 2003, 325, 175–188. [Google Scholar] [CrossRef]
- Lacerda, A.F.; Vasconcelos, E.A.; Pelegrini, P.B.; Grossi de Sa, M.F. Antifungal defensins and their role in plant defense. Front. Microbiol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Dracatos, P.M.; van der Weerden, N.L.; Carroll, K.T.; Johnson, E.D.; Plummer, K.M.; Anderson, M.A. Inhibition of cereal rust fungi by both class I and II defensins derived from the flowers of Nicotiana alata. Mol. Plant Pathol. 2014, 15, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Van der Weerden, N.L.; Hancock, R.E.W.; Anderson, M.A. Permeabilization of fungal hyphae by the plant defensin NaD1 occurs through a cell wall-dependent process. J. Biol. Chem. 2010, 285, 37513–37520. [Google Scholar] [CrossRef] [PubMed]
- Hayes, B.M.; Bleackley, M.R.; Wiltshire, J.L.; Anderson, M.A.; Traven, A.; van der Weerden, N.L. Identification and mechanism of action of the plant defensin NaD1 as a new member of the antifungal drug arsenal against Candida albicans. Antimicrob. Agents Chemother. 2013, 57, 3667–3675. [Google Scholar] [CrossRef] [PubMed]
- Bleackley, M.; Payne, J.; Hayes, B.; Durek, T.; Craik, D.; Shafee, T.; Poon, I.; Hulett, M.; Weerden, N.v.d.; Anderson, M. Nicotiana alata defensin chimeras reveal differences in the mechanism of fungal and tumour cell killing and an enhanced antifungal variant. Antimicrob. Agents Chemother. 2016. [Google Scholar] [CrossRef]
- Payne, J.A.; Bleackley, M.R.; Lee, T.H.; Shafee, T.M.; Poon, I.K.; Hulett, M.D.; Aguilar, M.I.; van der Weerden, N.L.; Anderson, M.A. The plant defensin NaD1 introduces membrane disorder through a specific interaction with the lipid, phosphatidylinositol 4,5-bisphosphate. Biochim. Biophys. Acta 2016, 1858, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Poon, I.; Baxter, A.A.; Lay, F.T.; Mills, G.D.; Adda, C.G.; Payne, J.A.; Phan, T.K.; Ryan, G.F.; White, J.A.; Veneer, P.K.; et al. Phosphoinositide-mediated oligomerization of a defensin induces cell lysis. eLife 2014. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.C.; Lee, B.; Young, M.E.; Koo, S.C.; Cooper, J.A.; Baek, D.; Lim, C.O.; Lee, S.Y.; Yun, D.-J.; Cho, M.J. Pn-amp1, a plant defense protein, induces actin depolarization in yeasts. Plant Cell. Physiol 2004, 45, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Mora-Montes, H.M.; Gow, N.A.R.; Coote, P.J. Loss of mannosylphosphate from Candida albicans cell wall proteins results in enhanced resistance to the inhibitory effect of a cationic antimicrobial peptide via reduced peptide binding to the cell surface. Microbiology 2009, 155, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Hamaguchi, T.; Sameshima, Y.; Goto, M.; Furukawa, K. Molecular characterization of protein O-mannosyltransferase and its involvement in cell-wall synthesis in Aspergillus nidulans. Microbiology 2004, 150, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Harada, Y.; Oka, T.; Matsumoto, S.; Takegawa, K.; Furukawa, K. Protein O-mannosyltransferases B and C support hyphal development and differentiation in Aspergillus nidulans. Eukaryot. Cell 2009, 8, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Shaw, B.D.; Momany, M. Aspergillus nidulans polarity mutant swoA is complemented by protein O-mannosyltransferase pmta. Fungal Genet. Biol. 2002, 37, 263–270. [Google Scholar] [CrossRef]
- Perez-Nadales, E.; di Pietro, A. The membrane mucin Msb2 regulates invasive growth and plant infection in Fusarium oxysporum. Plant Cell 2011, 23, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Gustin, M.C.; Albertyn, J.; Alexander, M.; Davenport, K. Map kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 1998, 62, 1264–1300. [Google Scholar] [PubMed]
- Heilmann, C.J.; Sorgo, A.G.; Mohammadi, S.; Sosinska, G.J.; de Koster, C.G.; Brul, S.; de Koning, L.J.; Klis, F.M. Surface stress induces a conserved cell wall stress response in the pathogenic fungus Candida albicans. Eukaryot. Cell 2013, 12, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.E. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2005, 69, 262–291. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Peña, J.M.; García, R.; Nombela, C.; Arroyo, J. The high-osmolarity glycerol (HOG) and cell wall integrity (CWI) signalling pathways interplay: A yeast dialogue between mapk routes. Yeast 2010, 27, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Thevissen, K.; de Mello Tavares, P.; Xu, D.; Blankenship, J.; Vandenbosch, D.; Idkowiak-Baldys, J.; Govaert, G.; Bink, A.; Rozental, S.; de Groot, P.W.J.; et al. The plant defensin RsAFP2 induces cell wall stress, septin mislocalization and accumulation of ceramides in Candida albicans. Mol. Microbiol. 2012, 84, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, V.; Zhao, X.; Snyder, A.K.; Xu, J.R.; Shah, D.M. Two mitogen-activated protein kinase signalling cascades mediate basal resistance to antifungal plant defensins in Fusarium graminearum. Cell. Microbiol. 2007, 9, 1491–1506. [Google Scholar] [CrossRef] [PubMed]
- Van der Weerden, N.L.; Lay, F.T.; Anderson, M.A. The plant defensin, NaD1, enters the cytoplasm of Fusarium oxysporum hyphae. J. Biol. Chem. 2008, 283, 14445–14452. [Google Scholar] [CrossRef] [PubMed]
- Spelbrink, R.G.; Dilmac, N.; Allen, A.; Smith, T.J.; Shah, D.M.; Hockerman, G.H. Differential antifungal and calcium channel-blocking activity among structurally related plant defensins. Plant Physiol. 2004, 135, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, L.N.; Angeli, R.; Sarzedas, C.G.; Barreto-Bergter, E.; Valente, A.P.; Kurtenbach, E.; Almeida, F.C.L. Backbone dynamics of the antifungal PsD1 pea defensin and its correlation with membrane interaction by NMR spectroscopy. Biochim. Biophys. Acta Biomembr. 2010, 1798, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Sagaram, U.S.; El-Mounadi, K.; Buchko, G.W.; Berg, H.R.; Kaur, J.; Pandurangi, R.S.; Smith, T.J.; Shah, D.M. Structural and functional studies of a phosphatidic acid-binding antifungal plant defensin MtDef4: Identification of an RGFRRR motif governing fungal cell entry. PLoS ONE 2013, 8, e82485. [Google Scholar] [CrossRef] [PubMed]
- De Paula, V.S.; Razzera, G.; Barreto-Bergter, E.; Almeida, F.C.L.; Valente, A.P. Portrayal of complex dynamic properties of sugarcane defensin 5 by NMR: Multiple motions associated with membrane interaction. Structure 2011, 19, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Kriangkripipat, T.; Momany, M. Aspergillus nidulans Pmts form heterodimers in all pairwise combinations. FEBS Open Biol. 2014, 4, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Futagami, T.; Nakao, S.; Kido, Y.; Oka, T.; Kajiwara, Y.; Takashita, H.; Omori, T.; Furukawa, K.; Goto, M. Putative stress sensors WscA and WscB are involved in hypo-osmotic and acidic pH stress tolerance in Aspergillus nidulans. Eukaryot. Cell 2011, 10, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Argimon, S.; Fanning, S.; Blankenship, J.R.; Mitchell, A.P. Interaction between the Candida albicans high-osmolarity glycerol (HOG) pathway and the response to Human β-Defensins 2 and 3. Eukaryot. Cell 2011, 10, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Vylkova, S.; Jang, W.S.; Li, W.; Nayyar, N.; Edgerton, M. Histatin 5 initiates osmotic stress response in Candida albicans via activation of the HOG1 mitogen-activated protein kinase pathway. Eukaryot. Cell 2007, 6, 1876–1888. [Google Scholar] [CrossRef] [PubMed]
- Bleackley, M.R.; Wiltshire, J.L.; Perrine-Walker, F.; Vasa, S.; Burns, R.L.; van der Weerden, N.L.; Anderson, M.A. Agp2p, the plasma membrane transregulator of polyamine uptake, regulates the antifungal activities of the plant defensin NaD1 and other cationic peptides. Antimicrob. Agents Chemother. 2014, 58, 2688–2698. [Google Scholar] [CrossRef] [PubMed]
- Kriangkripipat, T.; Momany, M. Aspergillus nidulans protein O-mannosyltransferases play roles in cell wall integrity and developmental patterning. Eukaryot. Cell 2009, 8, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, A.; Garcia-MacEira, F.I.; Meglecz, E.; Roncero, M.I. A map kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol. Microbiol. 2001, 39, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Luque, D.S.; di Pietro, A.; Pérez-Nadales, E.; Turrà, D. Three Fusarium. oxysporum MAPKs have distinct and complementary roles in stress adaptation and cross-kingdom pathogenicity. Mol. Plant Pathol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Turra, D.; El Ghalid, M.; Rossi, F.; di Pietro, A. Fungal pathogen uses sex pheromone receptor for chemotropic sensing of host plant signals. Nature 2015, 527, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Rispail, N.; di Pietro, A. The two-component histidine kinase Fhk1 controls stress adaptation and virulence of Fusarium oxysporum. Mol. Plant Pathol. 2010, 11, 395–407. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).