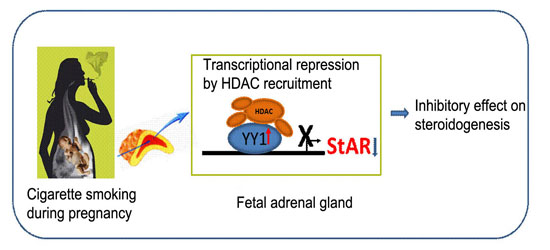
Nicotine Suppressed Fetal Adrenal StAR Expression via YY1 Mediated-Histone Deacetylation Modification Mechanism
Abstract
:1. Introduction
2. Results
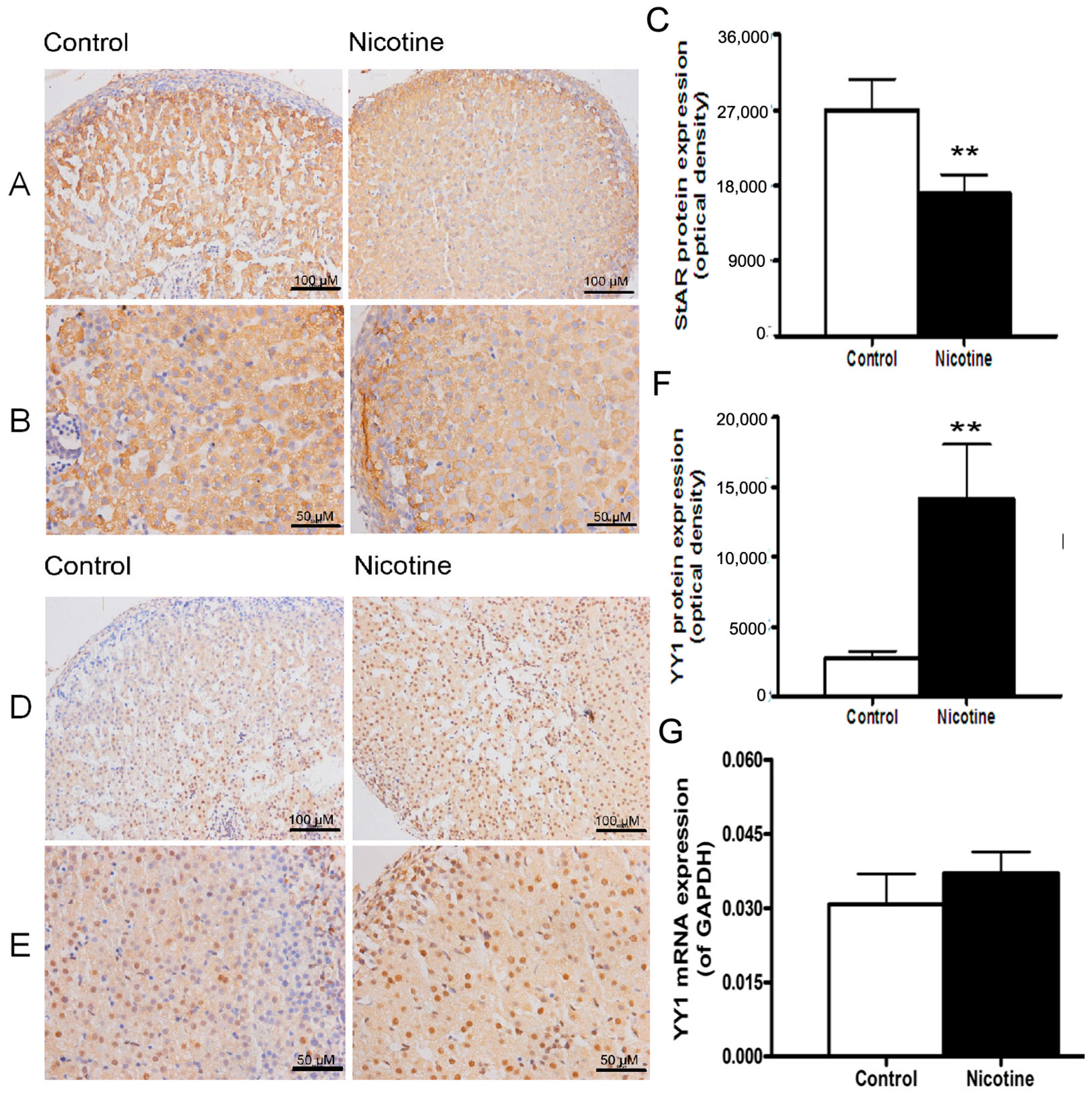
2.1. Expression Patterns of Steroidogenic Acute Regulatory (StAR) and Yin Yang 1 (YY1) in Nicotine-Treated Fetal Rat Adrenal Glands
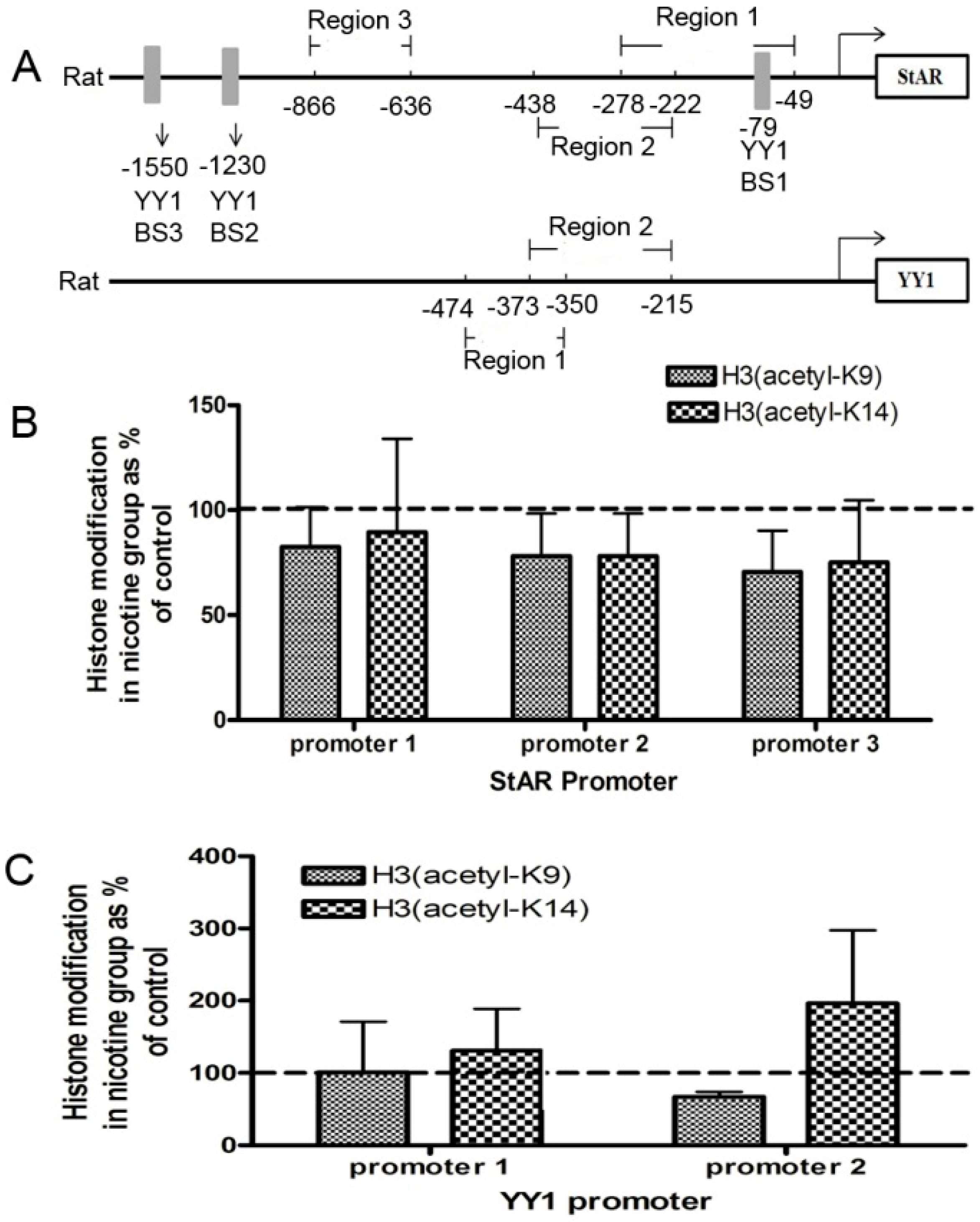
2.2. Histone Modification of StAR and YY1 in Fetal Rat Adrenal Glands
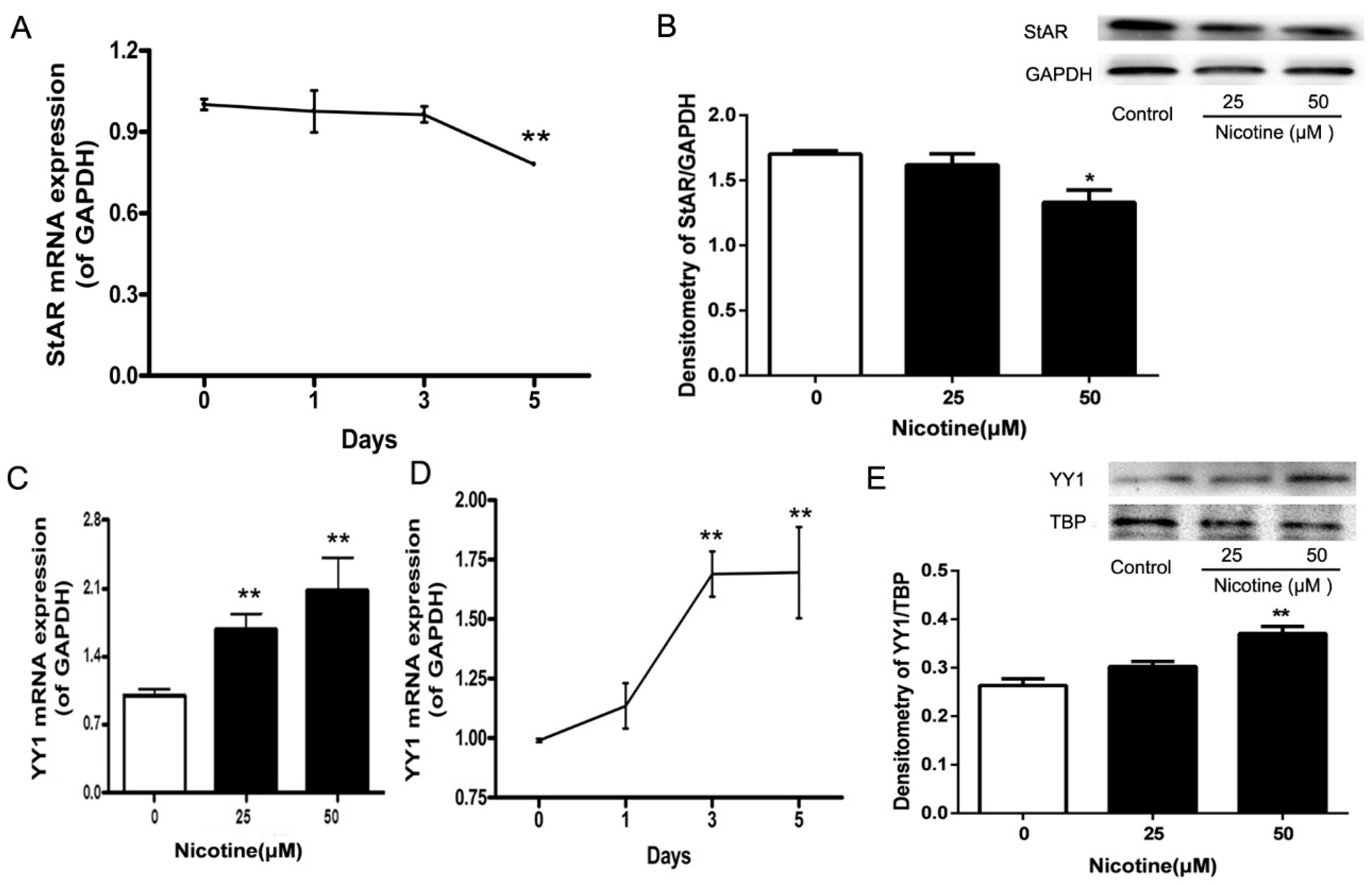
2.3. Expression Patterns of StAR and YY1 in Nicotine-Treated NCI-H295A Cells
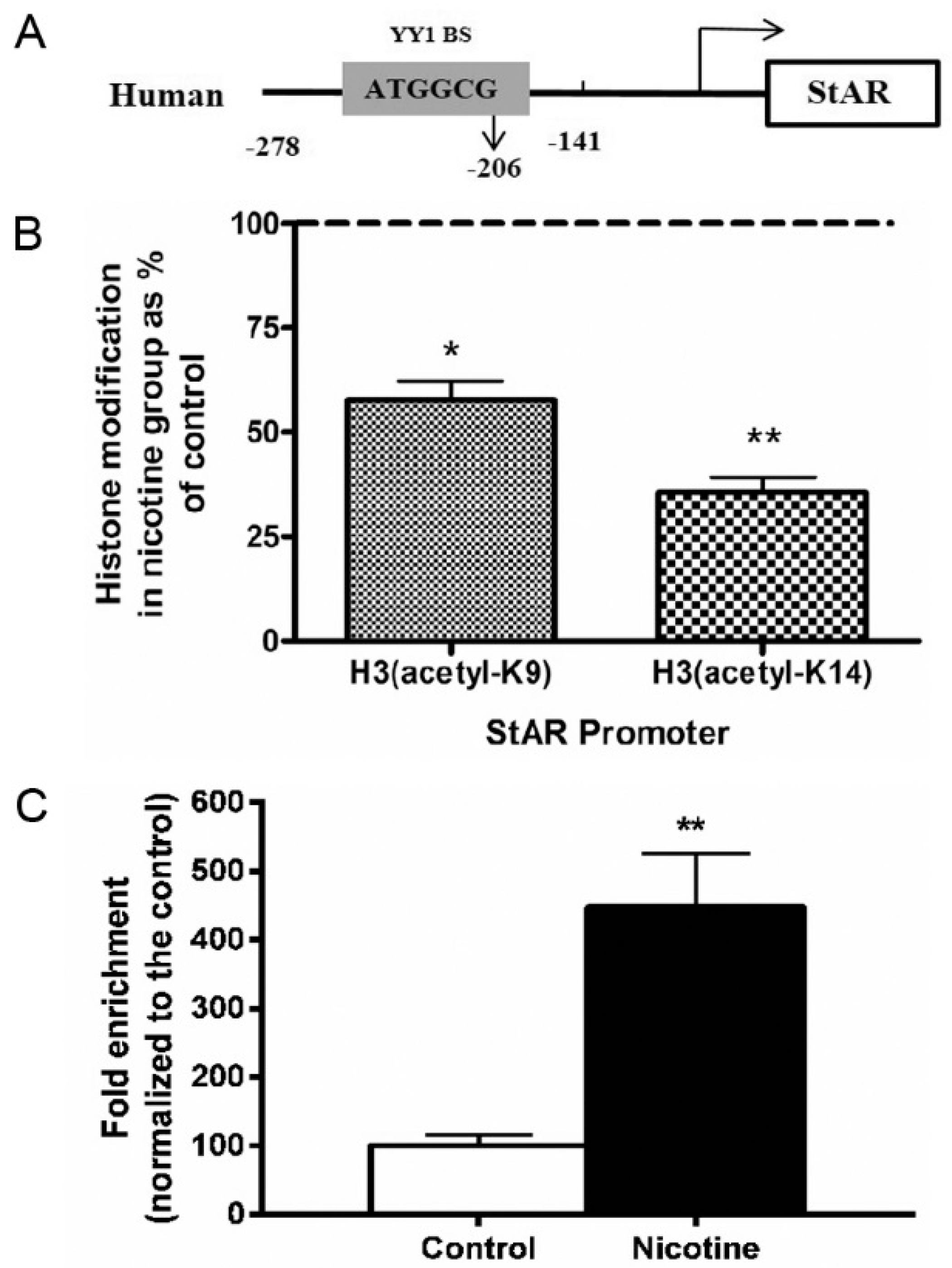
2.4. Histone Modification of StAR in Nicotine-Treated NCI-H295A Cells
2.5. YY1 and StAR Promoter Interaction in Nicotine-Treated NCI-H295A Cells
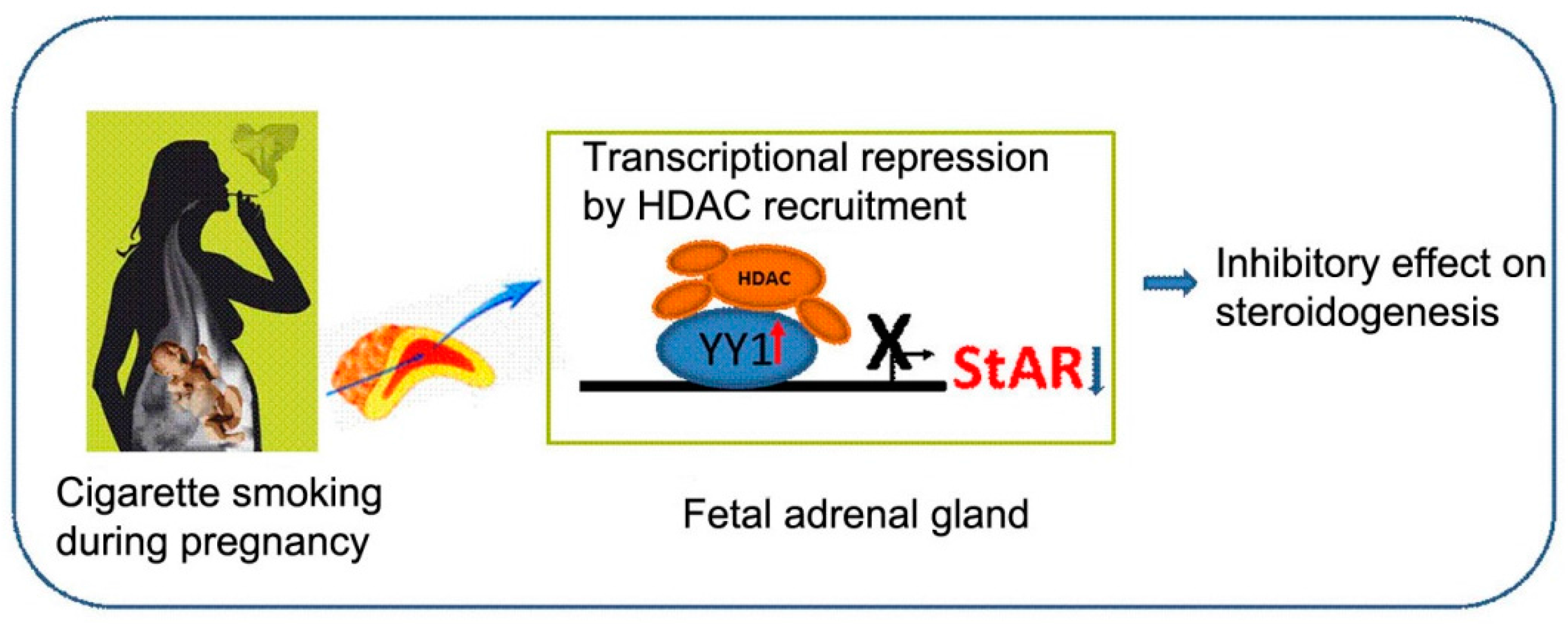
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. Human Adrenocortical Carcinoma (NCI-H295A) Cell Culture and Drug Treatment
4.3. Immunohistochemistry Observations
4.4. Quantitative Real-Time PCR (qRT-PCR)
4.5. Western Blotting Analysis
4.6. Chromatin Immunoprecipitation (ChIP)
4.7. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fowden, A.L.; Forhead, A.J. Endocrine mechanisms of intrauterine programming. Reproduction 2004, 127, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.; Seckl, J. Glucocorticoids, prenatal stress and the programming of disease. Horm. Behav. 2011, 59, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, B.J.; Liang, G.; Ping, J.; Kou, H.; Li, X.J.; Xiong, J.; Hu, D.C.; Chen, L.B.; Magdalou, J.; et al. Caffeine-Induced Activated Glucocorticoid Metabolism in the Hippocampus Causes Hypothalamic-Pituitary-Adrenal Axis Inhibition in Fetal Rats. PLoS ONE 2012, 7, e44497. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, B.; Patro-Malysza, J.; Poniedzialek-Czajkowska, E.; Kimber-Trojnar, Z.; Leszczyska-Gorzelak, B.; Oleszczuk, J. Glucocorticoids in Pregnancy. Curr. Pharm. Biotechnol. 2011, 12, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, M.; Peng, R.X.; Le, J. Influences of 3-methylcholanthrene, phenobarbital and dexamethasone on xenobiotic metabolizing-related cytochrome P450 enzymes and steroidogenesis in human fetal adrenal cortical cells. Acta Pharmacol. Sin. 2006, 27, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, T.; Liao, Z.X.; Pan, X.L.; Feng, Y.H.; Wang, H. Nicotine-induced prenatal overexposure to maternal glucocorticoid and intrauterine growth retardation in rat. Exp. Toxicol. Pathol. 2007, 59, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Chen, M.; Pan, X.L.; Zheng, J.; Wang, H. Ethanol-induced inhibition of fetal hypothalamic-pituitary-adrenal axis due to prenatal overexposure to maternal glucocorticoid in mice. Exp. Toxicol. Pathol. 2011, 63, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Chen, M.; Liu, L.; Cheng, H.Y.; Yan, Y.E.; Feng, Y.H.; Wang, H. Nicotine induced CpG methylation of Pax6 binding motif in StAR promoter reduces the gene expression and cortisol production. Toxicol. Appl. Pharm. 2011, 257, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.E.; Liu, L.; Wang, J.F.; Liu, F.; Li, X.H.; Qin, H.Q.; Wang, H. Prenatal nicotinic exposure suppresses fetal adrenal steroidogenesis via steroidogenic factor 1 (SF-1) deacetylation. Toxicol. Appl. Pharmacol. 2014, 277, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Stocco, D.M. StAR protein and the regulation of steroid hormone biosynthesis. Annu. Rev. Physiol. 2001, 63, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.R.; Dyson, M.T.; Stocco, D.M. Regulation of the steroidogenic acute regulatory protein gene expression: present and future perspectives. Mol. Hum. Reprod. 2009, 15, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Seto, E.; Chang, L.S.; Shenk, T. Transcriptional repression by YY1, a human GLI-Kruppel-related protein, and relief of repression by adenovirus E1A protein. Cell 1991, 67, 377–388. [Google Scholar] [CrossRef]
- Satyamoorthy, K.; Park, K.; Atchison, M.L.; Howe, C.C. The intracisternal A-particle upstream element interacts with transcription factor YY1 to activate transcription: Pleiotropic effects of YY1 on distinct DNA promoter elements. Mol. Cell. Biol. 1993, 13, 6621–6628. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Cao, P.; Wan, M.M.; Sui, G. Yin Yang 1: A multifaceted protein beyond a transcription factor. Transcription 2010, 1, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Christenson, L.K.; Osborne, T.F.; McAllister, J.M.; Strauss, J.F., III. Conditional response of the human steroidogenic acute regulatory protein gene promoter to sterol regulatory element binding protein-1a. Endocrinology 2001, 142, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Nackley, A.C.; Shea-Eaton, W.; Lopez, D.; McLean, M.P. Repression of the steroidogenic acute regulatory gene by the multifunctional transcription factor Yin Yang 1. Endocrinology 2002, 143, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Galvin, K.M.; Shi, Y. Multiple mechanisms of transcriptional repression by YY1. Mol. Cell. Biol. 1997, 17, 3723–3732. [Google Scholar] [CrossRef] [PubMed]
- Natesan, S.; Gilman, M.Z. DNA bending and orientation-dependent function of YY1 in the c-fos promoter. Genes Dev. 1993, 7, 2497–2509. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.M.; Yao, Y.L.; Sun, J.M.; Davie, J.R.; Seto, E. Isolation and characterization of cDNAs corresponding to an additional member of the human histone deacetylase gene family. J. Biol. Chem. 1997, 272, 28001–28007. [Google Scholar] [CrossRef] [PubMed]
- Karki, P.; Webb, A.; Smith, K.; Johnson, J., Jr.; Lee, K.; Son, D.S.; Aschner, M.; Lee, E. Yin Yang 1 is a repressor of glutamate transporter EAAT2, and it mediates manganese-induced decrease of EAAT2 expression in astrocytes. Mol. Cell. Biol. 2014, 34, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Merkler, K.A.; Zhang, X.; McLean, M.P. Prostaglandin F2α suppresses rat steroidogenic acute regulatory protein expression via induction of Yin Yang 1 protein and recruitment of histone deacetylase 1 protein. Endocrinology 2007, 148, 5209–5219. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.J.; Seto, E. Unlocking the mechanisms of transcription factor YY1: Are chromatin modifying enzymes the key? Gene 1999, 236, 197–208. [Google Scholar] [CrossRef]
- Landais, E.; El-Khoury, V.; Prevost, A.; Dufer, J.; Liautaud-Roger, F. Nicotine induces chromatin changes and c-Jun up-regulation in HL-60 leukemia cells. Oncol. Rep. 2005, 14, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Pastor, V.; Host, L.; Zwiller, J.; Bernabeu, R. Histone deacetylase inhibition decreases preference without affecting aversion for nicotine. J. Neurochem. 2011, 116, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.B.; Matter, M.L. Chromatin Immunoprecipitation Assay: Examining the Interaction of NFκB with the VEGF Promoter. Methods Mol. Biol. 2015, 1332, 75–87. [Google Scholar] [PubMed]
- Sak, A.; Kubler, D.; Bannik, K.; Groneberg, M.; Stuschke, M. Dependence of radiation-induced H2AX phosphorylation on histone methylation: Evidence from the chromatin immunoprecipitation assay. Int. J. Radiat. Biol. 2015, 91, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Rai, L.S.; Chatterjee, G.; Sanyal, K. Chromatin Immunoprecipitation (ChIP) Assay in Candida albicans. Methods Mol. Biol. 2016, 1356, 43–57. [Google Scholar] [PubMed]
- Dwyer, J.B.; Broide, R.S.; Leslie, F.M. Nicotine and brain development. Birth Defects Res. C Embryo Today 2008, 84, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Alkondon, M.; Pereira, E.F.; Almeida, L.E.; Randall, W.R.; Albuquerque, E.X. Nicotine at concentrations found in cigarette smokers activates and desensitizes nicotinic acetylcholine receptors in CA1 interneurons of rat hippocampus. Neuropharmacology 2000, 39, 2726–2739. [Google Scholar] [CrossRef]
- Somm, E.; Schwitzgebel, V.M.; Vauthay, D.M.; Camm, E.J.; Chen, C.Y.; Giacobino, J.P.; Sizonenko, S.V.; Aubert, M.L.; Huppi, P.S. Prenatal nicotine exposure alters early pancreatic islet and adipose tissue development with consequences on the control of body weight and glucose metabolism later in life. Endocrinology 2008, 149, 6289–6299. [Google Scholar] [CrossRef] [PubMed]
- De Weerd, S.; Thomas, C.M.; Kuster, J.E.; Cikot, R.J.; Steegers, E.A. Variation of serum and urine cotinine in passive and active smokers and applicability in preconceptional smoking cessation counseling. Environ. Res. 2002, 90, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Feyerabend, C.; Ings, R.M.; Russel, M.A. Nicotine pharmacokinetics and its application to intake from smoking. Br. J. Clin. Pharmacol. 1985, 19, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, A.; Wang, L.; Zhao, B. Nornicotine and Nicotine Induced Neovascularization via Increased VEGF/PEDF Ratio. Ophthalmic Res. 2015, 55, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Laytragoon-Lewin, N.; Bahram, F.; Rutqvist, L.E.; Turesson, I.; Lewin, F. Direct effects of pure nicotine, cigarette smoke extract, Swedish-type smokeless tobacco (Snus) extract and ethanol on human normal endothelial cells and fibroblasts. Anticancer Res. 2011, 31, 1527–1534. [Google Scholar] [PubMed]
- De B Machado, J.; Plinio Filho, V.M.; Petersen, G.O.; Chatkin, J.M. Quantitative effects of tobacco smoking exposure on the maternal-fetal circulation. BMC Pregnancy Childbirth 2011, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Zhou, L.N.; Li, Z.; Zhang, T.; Liu, W.P.; Liu, Z.; Yuan, Y.T.C.; Su, F.; Xu, L.; Wang, Y.; et al. YY1 suppresses FEN1 over-expression and drug resistance in breast cancer. BMC Cancer 2015, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Saeki, N.; Ono, H.; Yanagihara, K.; Aoyagi, K.; Sasaki, H.; Sakamoto, H.; Yoshida, T. RS2294008T, a risk allele for gastric and gallbladder cancers, suppresses the PSCA promoter by recruiting the transcription factor YY1. Genes Cells 2015, 20, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Choi, Y.H.; Lee, S.H.; Jin, Y.H.; Cheong, H.; Lee, K.Y. Yin Yang 1 is a multi-functional regulator of adipocyte differentiation in 3T3-L1 cells. Mol. Cell. Endocrinol. 2015, 413, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yao, H.W.; Lin, X.; Xu, H.D.; Dean, D.; Zhu, Z.; Liu, G.; Sime, P. IL-13 Induces YY1 through the AKT Pathway in Lung Fibroblasts. PLoS ONE 2015, 10, e0119039. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.M.; Hao, A.X.; Zhang, Q.; Sui, G.C. The Role of YY1 in Oncogenesis and Its Potential as a Drug Target in Cancer Therapies. Curr. Cancer Drug Targets 2015, 15, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Z.; Goff, S.P. Regulation of Yin Yang 1 by Tyrosine Phosphorylation. J. Biol. Chem. 2015, 290, 21890–21900. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.K.; Clark, B.J. Angiotensin II-Dependent Transcriptional Activation of Human Steroidogenic Acute Regulatory Protein Gene by a 25-kDa cAMP-Responsive Element Modulator Protein Isoform and Yin Yang 1. Endocrinology 2012, 153, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- LaVoie, H.A. Epigenetic control of ovarian function: The emerging role of histone modifications. Mol. Cell. Endocrinol. 2005, 243, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Arguelles, D.B.; Papadopoulos, V. Epigenetic regulation of the expression of genes involved in steroid hormone biosynthesis and action. Steroids 2010, 75, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, H.; Christenson, L.K.; Chang, L.; Sammel, M.D.; Berger, S.L.; Strauss, J.F. Temporal and spatial changes in transcription factor binding and histone modifications at the steroidogenic acute regulatory protein (StAR) locus associated with StAR transcription. Mol. Endocrinol. 2004, 18, 791–806. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Asada, H.; Kizuka, F.; Tamura, I.; Maekawa, R.; Taketani, T.; Sato, S.; Yamagata, Y.; Tamura, H.; Sugino, N. Changes in histone modification and DNA methylation of the StAR and Cyp19a1 promoter regions in granulosa cells undergoing luteinization during ovulation in rats. Endocrinology 2013, 154, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Hankel, I.L.; Hostager, B.S.; Swartzendruber, J.A.; Friedman, A.D.; Brenton, J.L.; Rothman, P.B.; Colgan, J.D. The developmental regulator protein Gon4l associates with protein YY1, co-repressor Sin3a, and histone deacetylase 1 and mediates transcriptional repression. J. Biol. Chem. 2011, 286, 18311–18319. [Google Scholar] [CrossRef] [PubMed]
- Luke, M.P.; Sui, G.; Liu, H.; Shi, Y. Yin Yang 1 physically interacts with Hoxa11 and represses Hoxa11-dependent transcription. J. Biol. Chem. 2006, 281, 33226–33232. [Google Scholar] [CrossRef] [PubMed]
- Sucharov, C.C.; Langer, S.; Bristow, M.; Leinwand, L. Shuttling of HDAC5 in H9C2 cells regulates YY1 function through CaMKIV/PKD and PP2A. Am. J. Physiol. Cell Physiol. 2006, 291, C1029–C1037. [Google Scholar] [CrossRef] [PubMed]
- De Nigris, F.; Botti, C.; Rossiello, R.; Crimi, E.; Sica, V.; Napoli, C. Cooperation between Myc and YY1 provides novel silencing transcriptional targets of α3β1-integrin in tumour cells. Oncogene 2007, 26, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Liang, G.; Yan, Y.E.; He, W.W.; Liu, Y.S.; Chen, L.B.; Magdalou, J.; Wang, H. Nicotine-induced over-exposure to maternal glucocorticoid and activated glucocorticoid metabolism causes hypothalamic-pituitary-adrenal axis-associated neuroendocrine metabolic alterations in fetal rats. Toxicol. Lett. 2012, 209, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Ozerdem, U.; Wojcik, E.M.; Barkan, G.A.; Duan, X.; Ersahin, C. A practical application of quantitative vascular image analysis in breast pathology. Pathol. Res. Pract. 2013, 209, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Son, H.J.; Kim, J.Y.; Hahn, Y.; Seo, S.B. Negative regulation of JAK2 by H3K9 methyltransferase G9a in leukemia. Mol. Cell. Biol. 2012, 32, 3681–3694. [Google Scholar] [CrossRef] [PubMed]
| Genes | Forward Primer | Reverse Primer | Product (bp) | Annealing |
|---|---|---|---|---|
| Rat StAR | GGGAGATGCCTGAGCAAAGC | GCTGGCGAACTCTATCTGGGT | 188 | 65 °C, 20 s |
| Rat YY1 | CAGAAGCAGGTGCAGATCAAGAC | CCCTGAACATCTTTGTGCAGCC | 307 | 68 °C, 30 s |
| Rat GAPDH | GCAAGTTCAACGGCACAG | GCCAGTAGACTCCACGACA | 107 | 63 °C, 30 s |
| Human StAR | GATTTTGCCAACCACCTGC | GGATTCTCCTGATGAGCGTGT | 127 | 60 °C, 25 s |
| Human YY1 | CAGAAGCAGGTGCAGATCAAGAC | CCCTGAACATCTTTGTGCAGCC | 307 | 68 °C, 30 s |
| Human GAPDH | GAAATCCCATCACCATCTTCCAG | ATGAGTCCTTCCACGATACCAAAG | 313 | 59 °C, 15 s |
| Genes | Forward Primer | Reverse Primer | Product (bp) | Annealing |
|---|---|---|---|---|
| Rat StAR-1 (−278~−49) | AATGCTGAACCTGGAGCTTG | GGAAGGCTGTGCATCATCAC | 229 bp | 63 °C, 30 s |
| Rat StAR-2 (−438~−222) | TGTCTGTTCCTGGGAGAGTTG | TCCAAAGTTTTAAATGGAGAC | 216 bp | 58 °C, 30 s |
| Rat StAR-3 (−866~−636) | CACAGGTCAAGTCCTGTAGC | CTTGGTTCCTTCCACATGTG | 230 bp | 63 °C, 30 s |
| Rat YY1-1 (−474~−350) | CCAGAACAGCGAGTGGGA | AACTCCTCAACCCGAGCC | 124 bp | 61 °C, 30 s |
| Rat YY1-2 (−373~−215) | ATCTGGGCTCGGGTTGAG | CCGCTGCTTGTCCTGCTC | 158 bp | 64 °C, 30 s |
| Human StAR (−278~−141) | AAACGGCCAAAGCAGCAGTGTGAGG | GACCTGGTGTTCTGCGTCTT | 59 bp | 63 °C, 30 s |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Wang, J.-F.; Fan, J.; Rao, Y.-S.; Liu, F.; Yan, Y.-E.; Wang, H. Nicotine Suppressed Fetal Adrenal StAR Expression via YY1 Mediated-Histone Deacetylation Modification Mechanism. Int. J. Mol. Sci. 2016, 17, 1477. https://doi.org/10.3390/ijms17091477
Liu L, Wang J-F, Fan J, Rao Y-S, Liu F, Yan Y-E, Wang H. Nicotine Suppressed Fetal Adrenal StAR Expression via YY1 Mediated-Histone Deacetylation Modification Mechanism. International Journal of Molecular Sciences. 2016; 17(9):1477. https://doi.org/10.3390/ijms17091477
Chicago/Turabian StyleLiu, Lian, Jian-Fei Wang, Jie Fan, Yi-Song Rao, Fang Liu, You-E Yan, and Hui Wang. 2016. "Nicotine Suppressed Fetal Adrenal StAR Expression via YY1 Mediated-Histone Deacetylation Modification Mechanism" International Journal of Molecular Sciences 17, no. 9: 1477. https://doi.org/10.3390/ijms17091477
APA StyleLiu, L., Wang, J.-F., Fan, J., Rao, Y.-S., Liu, F., Yan, Y.-E., & Wang, H. (2016). Nicotine Suppressed Fetal Adrenal StAR Expression via YY1 Mediated-Histone Deacetylation Modification Mechanism. International Journal of Molecular Sciences, 17(9), 1477. https://doi.org/10.3390/ijms17091477