Extraction, Chemical Composition, and Antifungal Activity of Essential Oil of Bitter Almond
Abstract
:1. Introduction
2. Results and Discussion
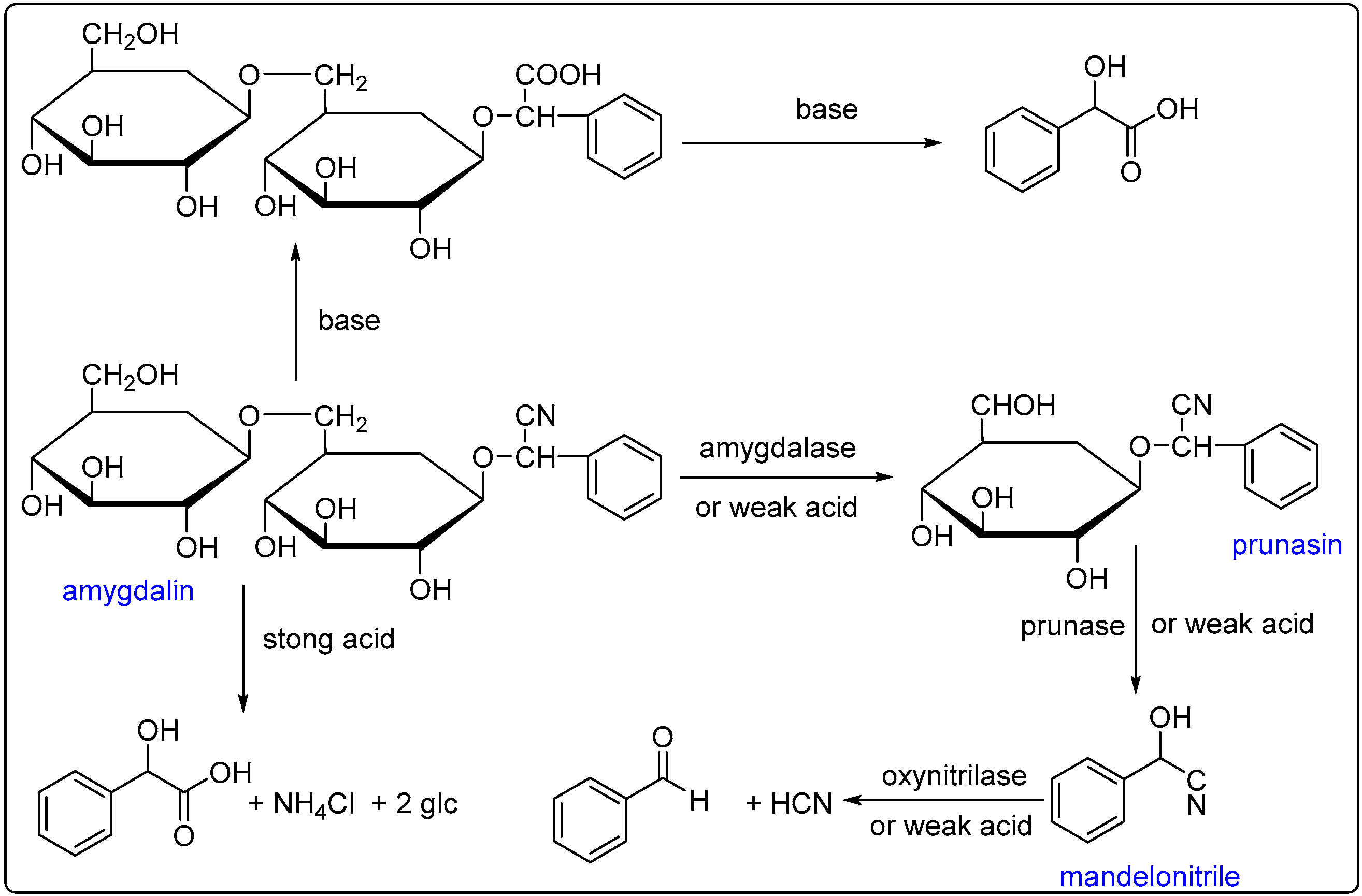
2.1. Extraction of BAEO
2.2. Chemical Composition of BAEO
2.3. The in Vitro Antifungal Activity
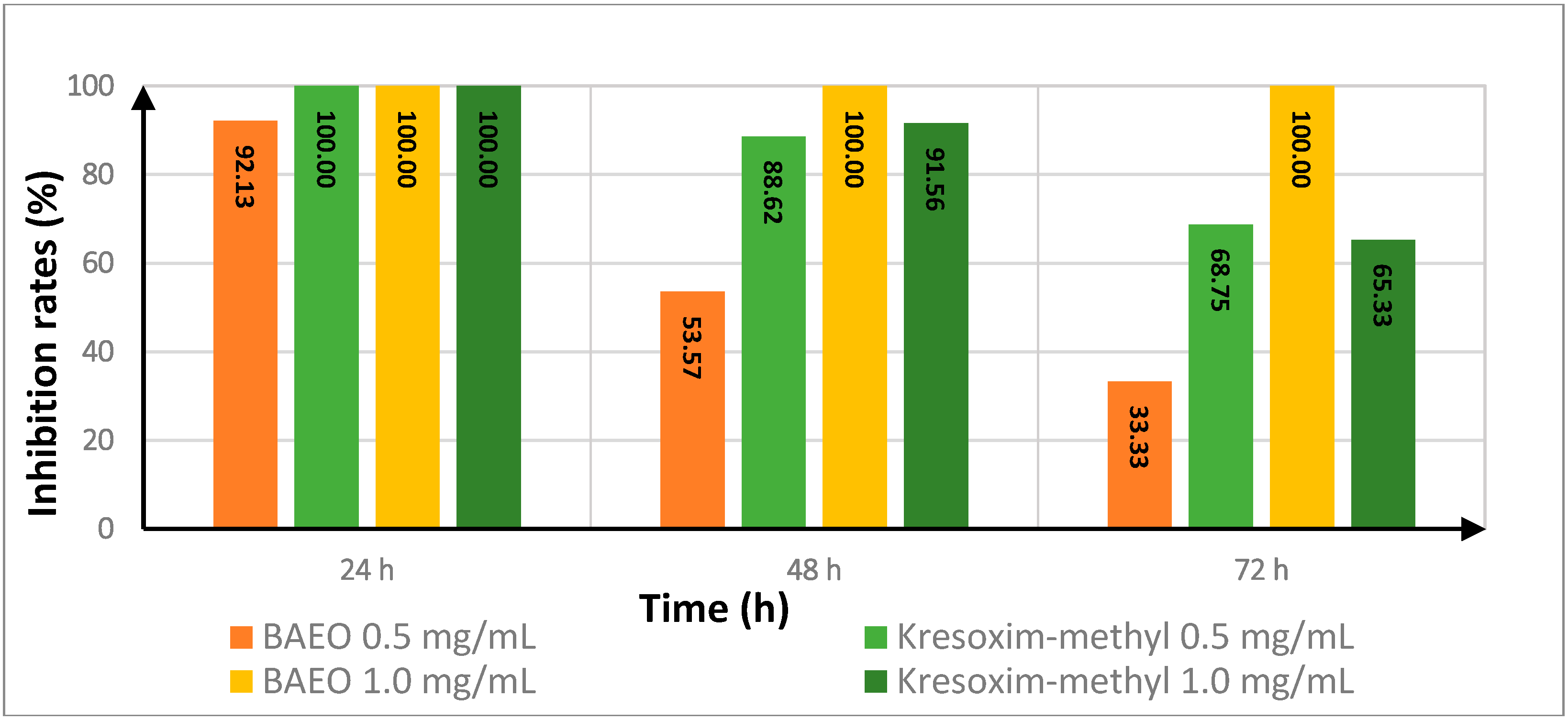
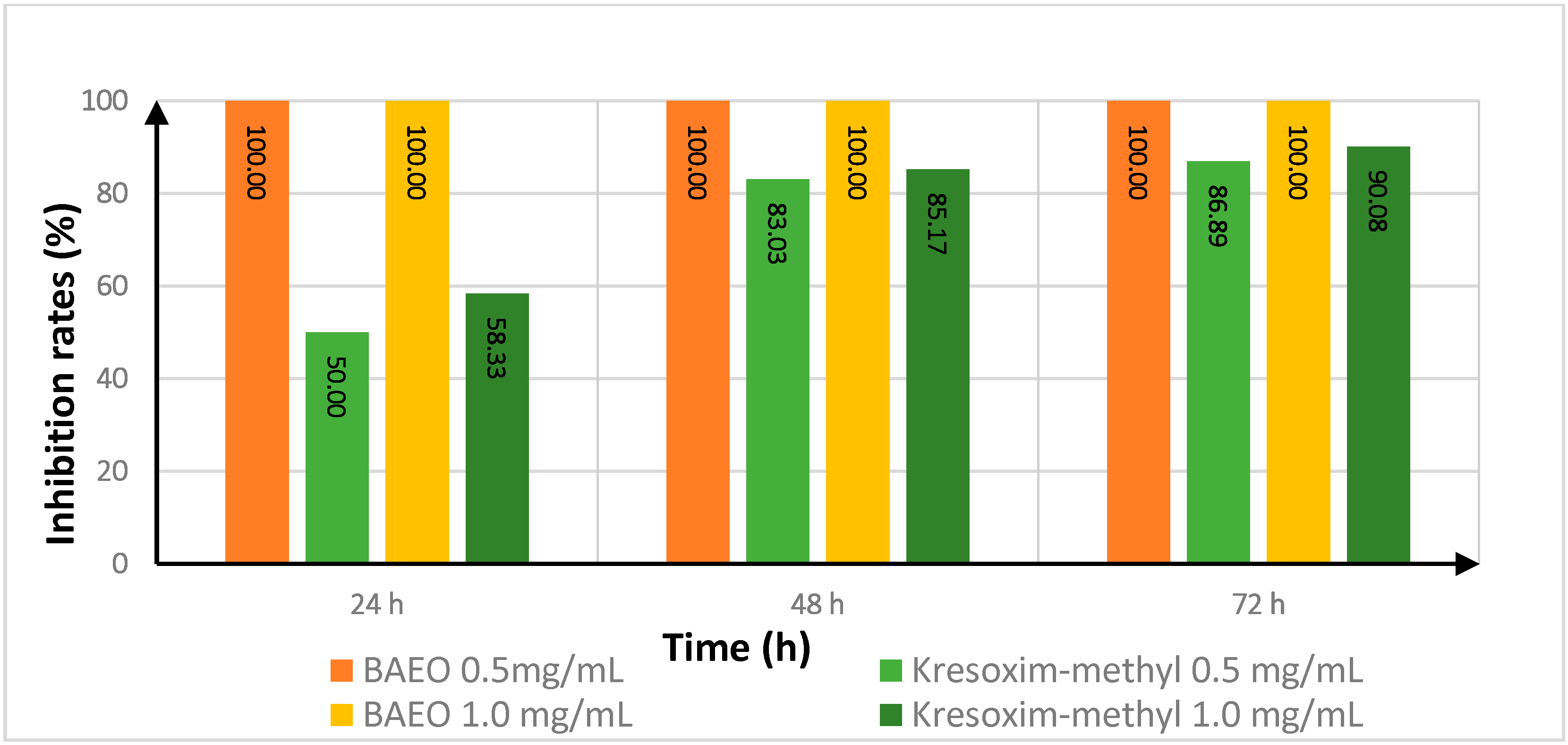
2.4. Effect of Treatment Time on the In Vitro Antifungal Activity of BAEO
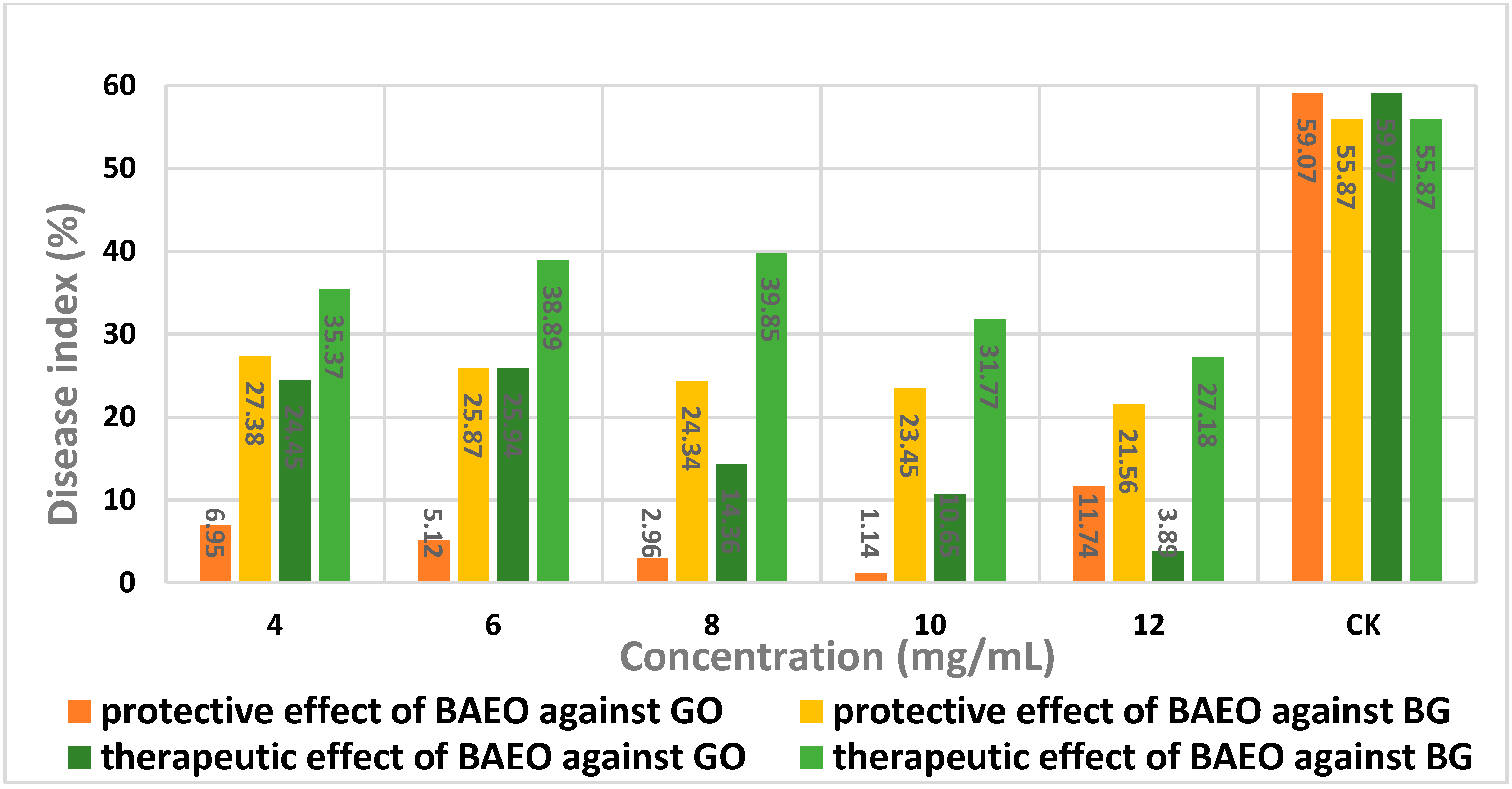
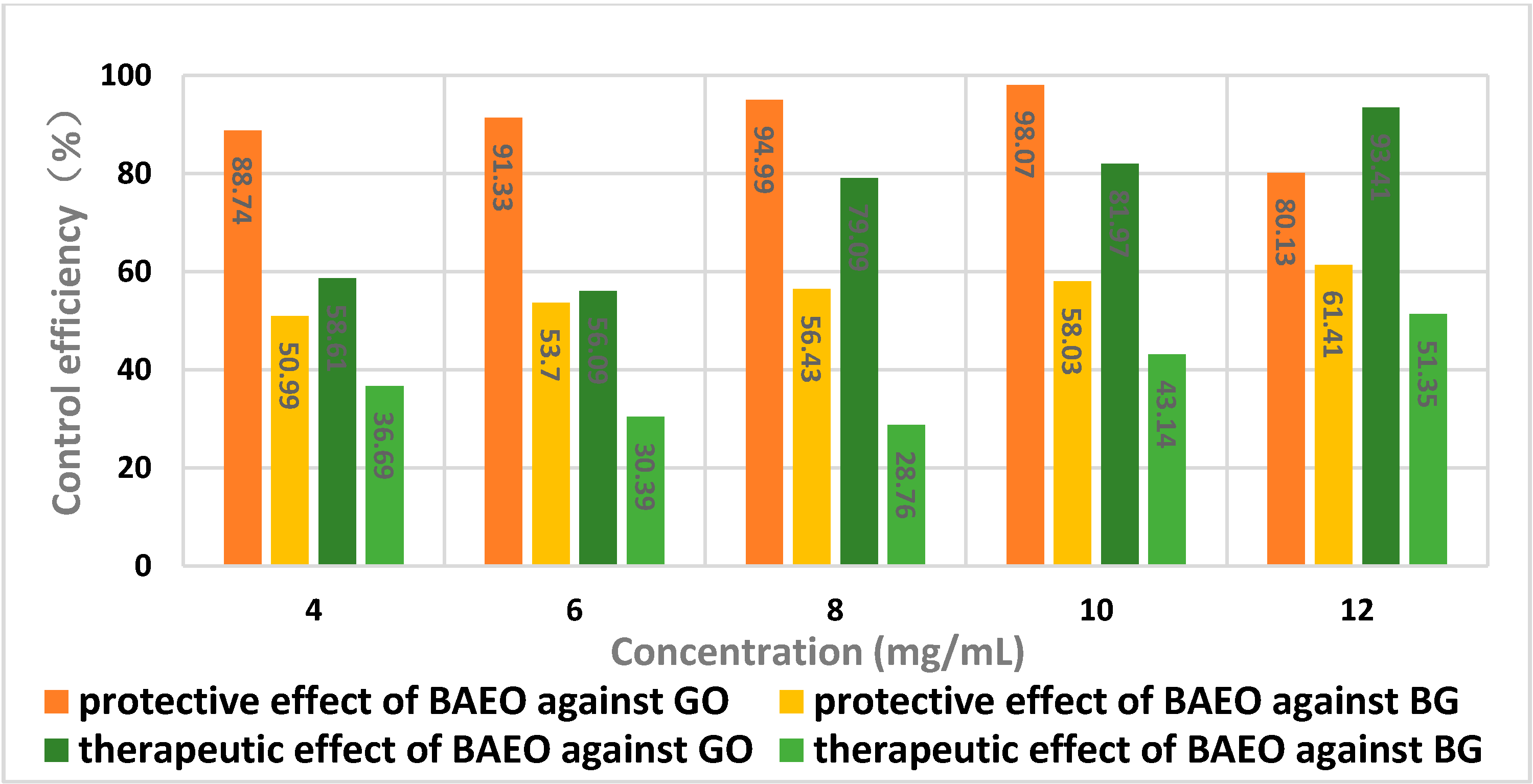
2.5. In Vivo Antifungal Activity
3. Materials and Methods
3.1. General
3.2. Materials
3.3. Extraction of BAEO
3.4. Analysis of the Chemical Composition of BAEO
3.5. Assay of the in Vitro Antifungal Activity of BAEO
3.6. Assay of in Vivo Antifungal Activity of BAEO by Pot Test
3.6.1. Assay the Control Effect of BAEO against Gloeosporium orbiculare
- Level 0: The area of disease spots is zero.
- Level 1: The area of disease spots is below 5% of the total area of infected leaf.
- Level 3: The area of disease spots is 6%~10% of the total area of infected leaf.
- Level 5: The area of disease spots is 11%~25% of the total area of infected leaf.
- Level 7: The area of disease spots is 26%~50% of the total area of infected leaf.
- Level 9: The area of disease spots is above 50% of the total area of infected leaf.
3.6.2. Assay of the Control Effect of BAEO against Blumeria graminis f. sp. tritici
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Agrios, G.N. Significance of Plant Diseases; Plant Pathology; Academic Press: London, UK, 2000; pp. 25–37. [Google Scholar]
- Lu, A.; Ma, Y.; Wang, Z.; Zhou, Z.; Wang, Q. Application of “hydrogen-bonding interaction” in drug design. Part 2: Design, synthesis, and structure—Activity relationships of thiophosphoramide derivatives as novel antiviral and antifungal agents. J. Agric. Food Chem. 2015, 63, 9435–9440. [Google Scholar] [CrossRef] [PubMed]
- Bräse, S.; Encinas, A.; Keck, J.; Nising, C.F. Chemistry and biology of mycotoxins and related fungal metabolites. Chem. Rev. 2009, 109, 3903–3990. [Google Scholar] [CrossRef] [PubMed]
- Brent, K.J.; Hollomon, D.W. Fungicide Resistance: The Assessment of Risk; FRAC, Global Crop Protection Federation: Brussels, Belgium, 1998; pp. 1–48. [Google Scholar]
- Ormancey, X.; Sisalli, S.; Coutiere, P. Formulation of essential oils in functional perfumery. Perfum. Cosmet. Actual. 2001, 157, 30–40. [Google Scholar]
- Atiqur, R.; Sharif, M.A.; Sun, C.K. Antifungal activity of essential oil and extracts of Piper chaba hunter against phytopathogenic fungi. J. Am. Oil Chem. Soc. 2011, 88, 573–579. [Google Scholar]
- Amiri, A.; Dugas, R.; Pichot, A.L.; Bompeix, G. In vitro and in vitro activity of eugenol oil (Eugenia caryophylata) against four important postharvest apple pathogens. Int. J. Food Microbiol. 2008, 126, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Dikbas, N.; Kotan, R.; Dadasoglu, F.; Sahin, F. Control of Aspergillus flavus with essential oil and methanol extract of Satureja. hortensis. Int. J. Food Microbiol. 2008, 124, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.K.; Kumar, R.; Chansouria, J.P.N.; Dubey, N.K. Evaluation of Amomum subulatum Roxb oil as a source of botanical fungitoxicant for the protection of mango fruits from fungal rotting. J. Food Saf. 2008, 28, 400–412. [Google Scholar] [CrossRef]
- Tzortzakis, N.G. Impact of cinnamon oil-enrichment on microbial spoilage of fresh produce. Innov.Food Sci. Emerg. Technol. 2009, 10, 97–102. [Google Scholar] [CrossRef]
- Tzortzakis, N.G.; Economakis, C.D. Antifungal activity of lemongrass (Cympopogon citratus L.) essential oil against key postharvest pathogens. Innov. Food Sci. Emerg. Technol. 2007, 8, 253–258. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, Z.; Li, K.; Ma, X.; Guo, C.; Wei, L. Oil content and composition of almond from different producing area. J. Chin. Cereals Oils Assoc. 2009, 24, 70–73. [Google Scholar]
- Li, K.; Shi, Q.; Zhu, H.; Tang, D. Chemical compositions in bitter almond. J. Northwest For. Univ. 2004, 19, 124–126. [Google Scholar]
- Li, K.; Shi, Q.; Zhu, H.; Tang, D. Study on main nutrient composition of bitter almond. Acta Agric. Boreal. Occident. Sin. 2003, 12, 119–121. [Google Scholar]
- Sun, J.; Feng, X.; Zhang, X. Studies of the properties and constituents of almond oil. J. Inn. Mong. Inst. Agric. Anim. Husb. 1994, 15, 123–124. [Google Scholar]
- Ma, Y.; Zhao, Z.; Li, K.; Ma, X.; Shi, Q.; Zhu, H. Physicochemical property and fatty acid composition of bitter almond oil. J. Chin. Cereals Oils Assoc. 2008, 23, 99–102. [Google Scholar]
- Li, K.; Shi, Q.; Zhu, H.; Tang, D. Comprehensive exploitation and utilization of bitter almond. J. Northwest For. Univ. 2003, 18, 63–65. [Google Scholar]
- Lim, T.K. Prunus armeniaca. In Edible Medicinal and Non-Medicinal Plants; Lim, T.K., Ed.; Springer: New York, NY, USA, 2012; pp. 442–450. [Google Scholar]
- Mehdi, A.; Hamid-Reza, K. Characterization and transesterification of Iranian bitter almond oil for biodiesel production. Appl. Energy 2011, 88, 2377–2381. [Google Scholar]
- Korekar, G.; Stobdan, T.; Arora, R.; Yadav, A.; Singh, S.B. Antioxidant capacity and phenolics content of apricot (Prunus armeniaca L.) kernel as a function of genotype. Plant Foods Hum. Nutr. 2011, 66, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Ghazavi, A.; Abtahi, H.; Karimi, M.; Mollaghasemi, S.; Mosayebi, G. Antimicrobial activities of water and methanol extracts of bitter apricot seeds. J. Med. Sci. 2008, 8, 433–436. [Google Scholar] [CrossRef]
- Do, J.S.; Hwang, J.K.; Seo, H.J.; Woo, W.H.; Nam, S.Y. Antiasthmatic activity and selective inhibition of type 2 helper T cell response by aqueous extract of semen Armeniacae amarum. Immunopharmacol. Immunotoxicol. 2006, 28, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.K.; Shin, M.S.; Yang, H.Y. Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expressions in human DU145 and LNCaP prostate cancer cells. Biol. Pharm. Bull. 2006, 29, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.K.; Yang, H.Y.; Lee, T.H. Armeniacae semen extract suppresses lipopolysaccharide-induced expressions of cyclooxygenase-2 and inducible nitric oxide synthase in mouse BV2 microglial cells. Biol. Pharm. Bull. 2005, 28, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Bensky, D.; Clavey, S.; Stöger, E. Chinese Herbal Medicine: Materia Medica, 3rd ed.; Eastland Press: Seattle, WA, USA, 2004; pp. 437–440. [Google Scholar]
- Femenia, A.; Rossello, C.; Mulet, A.; Canellas, J. Chemical composition of bitter and sweet apricot kernels. J. Agric. Food Chem. 1995, 43, 356–361. [Google Scholar] [CrossRef]
- Brühne, F.; Wright, E. Benzaldehyde. In Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; Wiley-VCH: Weinheim, Germany, 2007; p. 11. [Google Scholar]
- Lee, H.; Ahn, J.; Kwon, A.; Lee, E.S.; Kwak, J.; Min, Y. Chemical composition and antimicrobial activity of the essential oil of apricot seed. Phytother. Res. 2014, 28, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Gupta, A.; Kaundal, K.; Verma, A.K. Antifungal property of essential oil extracted from bitter apricot kernel press cake. Indian Perfum. 2013, 57, 27–31. [Google Scholar]
- Yang, R.; Gao, Z.-F.; Zhao, J.-Y.; Li, W.-B.; Zhou, L.; Miao, F. New class of 2-aryl-6-chloro-3,4-dihydroiso-quinolinium salts as potential antifungal agents for plant protection: Synthesis, bioactivity and structure-activity relationships. J. Agric. Food Chem. 2015, 63, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G. Study on the Chemical Prevention and dsRNA of Gaeumannomyces graminsis. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2013. [Google Scholar]
- Fang, Z.-D. Methodology for Plant Pathology; China Agricultural Press: Beijing, China, 1998; pp. 8–11. [Google Scholar]
| Compound | TR (min) a | Formula | CAS Number | Relative Content (%) b | SI (min) c |
|---|---|---|---|---|---|
| ethylbenzene | 4.495 | C8H10 | 100-41-4 | 0.69 | 93 |
| 1,2-xylene | 4.560 | C8H10 | 95-47-6 | 0.18 | 89 |
| 1,3-xylene | 4.720 | C8H10 | 108-38-3 | 1.56 | 95 |
| 2-methylundecane | 4.865 | C12H26 | 7045-71-8 | 0.26 | 92 |
| tetradecane | 5.040 | C14H30 | 629-59-4 | 0.61 | 93 |
| 1,4-xylene | 5.405 | C8H10 | 106-42-3 | 0.90 | 95 |
| dodecane | 5.585 | C12H36 | 112-40-3 | 1.50 | 95 |
| 1-ethyl-3-methyl-benzene | 6.145 | C9H12 | 620-14-4 | 1.61 | 84 |
| hexadecane | 6.345 | C16H34 | 544-76-3 | 3.97 | 94 |
| mesitylene | 7.275 | C9H12 | 108-67-8 | 1.62 | 94 |
| 1,2,4-trimethylbenzene | 7.320 | C9H12 | 95-63-6 | 0.23 | 88 |
| eicosane | 10.600 | C20H42 | 112-95-8 | 2.26 | 94 |
| heptadecane | 10.705 | C17H36 | 629-78-7 | 0.39 | 94 |
| benzaldehyde | 11.955 | C7H6O | 100-52-7 | 62.52 | 98 |
| dodecyl aldehyde | 14.730 | C12H24O | 112-54-9 | 1.18 | 92 |
| 3-(hydroxy-phenyl-methyl)-2,3-dimethyl-octan-4-one | 16.025 | C17H26O2 | 911800-56-1 | 1.23 | 84 |
| benzyl alcohol | 16.865 | C7H8O | 100-51-6 | 1.59 | 87 |
| benzyl cyanide | 17.505 | C8H7N | 140-29-4 | 1.06 | 92 |
| 3-ethyl-2-undecanone | 19.995 | C13H26O | 328078-05-3 | 0.50 | 83 |
| 3,5-di-tert-butylphenol | 21.335 | C14H22O | 1138-52-9 | 1.24 | 82 |
| benzoic acid | 22.835 | C7H6O2 | 65-85-0 | 14.80 | 94 |
| Fungi | Average Inhibition Rate ± SD (%) (n = 3) | ||
|---|---|---|---|
| BAEO | Benzaldehyde | Kresoxim-Methyl | |
| FOC | 68.3 ± 0.9a | 62.3 ± 0.5b | 67.2 ± 0.9a |
| VM | 50.5 ± 3.0b | 51.4 ± 0.0b | 81.0 ± 1.6a |
| PO | 95.3 ± 0.6a | 54.7 ± 2.3c | 82.2 ± 0.2b |
| FG | 87.0 ± 0.3b | 81.4 ± 0.4c | 93.2 ± 2.3a |
| AA | 80.1 ± 0.2c | 86.7 ± 0.1b | 93.4 ± 0.1a |
| AS | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 |
| PC | 49.2 ± 2.7b | 39.2 ± 0.9c | 85.1 ± 0.4a |
| GF | 91.9 ± 2.0b | 100.0 ± 0.0a | 100.0 ± 0.0a |
| FOL | 49.5 ± 0.8b | 35.5 ± 0.6c | 88.8 ± 0.2a |
| GO | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 |
| VD | 72.5 ± 0.8b | 64.2 ± 0.6c | 82.3 ± 0.1a |
| GG | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 |
| BC | 100.0 ± 0.0a | 100.0 ± 0.0a | 88.8 ± 2.1b |
| FOV | 81.5 ± 0.0 | 40.7 ± 0.0 | 63.0 ± 0.0 |
| CL | 88.7 ± 0.1b | 85.8 ± 0.1c | 94.3 ± 0.0a |
| FOS | 46.7 ± 1.7c | 74.7 ± 0.8b | 81.2 ± 0.5a |
| CG | 44.8 ± 0.6c | 55.8 ± 0.5b | 100.0 ± 0.0a |
| FON | 56.4 ± 0.4c | 83.2 ± 0.2b | 100.0 ± 0.0a |
| AB | 100.0 ± 0.0a | 28.6 ± 0.0c | 83.2 ± 0.8b |
| Fungi | Toxicity Regression Equation a | R2 | EC50 (μg/mL) | 95% CI of LC50 b |
|---|---|---|---|---|
| FOC | y = 1.4963x + 0.9404 | 0.98 | 511.7 | 451.4–579.9 |
| VM | y = 2.4458x − 1.7149 | 0.93 | 610.8 | 531.4–702.1 |
| PO | y = 4.0988x − 5.7808 | 0.98 | 429.3 | 408.7– 450.9 |
| FG | y = 4.3710x − 7.1765 | 0.97 | 627.9 | 579.0–680.8 |
| AA | y = 2.4905x − 1.9416 | 0.96 | 642.0 | 582.6–707.5 |
| AS | y = 1.4637x + 2.1046 | 0.96 | 103.2 | 73.4–144.9 |
| PC | y = 1.5343x + 0.7563 | 0.92 | 600.5 | 376.0–959.2 |
| GF | y = 2.3698x − 0.4715 | 0.93 | 225.9 | 196.7–259.5 |
| FOL | y = 1.0912x + 2.2974 | 0.92 | 295.1 | 229.1–379.9 |
| GO | y = 2.0324x + 0.3784 | 0.94 | 273.7 | 230.5–325.0 |
| VD | y = 1.9931x − 0.0070 | 0.93 | 325.2 | 0.3082–0.5464 |
| GG | y = 0.9111x + 2.9284 | 0.98 | 192.0 | 104.2–353.7 |
| BC | y = 2.0393x + 0.5824 | 0.99 | 217.0 | 155.2–303.4 |
| FOV | y = 2.2360x − 1.0803 | 0.95 | 526.7 | 491.1–564.8 |
| CL | y = 1.8933x − 0.0960 | 0.97 | 509.5 | 458.2–566.5 |
| FOS | y = 2.8836x − 2.4579 | 0.93 | 423.8 | 363.8–493.8 |
| CG | y = 1.2270x + 1.8435 | 0.98 | 381.8 | 353.9–411.8 |
| FON | y = 2.2968x − 1.2753 | 0.92 | 569.3 | 490.9–660.2 |
| AB | y = 0.8082x + 3.6361 | 0.96 | 50.2 | 42.4–59.3 |
| Fungi | Toxicity Regression Equation a | R2 | EC50 (μg/mL) | 95% CI of LC50 b |
|---|---|---|---|---|
| FOC | y = 0.5028x + 4.4763 | 0.95 | 10.89 | 8.50–13.97 |
| VM | y = 0.5030x + 3.9375 | 0.96 | 121.20 | 119.54–120.54 |
| PO | y = 0.2362x + 4.5941 | 0.97 | 51.85 | 49.31–52.06 |
| FG | y = 0.2814x + 4.8887 | 0.98 | 2.46 | 1.40–5.59 |
| AA | y = 0.2910x + 4.7420 | 0.95 | 7.737 | 6.28–9.54 |
| AS | y = 1.3929x + 4.4564 | 0.96 | 2.27 | 1.95–2.65 |
| PC | y = 0.3661x + 5.0734 | 0.95 | 0.68 | 0.57–0.85 |
| GF | y = 0.7777x + 4.4152 | 0.95 | 5.87 | 4.60–6.43 |
| FOL | y = 0.4702x + 5.0878 | 0.97 | 0.71 | 0.54–0.93 |
| GO | y = 0.6174x + 4.5918 | 0.96 | 4.60 | 3.96–5.34 |
| VD | y = 2.3929x +5.4564 | 0.96 | 3.78 | 2.95–4.65 |
| GG | - | - | 6.26 c | - |
| BC | y = 0.5430x + 3.8613 | 0.95 | 132.3 | 129.81–135.30 |
| FOV | y = 0.3288x + 4.5625 | 0.97 | 21.50 | 20.34–25.19 |
| CL | y = 0.4778x + 4.0928 | 0.96 | 76.06 | 74.73–80.36 |
| FOS | y = 0.4315x + 4.5359 | 0.98 | 11.89 | 10.66–13.25 |
| CG | y = 2.3629x + 2.1090 | 0.98 | 15.45 | 14.06–16.98 |
| FON | y = 0.2123x + 4.6062 | 0.97 | 71.40 | 69.81–75.22 |
| AB | y = 0.4492x + 4.3807 | 0.98 | 24.14 | 21.28–27.38 |
| Fungicide | Treatment Time (h) | Inhibition Rate a | |||
|---|---|---|---|---|---|
| FON | AS | ||||
| 0.5 (mg/mL) | 1.0 (mg/mL) | 0.5 (mg/mL) | 1.0 (mg/mL) | ||
| kresoxim-methyl | 24 | 100.00 | 100.00 | 50.00 | 58.33 |
| 48 | 88.62 | 91.56 | 83.03 | 85.17 | |
| 72 | 68.75 | 65.33 | 86.89 | 90.08 | |
| BAEO | 24 | 92.13 | 100.00 | 100.00 | 100.00 |
| 48 | 53.57 | 100.00 | 100.00 | 100.00 | |
| 72 | 33.33 | 100.00 | 100.00 | 100.00 | |
| Concentration (mg/mL) | Disease Index (%) a | Protective Effect (%) b |
|---|---|---|
| 4 | 6.95 | 88.74 |
| 6 | 5.12 | 91.33 |
| 8 | 2.96 | 94.99 |
| 10 | 1.14 | 98.07 |
| 12 | 11.74 | 80.13 |
| Control | 59.07 | - |
| Concentration (mg/mL) | Disease Index (%) a | Therapeutic Effect (%) b |
|---|---|---|
| 4 | 24.45 | 58.61 |
| 6 | 25.94 | 56.09 |
| 8 | 14.36 | 79.09 |
| 10 | 10.65 | 81.97 |
| 12 | 3.89 | 93.41 |
| Control | 59.07 | - |
| Concentration (mg/mL) | Disease Index (%) a | Protective Effect (%) b |
|---|---|---|
| 4.0 | 27.38 | 50.99 |
| 6.0 | 25.87 | 53.70 |
| 8.0 | 24.34 | 56.43 |
| 10 | 23.45 | 58.03 |
| 12 | 21.56 | 61.41 |
| Control | 55.87 | - |
| Concentration (mg/mL) | Disease Index (%) a | Therapeutic Effect (%) b |
|---|---|---|
| 4.0 | 35.37 | 36.69 |
| 6.0 | 38.89 | 30.39 |
| 8.0 | 39.85 | 28.76 |
| 10 | 31.77 | 43.14 |
| 12 | 27.18 | 51.35 |
| Control | 55.87 | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, H.; Yu, X.; Lu, A.; Cao, H.; Zhou, B.; Zhou, L.; Zhao, Z. Extraction, Chemical Composition, and Antifungal Activity of Essential Oil of Bitter Almond. Int. J. Mol. Sci. 2016, 17, 1421. https://doi.org/10.3390/ijms17091421
Geng H, Yu X, Lu A, Cao H, Zhou B, Zhou L, Zhao Z. Extraction, Chemical Composition, and Antifungal Activity of Essential Oil of Bitter Almond. International Journal of Molecular Sciences. 2016; 17(9):1421. https://doi.org/10.3390/ijms17091421
Chicago/Turabian StyleGeng, Huiling, Xinchi Yu, Ailin Lu, Haoqiang Cao, Bohang Zhou, Le Zhou, and Zhong Zhao. 2016. "Extraction, Chemical Composition, and Antifungal Activity of Essential Oil of Bitter Almond" International Journal of Molecular Sciences 17, no. 9: 1421. https://doi.org/10.3390/ijms17091421
APA StyleGeng, H., Yu, X., Lu, A., Cao, H., Zhou, B., Zhou, L., & Zhao, Z. (2016). Extraction, Chemical Composition, and Antifungal Activity of Essential Oil of Bitter Almond. International Journal of Molecular Sciences, 17(9), 1421. https://doi.org/10.3390/ijms17091421





