Matrine Exerts a Strong Anti-Arthritic Effect on Type II Collagen-Induced Arthritis in Rats by Inhibiting Inflammatory Responses
Abstract
:1. Introduction
2. Results
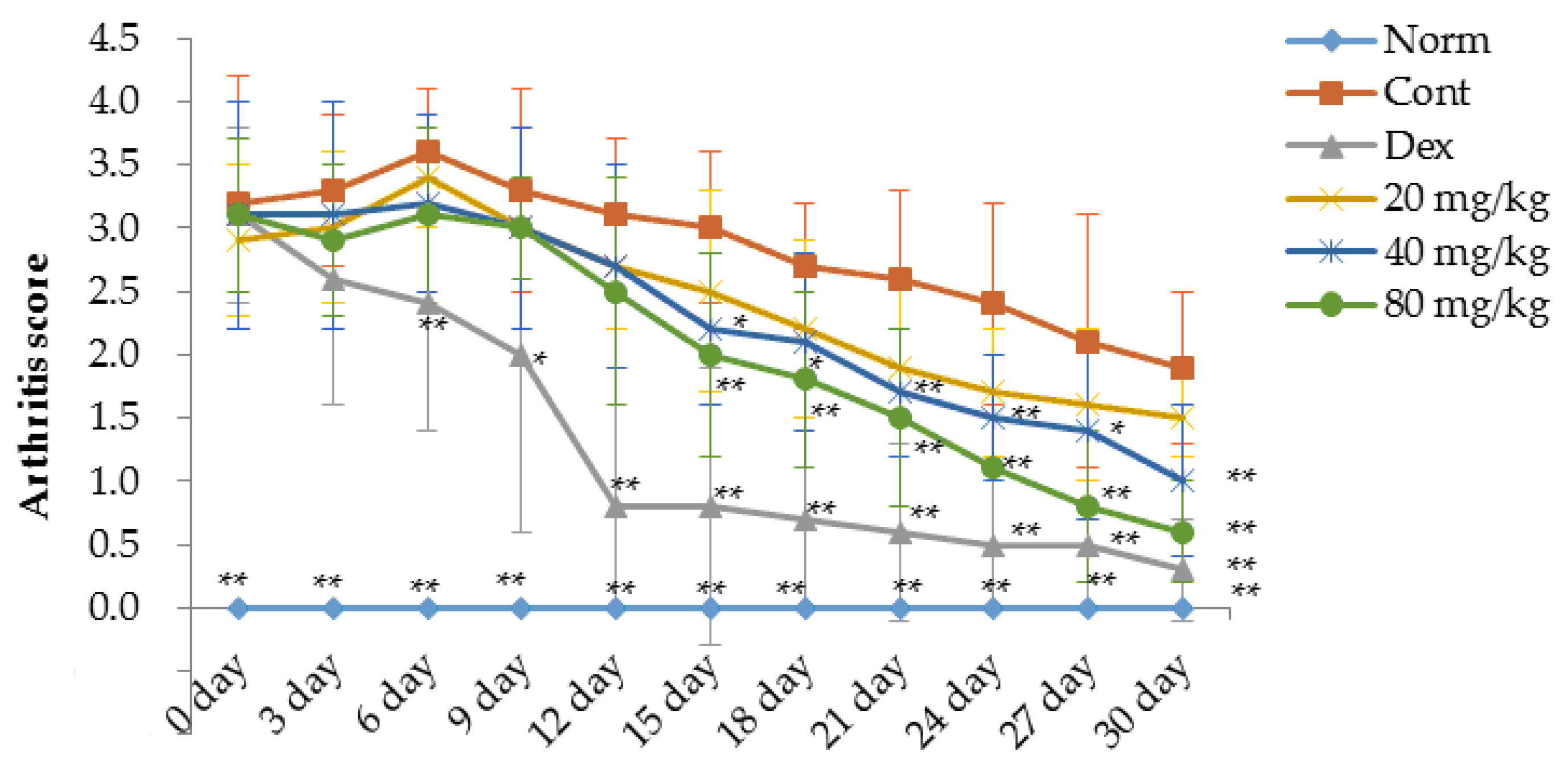
2.1. Matrine Decreases Paw Swelling, Arthritis Indices and Weight Loss in Collagen-Induced Arthritis (CIA) Rats
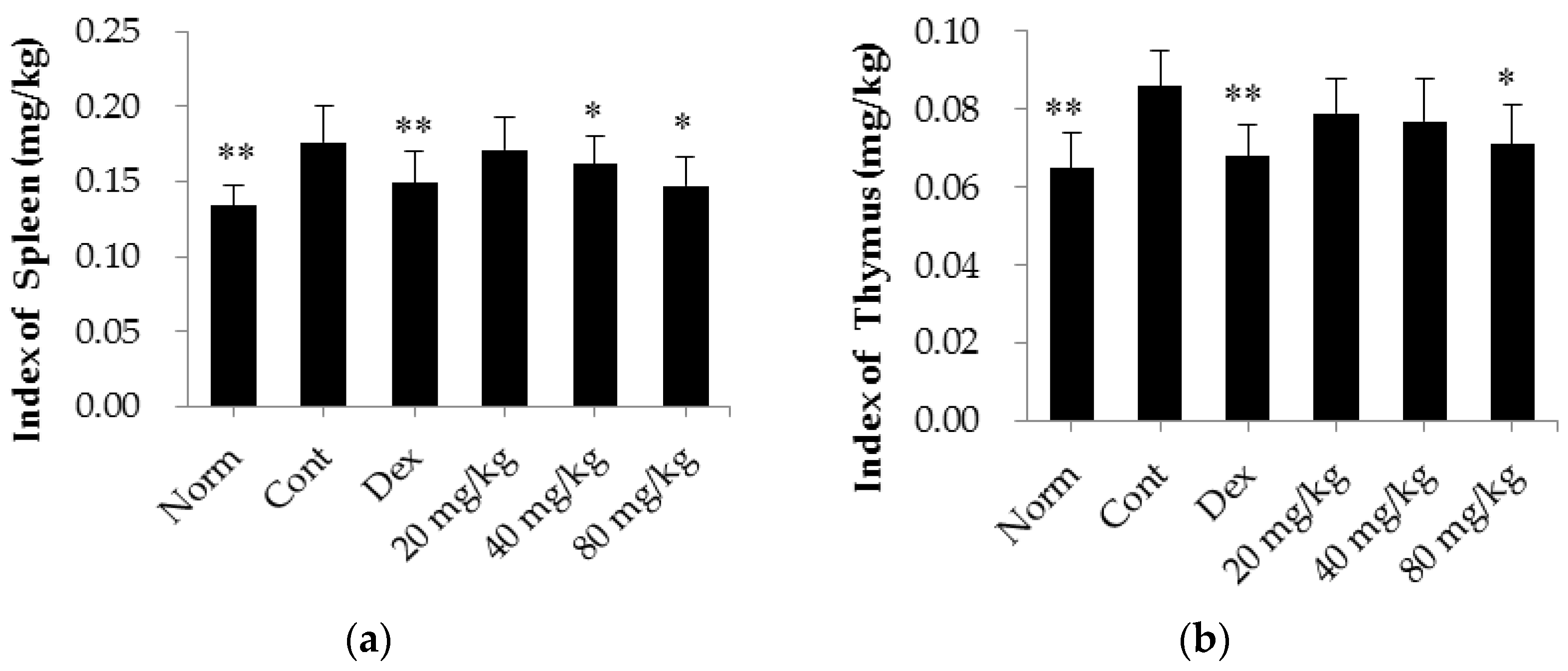
2.2. Matrine Decreases Thymus and Spleen Indices in CIA Rats
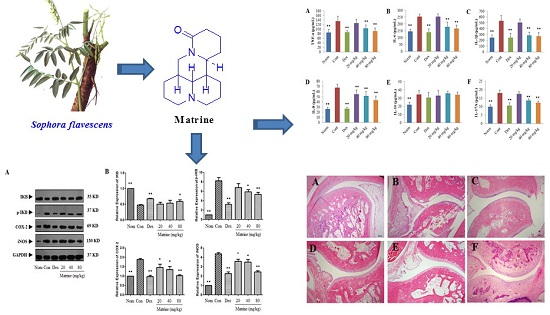
2.3. Matrine Decreases Inflammation as well as Joint and Synovial Tissue Destruction in CIA Rats
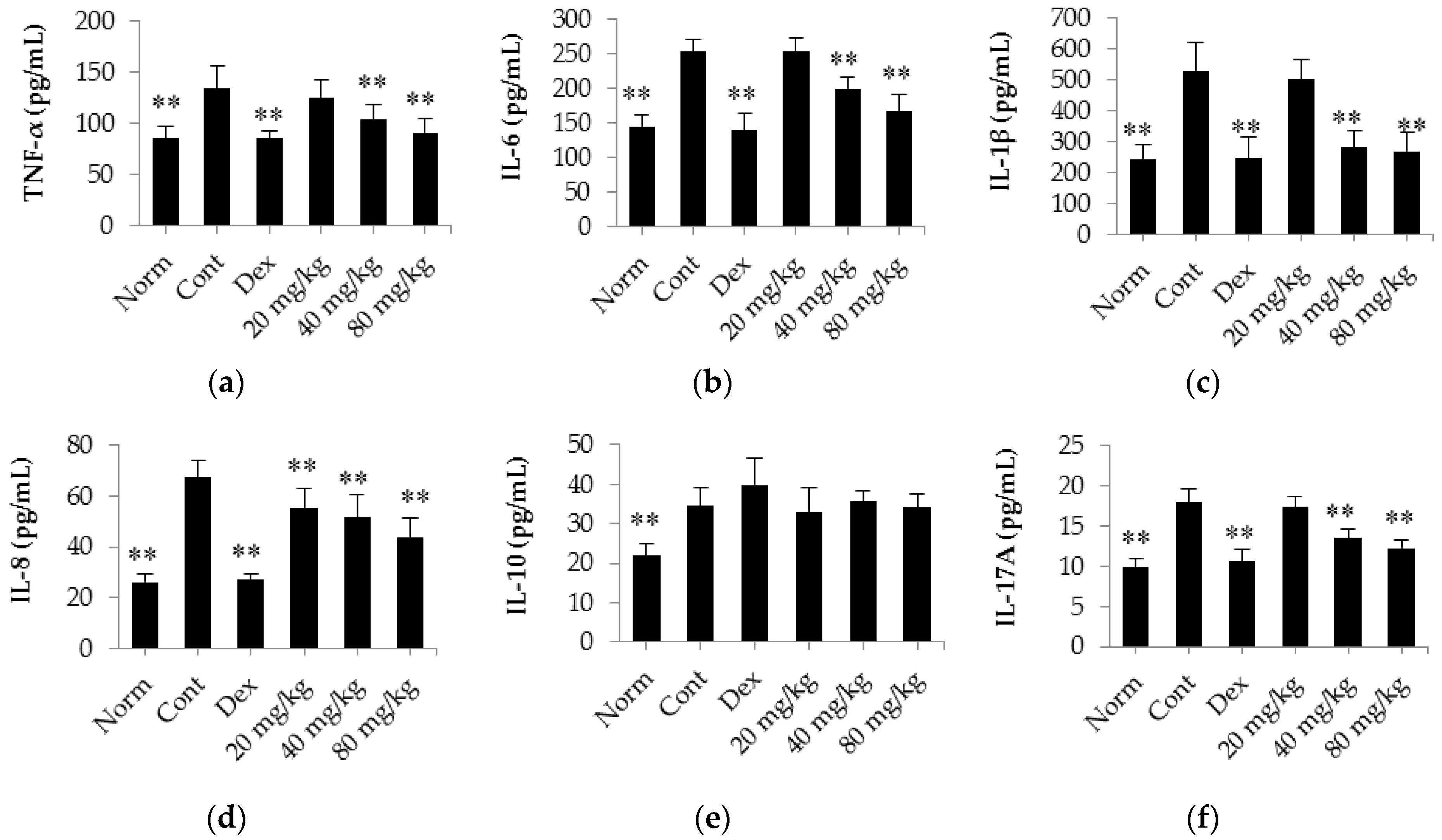
2.4. Matrine Decreases the Release of TNF-α, IL-6, IL-1β, IL-8, IL-17A and IL-10 into the Serum of CIA Rats
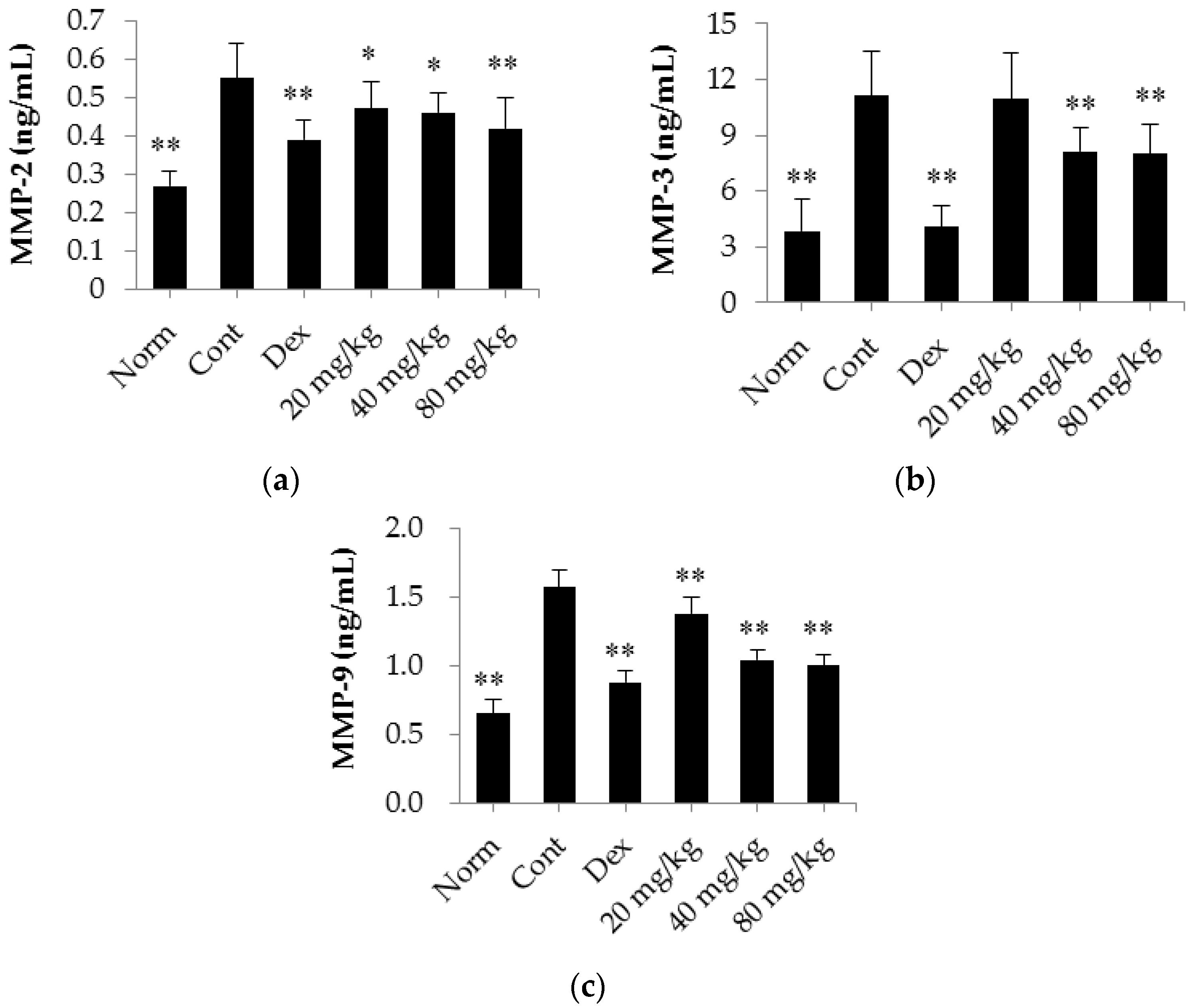
2.5. Matrine Decreases the Serum Levels of MMP-2, MMP-3 and MMP-9 in CIA Rats
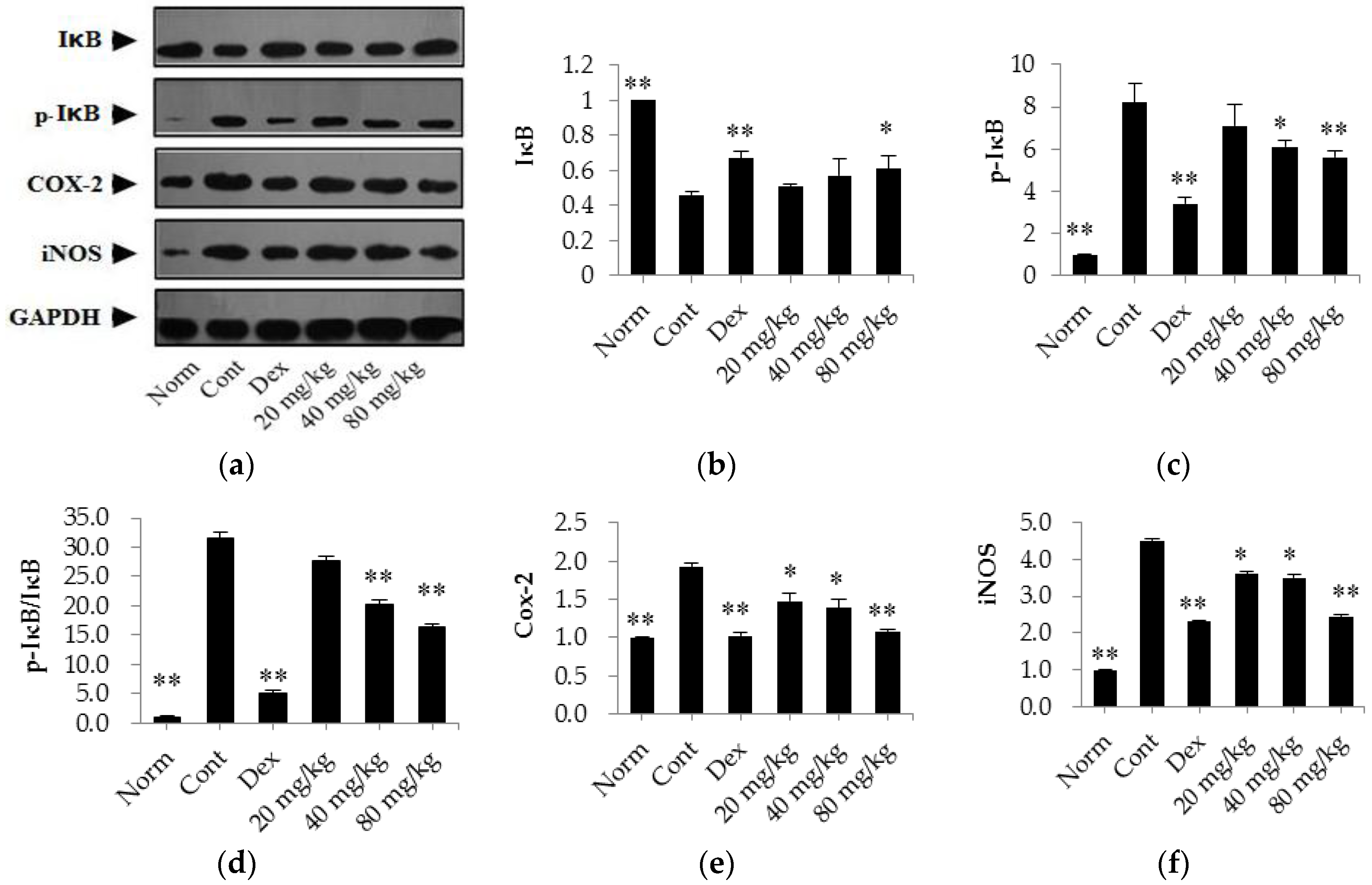
2.6. Matrine Down-Regulates the Expression Levels of p-IκB, Cox-2, and iNOS and Up-Regulates the Expression Level of IκB in the Synovial Tissues of CIA Rats
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Animals
4.3. Chemicals and Reagents
4.4. Preparation of Matrine
4.5. CII-Induced Arthritis (CIA) Animal Model Preparation and Experiment Protocols
4.6. Determination of Serum Cytokine Levels
4.7. Histopathological Examination
4.8. Western Blot Assays
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cunha, B.M.; Mota, L.M.; Pileggi, G.S.; Safe, I.P.; Lacerda, M.V. HIV/AIDS and rheumatoid arthritis. Autoimmun. Rev. 2015, 14, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Imboden, J.B. The immunopathogenesis of rheumatoid arthritis. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Solomon, D.H.; Liu, J.; Franklin, J.M.; Glynn, R.J.; Schneeweiss, S. Risk of venous thromboembolism in patients with rheumatoid arthritis: Initiating disease-modifying antirheumatic drugs. Am. J. Med. 2015, 128, 539.e7–539.e17. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Wang, K.J. Therapeutic effect of captopril on rheumatoid arthritis in rats. Asian Pac. J. Trop. Med. 2014, 7, 996–999. [Google Scholar] [CrossRef]
- Doan, T.; Massarotti, E. Rheumatoid arthritis: An overview of new and emerging therapies. J. Clin. Pharmacol. 2005, 45, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Kremers, H.M.; Nicola, P.; Crowson, C.S.; O’Fallon, W.M.; Gabriel, S.E. Therapeutic strategies in rheumatoid arthritis over a 40-year period. J. Rheumatol. 2004, 31, 2366–2373. [Google Scholar] [PubMed]
- Wang, Q.; Kuang, H.; Su, Y.; Sun, Y.; Feng, J.; Guo, R.; Chan, K. Naturally derived anti-inflammatory compounds from Chinese medicinal plants. J. Ethnopharmacol. 2013, 146, 9–39. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Y.; Hou, J.J.; Long, H.L.; Yang, W.Z.; Liang, J.; Guo, D.A. TCM-based new drug discovery and development in China. Chin. J. Nat. Med. 2014, 12, 241–250. [Google Scholar] [CrossRef]
- Zheng, C.J.; Zhao, X.X.; Ai, H.W.; Lin, B.; Han, T.; Jiang, Y.P.; Xing, X.; Qin, L.P. Therapeutic effects of standardized Vitex negundo seeds extract on complete Freund’s adjuvant induced arthritis in rats. Phytomedicine 2014, 21, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.H.; Kim, J.S.; Kang, S.S.; Son, K.H.; Chang, H.W.; Kim, H.P. Anti-inflammatory and anti-arthritic activity of total flavonoids of the roots of Sophora flavescens. J. Ethnopharmacol. 2010, 127, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Miyamoto, T.; Kawasuji, T.; Kuraishi, Y.; Suzuki, H. Antipruritic effects of Sophora flavescens on acute and chronic itch-related responses in mice. Biol. Pharm. Bull. 2003, 26, 722–724. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, A.P.; Wang, Y.Y.; Li, Y.D. Suppressive effects of a Chinese herbal medicine Qing-Luo-Yin extract on the angiogenesis of collagen-induced arthritis in rats. Am. J. Chin. Med. 2003, 31, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Chan, C.C.; Wu, S.J.; Chen, L.C.; Shen, J.J.; Kuo, M.L.; Chen, M.C.; Liou, C.J. Matrine attenuates allergic airway inflammation and eosinophil infiltration by suppressing eotaxin and Th2 cytokine production in asthmatic mice. J. Ethnopharmacol. 2014, 151, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Shi, L.J.; Song, G.Y.; Cai, Z.G.; Wang, C.; An, R.J. Protective effects of matrine against progression of high-fructose diet-induced steatohepatitis by enhancing antioxidant and anti-inflammatory defences involving Nrf2 translocation. Food Chem. Toxicol. 2013, 55, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Zhao, Y.; Han, P.; Yue, W.; Ma, X.Q.; Rahman, K.; Zheng, C.J.; Qin, L.P.; Han, T. Anti-arthritic activity of Xanthium strumarium L. extract on complete Freunds adjuvant induced arthritis in rats. J. Ethnopharmacol. 2014, 155, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Zhang, H.; Zhao, X.X.; Rahman, K.; Wang, Y.; Ma, X.Q.; Zheng, C.J.; Zhang, Q.Y.; Han, T.; Qin, L.P. Inhibitory effects of the root extract of Litsea cubeba (lour.) pers. on adjuvant arthritis in rats. J. Ethnopharmacol. 2013, 147, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Oh, H.M.; Park, J.H.; Choi, J.H.; Sa, K.H.; Kang, Y.M.; Park, P.H.; Shin, T.Y.; Rho, M.C.; Kim, S.H. Salvia plebeia extract inhibits the inflammatory response in human rheumatoid synovial fibroblasts and a murine model of arthritis. Phytomedicine 2015, 22, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Majithia, V.; Geraci, S.A. Rheumatoid arthritis: Diagnosis and management. Am. J. Med. 2007, 120, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Müller-Ladner, U.; Pap, T.; Gay, R.E.; Neidhart, M.; Gay, S. Mechanisms of disease: The molecular and cellular basis of joint destruction in rheumatoid arthritis. Nat. Clin. Pract. Rheumatol. 2005, 1, 102–110. [Google Scholar] [CrossRef] [PubMed]
- McNamee, K.; Williams, R.; Seed, M. Animal models of rheumatoid arthritis: How informative are they? Eur. J. Pharmacol. 2015, 759, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, M.; Xia, Y.; Dai, Y.; Chou, G.; Wang, Z. Therapeutic effect of norisoboldine, an alkaloid isolated from Radix Linderae, on collagen-induced arthritis in mice. Phytomedicine 2010, 17, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Trentham, D.E.; McCune, W.J.; Susman, P.; David, J.R. Autoimmunity to collagen in adjuvant arthritis of rats. J. Clin. Investig. 1980, 66, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jiang, Z.Z.; Wu, T.; Li, J.; Zhang, L.; Zhao, Y.; Li, X.J.; Zhang, L.Y.; Yang, S.Y. Anti-inflammatory effects and hepatotoxicity of Tripterygium-loaded solid lipid nanoparticles on adjuvant-induced arthritis in rats. Phytomedicine 2012, 19, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Chaabo, K.; Kirkham, B. Rheumatoid arthritis-anti-TNF. Int. Immunopharmacol. 2015, 27, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Mariaselvam, C.M.; Aoki, M.; Salah, S.; Boukouaci, W.; Moins-Teisserenc, H.; Charron, D.; Krishnamoorthy, R.; Tamouza, R.; Negi, V.S. Cytokine expression and cytokine-based T cell profiling in South Indian rheumatoid arthritis. Immunobiology 2014, 219, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Pasi, S.; Kant, R.; Gupta, S.; Surolia, A. Novel multimeric IL-1 receptor antagonist for the treatment of rheumatoid arthritis. Biomaterials 2015, 42, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Roeleveld, D.M.; Koenders, M.I. The role of the Th17 cytokines IL-17 and IL-22 in Rheumatoid Arthritis pathogenesis and developments in cytokine immunotherapy. Cytokine 2015, 74, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Chen, J.W.; Gao, J.S.; Li, L.; Xie, X. Resveratrol inhibits TNF-alpha-induced IL-1β, MMP-3 production in human rheumatoid arthritis fibroblast-like synoviocytes via modulation of PI3 kinase/Akt pathway. Rheumatol. Int. 2013, 33, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Burrage, P.S.; Mix, K.S.; Brinckerhoff, C.E. Matrix metallo proteinases: Role in arthritis. Front. Biosci. 2006, 11, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Cao, J.; Fang, L.; Zhao, H.; Liu, Z.; Ran, J.; Zheng, X.; Li, X.; Zhou, Y.; Ge, D.; et al. Geniposide suppresses LPS-induced nitric oxide, PGE2 and inflammatory cytokine by downregulating NF-κB, MAPK and AP-1 signaling pathways in macrophages. Int. Immunopharmacol. 2014, 20, 298–306. [Google Scholar] [CrossRef]
- Kong, X.; Liu, C.; Zhang, C.; Zhao, J.; Wang, J.; Wan, H.; Zhu, H.; Zhang, P.; Chen, W.; Xiao, Y.; et al. The suppressive effects of Saposhnikovia divaricata (Fangfeng) chromone extract on rheumatoid arthritis via inhibition of nuclear factor-κB and mitogen activated proteinkinases activation on collagen-induced arthritis model. J. Ethnopharmacol. 2013, 148, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Pamukcu, B.; Lip, G.Y.; Shantsila, E. The nuclear factor—κB pathway in atherosclerosis: A potential therapeutic target for atherothrombotic vascular disease. Thromb. Res. 2011, 128, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, M.F.; Liborio, A.B.; Teles, F.; Martins Cda, S.; Soares, P.M.; Meneses, G.C.; Rodrigues, F.A.; Leal, L.K.; Miron, D.; Silva, A.H.; et al. Red propolis ameliorates ischemic-reperfusion acute kidney injury. Phytomedicine 2015, 22, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xiao, J.; Wu, Z.W.; Wang, Z.M.; Hu, J.; Fu, H.Z.; Chen, Y.Y.; Qian, R.Q. Kirenol exerts a potent anti-arthritic effect in collagen-induced arthritis by modifying the T cells balance. Phytomedicine 2012, 19, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Ming, Q.L.; Han, P.; Zhang, Q.Y.; Jiang, Y.P.; Zheng, C.J.; Han, T.; Qin, L.P. Anti-allergic rhinitis effect of caffeoylxanthiazonoside isolated from fruits of Xanthium strumarium L. in rodent animals. Phytomedicine 2014, 21, 824–829. [Google Scholar] [CrossRef] [PubMed]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, J.; Fang, F.-F.; Li, X.-Q.; Shu, Z.-H.; Jiang, Y.-P.; Han, T.; Peng, W.; Zheng, C.-J. Matrine Exerts a Strong Anti-Arthritic Effect on Type II Collagen-Induced Arthritis in Rats by Inhibiting Inflammatory Responses. Int. J. Mol. Sci. 2016, 17, 1410. https://doi.org/10.3390/ijms17091410
Pu J, Fang F-F, Li X-Q, Shu Z-H, Jiang Y-P, Han T, Peng W, Zheng C-J. Matrine Exerts a Strong Anti-Arthritic Effect on Type II Collagen-Induced Arthritis in Rats by Inhibiting Inflammatory Responses. International Journal of Molecular Sciences. 2016; 17(9):1410. https://doi.org/10.3390/ijms17091410
Chicago/Turabian StylePu, Jiang, Fan-Fu Fang, Xiu-Qing Li, Zhi-Heng Shu, Yi-Ping Jiang, Ting Han, Wei Peng, and Cheng-Jian Zheng. 2016. "Matrine Exerts a Strong Anti-Arthritic Effect on Type II Collagen-Induced Arthritis in Rats by Inhibiting Inflammatory Responses" International Journal of Molecular Sciences 17, no. 9: 1410. https://doi.org/10.3390/ijms17091410
APA StylePu, J., Fang, F.-F., Li, X.-Q., Shu, Z.-H., Jiang, Y.-P., Han, T., Peng, W., & Zheng, C.-J. (2016). Matrine Exerts a Strong Anti-Arthritic Effect on Type II Collagen-Induced Arthritis in Rats by Inhibiting Inflammatory Responses. International Journal of Molecular Sciences, 17(9), 1410. https://doi.org/10.3390/ijms17091410