Binding and Fusion of Extracellular Vesicles to the Plasma Membrane of Their Cell Targets
Abstract
:1. Introduction
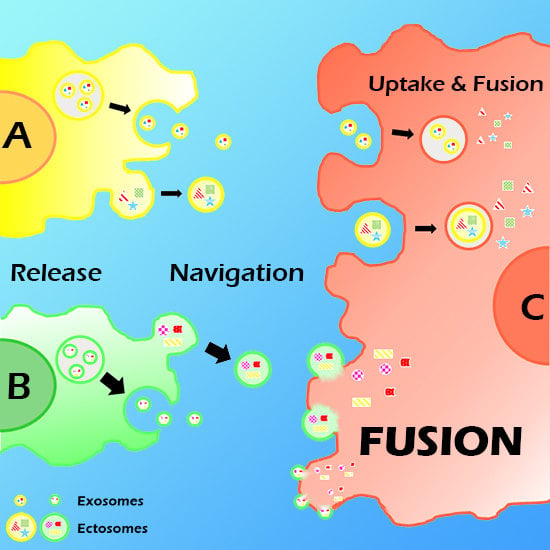
2. Vesicle (EV) Interactions with the Plasma Membrane of Target Cells
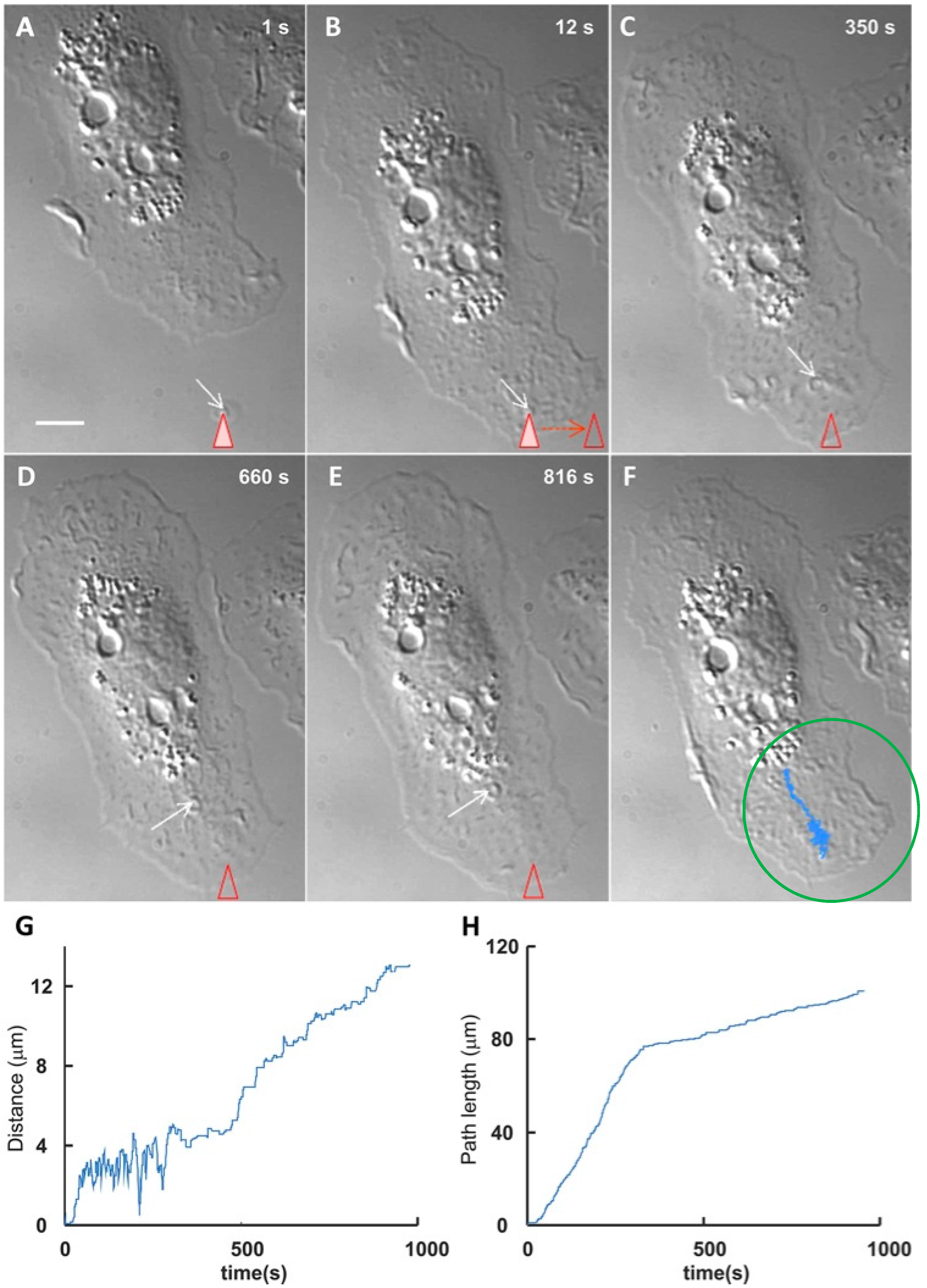
3. EV Binding to the Plasma Membrane of Target Cells
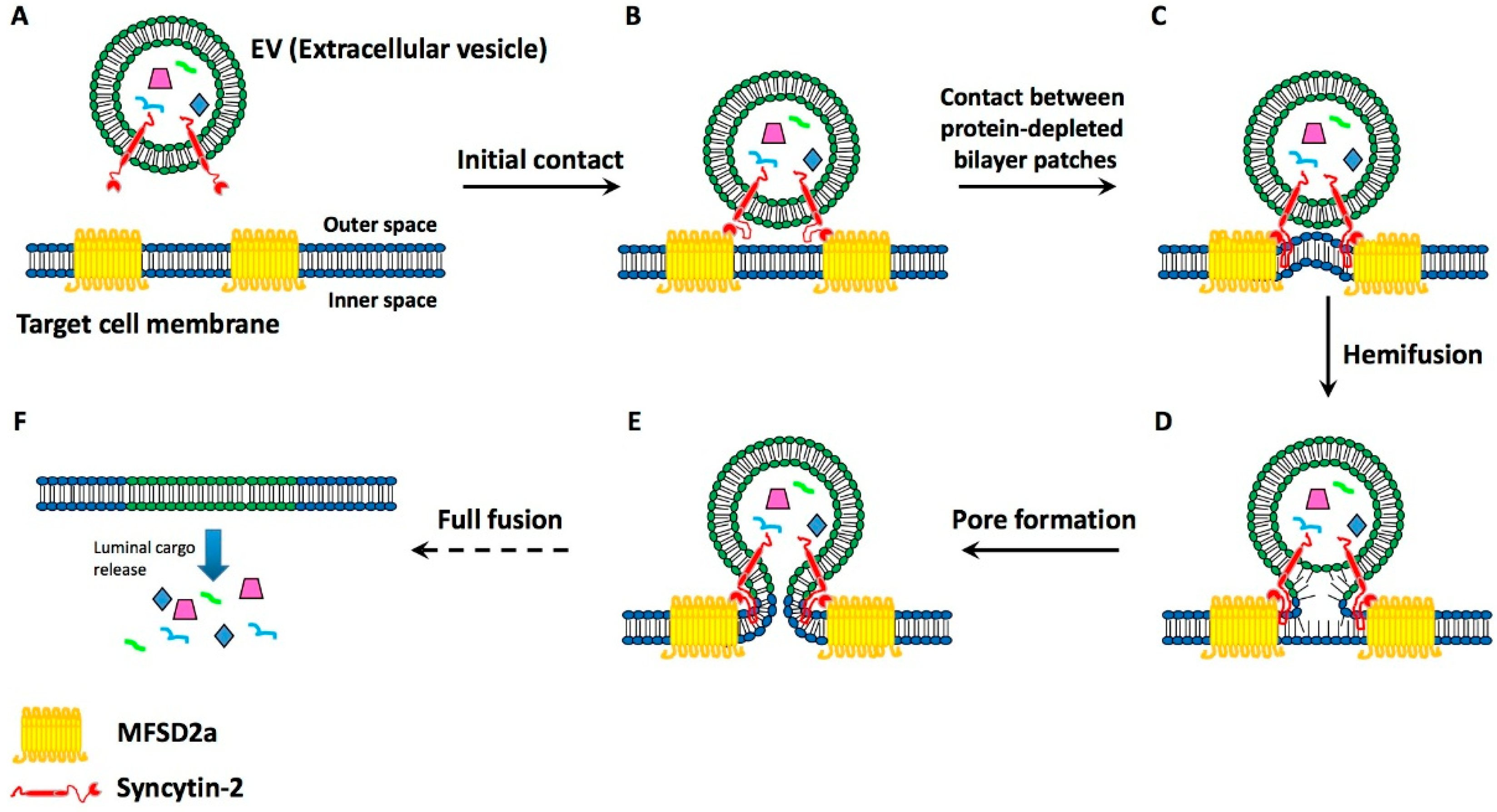
4. Present Knowledge about EV-Target Cell Surface Fusions
5. Perspectives of Therapy
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Denzer, K.; Kleijmeer, M.J.; Heijnen, H.F.; Stoorvogel, W.; Geuze, H.J. Exosome: From internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 2000, 113, 3365–3374. [Google Scholar] [PubMed]
- Stein, J.M.; Luzio, P. Ectocytosis caused by sublytic autologous complement attack on human neutrophils. The sorting of endogenous plasma membrane proteins and lipids into shed vesicles. Biochem. J. 1991, 274, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Meldolesi, J. Ectosomes and endosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.M.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Christianson, H.C.; Svensson, K.J.; Belting, M. Exosome and microvesicle mediated phene transfer in mammalian cells. Semin. Cancer Biol. 2014, 28, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Gasser, O.; Hess, C.; Miot, S.; Deon, C.; Sanchez, J.C.; Schifferli, J.A. Characterization and properties of ectsosomes released by human polymorphonuclear neutrophils. Exp. Cell Res. 2003, 285, 243–257. [Google Scholar] [CrossRef]
- Pluskota, E.; Woody, N.M.; Szpak, D.; Ballantyne, C.M.; Soloviev, D.A.; Simon, D.I.; Plow, E.F. Expression, activation, and function of integrin αMβ2 (Mac1) on neutrophil-derived microparticles. Blood 2008, 112, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Chivet, M.; Javalet, C.; Laulagnier, K.; Blot, B.; Hemming, F.J.; Sadoul, R. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J. Extracell. Vesicles 2014, 3, 24722. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhu, Y.L.; Hu, F.H.; Wang, Y.Y.; Huang, N.P.; Xiao, Z.D. Dynamics of exosome internalization and trafficking. J. Cell. Physiol. 2013, 228, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Prada, I.; Amin, L.; Furlan, R.; Legname, G.; Verderio, C.; Cojoc, D. A new approach to follow a single extracellular vesicle-cell interaction using optical tweezers. BioTechniques 2016, 60, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Podbilewicz, B. Virus and cell fusion mechanisms. Annu. Rev. Cell Dev. Biol. 2014, 30, 111–139. [Google Scholar] [CrossRef] [PubMed]
- Potgens, A.J.; Drewlo, S.; Kokozidou, M.; Kaufmann, P. Syncytin: The major regulator of trophoblast fusion? Recent developments and hypotheses on its action. Hum. Reprod. Update 2004, 10, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, J.M.; Schjenken, J.E.; Clifton, V.L.; Vargas, A.; Barbeau, B.; Lowry, P.; Maiti, K.; Smith, R. The endogenous retroviral envelope protein syncytin-1 inhibits LPS/PHA-stimulated cytokine responses in human blood and is sorted into placental exosomes. Placenta 2012, 33, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Bierregaard, B.; Lemmen, J.G.; Petersen, M.R.; Østrup, E.; Iversen, L.H.; Almstrup, K.; Larsson, L.I.; Ziebe, S. Syncytin-1 and its receptor are present in human gametes. J. Assist. Reprod. Genet. 2014, 31, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Søe, K.; Andersen, T.L.; Hobolt-Pedersen, A.S.; Bjerregaard, B.; Larsson, L.I.; Delaissé, J.M. Involvement of human endogenous retroviral syncytin-1 in human osteoclast fusion. Bone 2011, 48, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Bierregard, B.; Ziomkiewicz, I.; Schulz, A.; Larsson, L.I. Syncytin-1 in differentiating human myoblasts: Relationship to caveolin-3 and myogenin. Cell Tissue Res. 2014, 31, 533–539. [Google Scholar]
- Buslei, R.; Strissel, L.; Henke, C.; Schey, R.; Lang, N.; Ruebner, M.; Stolt, C.C.; Fabry, B.; Buchfelder, M.; Strick, R. Activation and regulation of endogenous retroviral genes in the human pituitary gland and related endocrine tumora. Neuropathol. Appl. Neurobiol. 2014, 41, 180–200. [Google Scholar] [CrossRef] [PubMed]
- Maliniemi, P.; Vincendeau, M.; Mayer, J.; Frank, O.; Hahtola, S.; Karenko, L.; Carlsson, E.; Mallet, F.; Seifarth, W.; Leib-Mösch, C.; et al. Expression of human endogenous retrovirus-w including syncytin-1 in cutaneous T-cell lymphoma. PLoS ONE 2013, 8, e76281. [Google Scholar]
- Esnault, C.; Priet, S.; Ribet, D.; Vernochet, C.; Bruls, T.; Lavialle, C.; Weissenbach, J.; Heidmann, T. A placenta-specific receptor for the fusogenic, endogenous retroviral-directed, human syncytin-2. Proc. Natl. Acad. Sci. USA 2008, 106, 17532–17537. [Google Scholar] [CrossRef] [PubMed]
- Toufaily, C.; Vargas, A.; Lemire, M.; Lafond, J.; Rassart, E.; Barbeau, B. MLSD2a, the syncytin-2 receptor, is important for trophoblast fusion. Placenta 2013, 34, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, H.; Lu, X.; Wang, R.; Zheng, R.; Li, Y.; Yang, X.; Jia, W.T.; Zhao, Y.; Wang, Y.; et al. Effects of individually silenced N-glycosylation sites and non-synonymous single-nucleotide polymorphisms on the fusogenic function of human syncytin-2. Cell Adhes. Migr. 2016, 10, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Vargas, J.; Krey, T.; Valansi, C.; Avinoam, O.; Haouz, A.; Jamin, M.; Raveh-Barak, H.; Podbilewicz, B.; Rey, F.A. Structural basis of eukaryotic cell-cell fusion. Cell 2014, 157, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Liu., Y.; Dai, X.; Li, W.; Cai, X.; Yin, Y.; Wang, Q.; Xue, Y.; Wang, C.; et al. Microvesicle-mediated transfer of microRNA-150 from monocytes to endothelial cells promote angiogenesis. J. Biol. Chem. 2013, 288, 23586–23596. [Google Scholar] [CrossRef] [PubMed]
- He, W.A.; Calore, F.; Londhe, P.; Canella, A.; Guttridge, D.C.; Croce, C.M. Microvesicles containing miRNAs promote muscle cell death in cancer cachexia via TLR7. Proc. Natl. Acad. Sci. USA 2014, 111, 4525–4529. [Google Scholar] [CrossRef] [PubMed]
- Muraluidharan-Chiari, V.; Clancy, J.W.; Sedgwick, A.; D’Souza-Schorey, C. Microvesicles: Mediators of extracellular communication during cancer progression. J. Cell Sci. 2010, 123, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Ishikawa, A.; Kuroda, M. Role of exosomes and microvesicles in disease pathogenesis. Adv. Drug Deliv. Rev. 2013, 65, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.H.; Ji, C.L.; Li, H.; Qiu, G.X.; Gao, C.J.; Weng, X.S. Membrane microparticles and diseases. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2420–2427. [Google Scholar] [PubMed]
- De Toro, J.; Herschlik, L.; Waldner, C.; Mongini, C. Emerging roles of exosomes in normal and pathological conditions: New insights for diagnosis and therapeutic applications. Front. Immunol. 2015, 6, 203. [Google Scholar] [CrossRef] [PubMed]
- Loyer, X.; Vion, A.C.; Tedgui, A.; Boulanger, C.M. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ. Res. 2014, 114, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.R.; Dias, M.S.; Hainaut, P. Tumor-cell-derived microvesicles as carriers of molecular information in cancer. Curr. Opin. Oncol. 2013, 25, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; El Andaloussi, S.; Wood, M.J. Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012, 21, R125–R134. [Google Scholar] [CrossRef] [PubMed]
- El Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2012, 12, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.E.; Leonard, J.N. Stabilization of exosome-tageted peptides via engineered glycosylation. J. Biol. Chem. 2015, 290, 8166–8172. [Google Scholar] [CrossRef] [PubMed]
- Van Dommelen, S.M.; Vader, P.; Lakhal, S.; Kooijmans, S.A.; van Solinge, W.W.; Wood, M.J.; Schiffelers, R.M. Microvesicles and exosomes: Opportunities for cell-derived membrane vesicles in drug delivery. J. Control. Release 2012, 161, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Aryani, A.; Denecke, B. Exosomes as a nanodelivery system: A key to the future of neuromedicine. Mol. Neurobiol. 2016, 53, 818–834. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prada, I.; Meldolesi, J. Binding and Fusion of Extracellular Vesicles to the Plasma Membrane of Their Cell Targets. Int. J. Mol. Sci. 2016, 17, 1296. https://doi.org/10.3390/ijms17081296
Prada I, Meldolesi J. Binding and Fusion of Extracellular Vesicles to the Plasma Membrane of Their Cell Targets. International Journal of Molecular Sciences. 2016; 17(8):1296. https://doi.org/10.3390/ijms17081296
Chicago/Turabian StylePrada, Ilaria, and Jacopo Meldolesi. 2016. "Binding and Fusion of Extracellular Vesicles to the Plasma Membrane of Their Cell Targets" International Journal of Molecular Sciences 17, no. 8: 1296. https://doi.org/10.3390/ijms17081296
APA StylePrada, I., & Meldolesi, J. (2016). Binding and Fusion of Extracellular Vesicles to the Plasma Membrane of Their Cell Targets. International Journal of Molecular Sciences, 17(8), 1296. https://doi.org/10.3390/ijms17081296