Bioinformatics and Microarray Analysis of miRNAs in Aged Female Mice Model Implied New Molecular Mechanisms for Impaired Fracture Healing
Abstract
:1. Introduction
2. Results
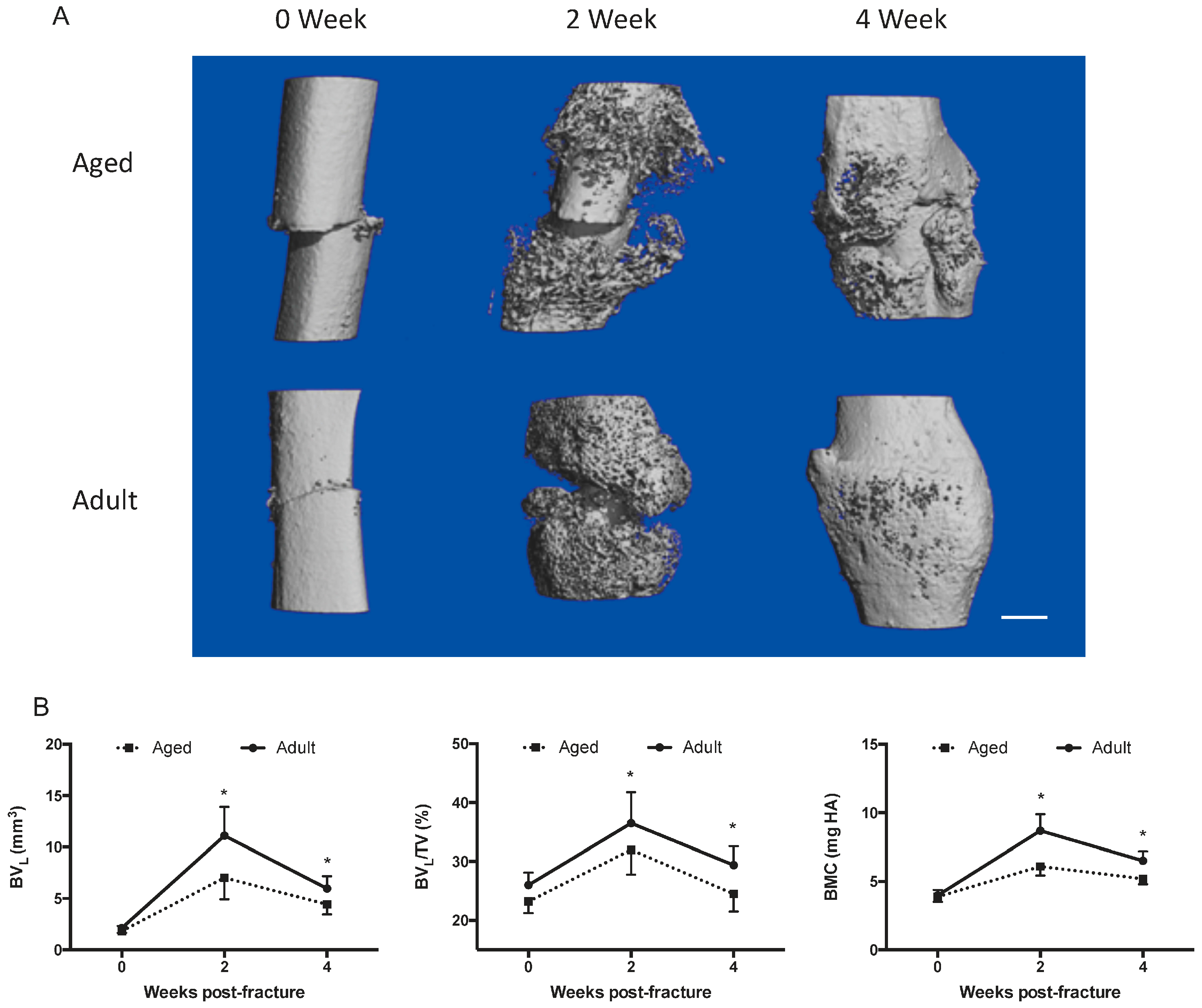
2.1. Fracture Healing Is Impaired in Aged Female Mice
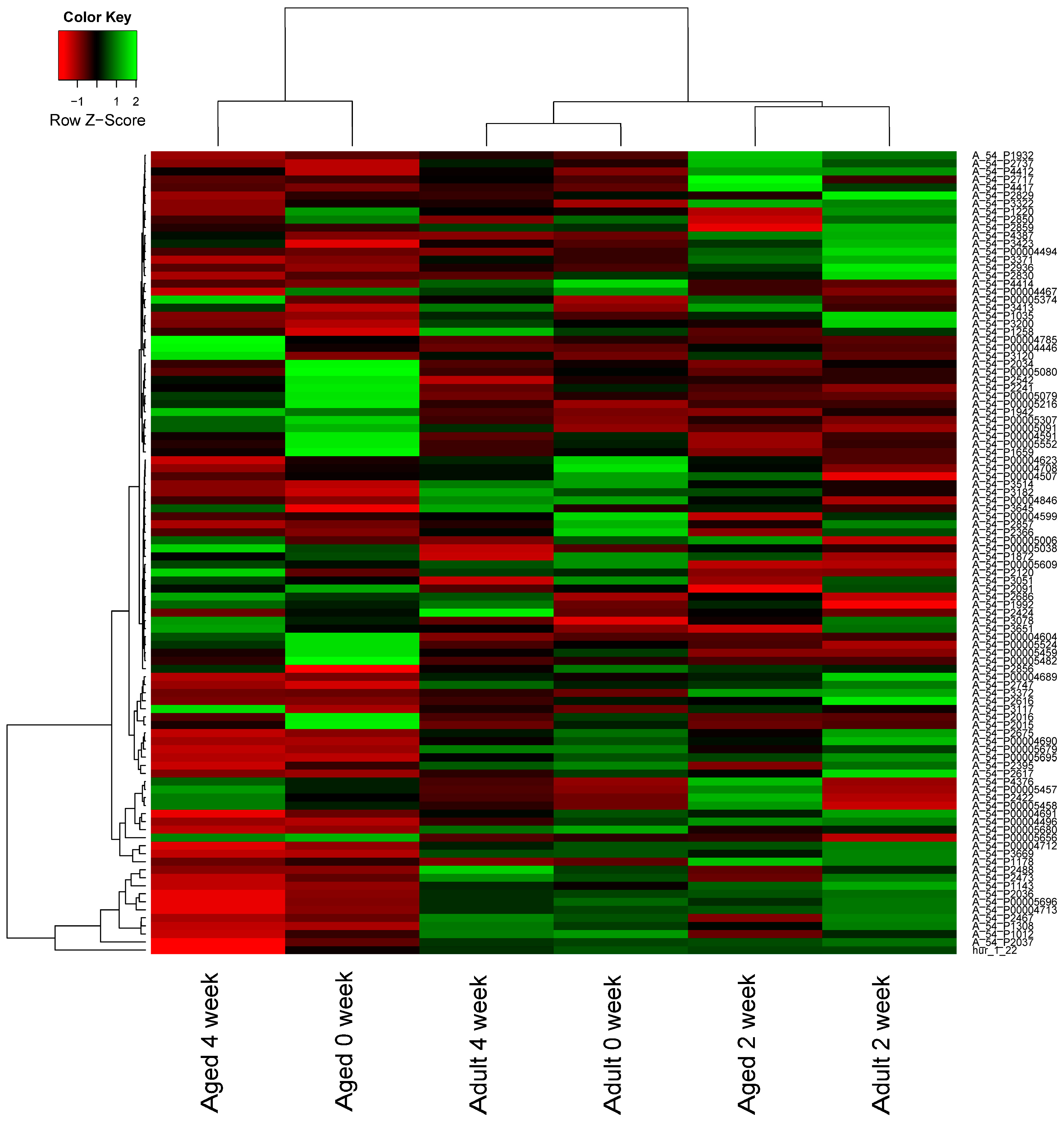
2.2. MicroRNA (miRNA) Expression Profiles
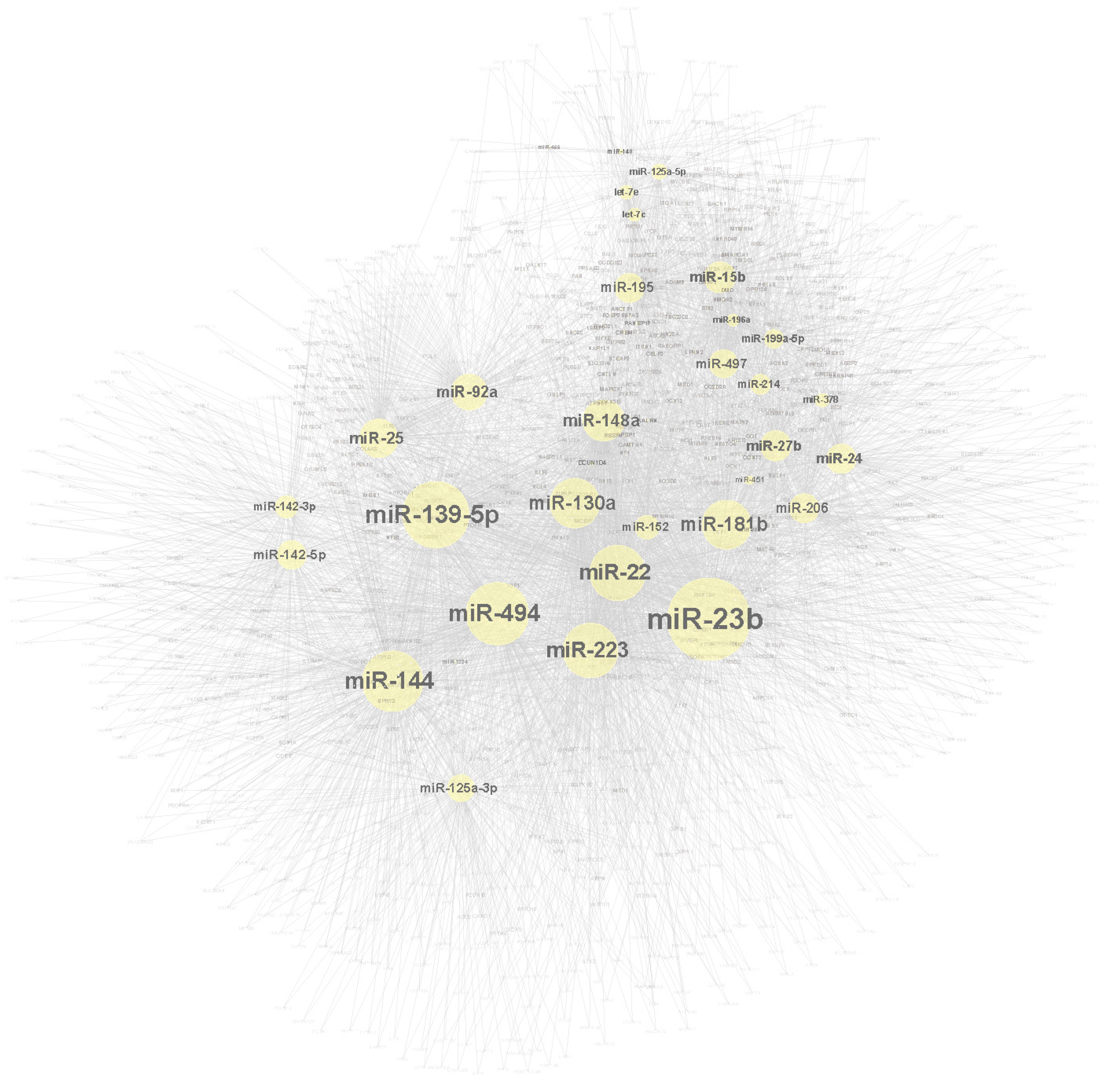
2.3. Target Genes and Molecular Network Construction
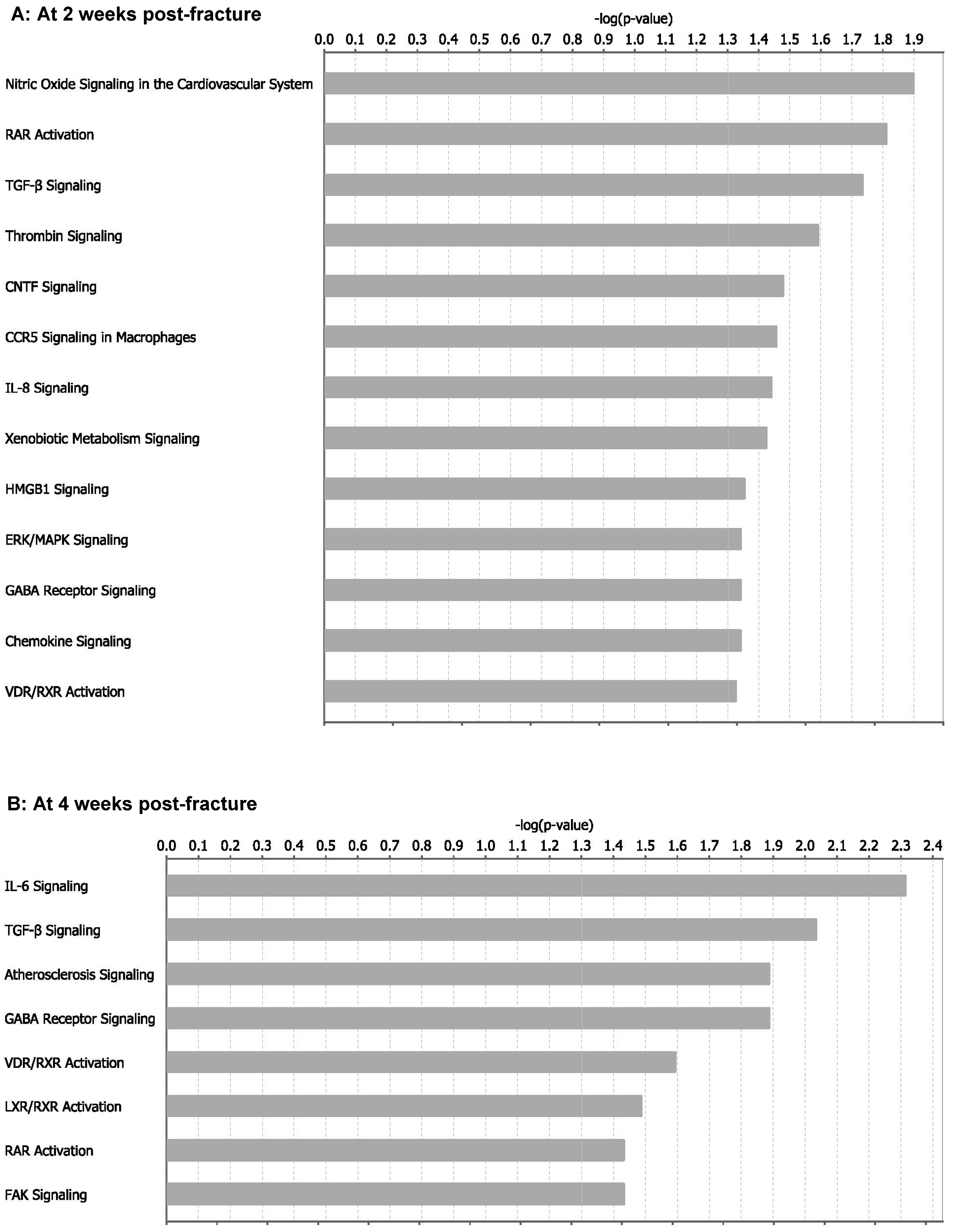
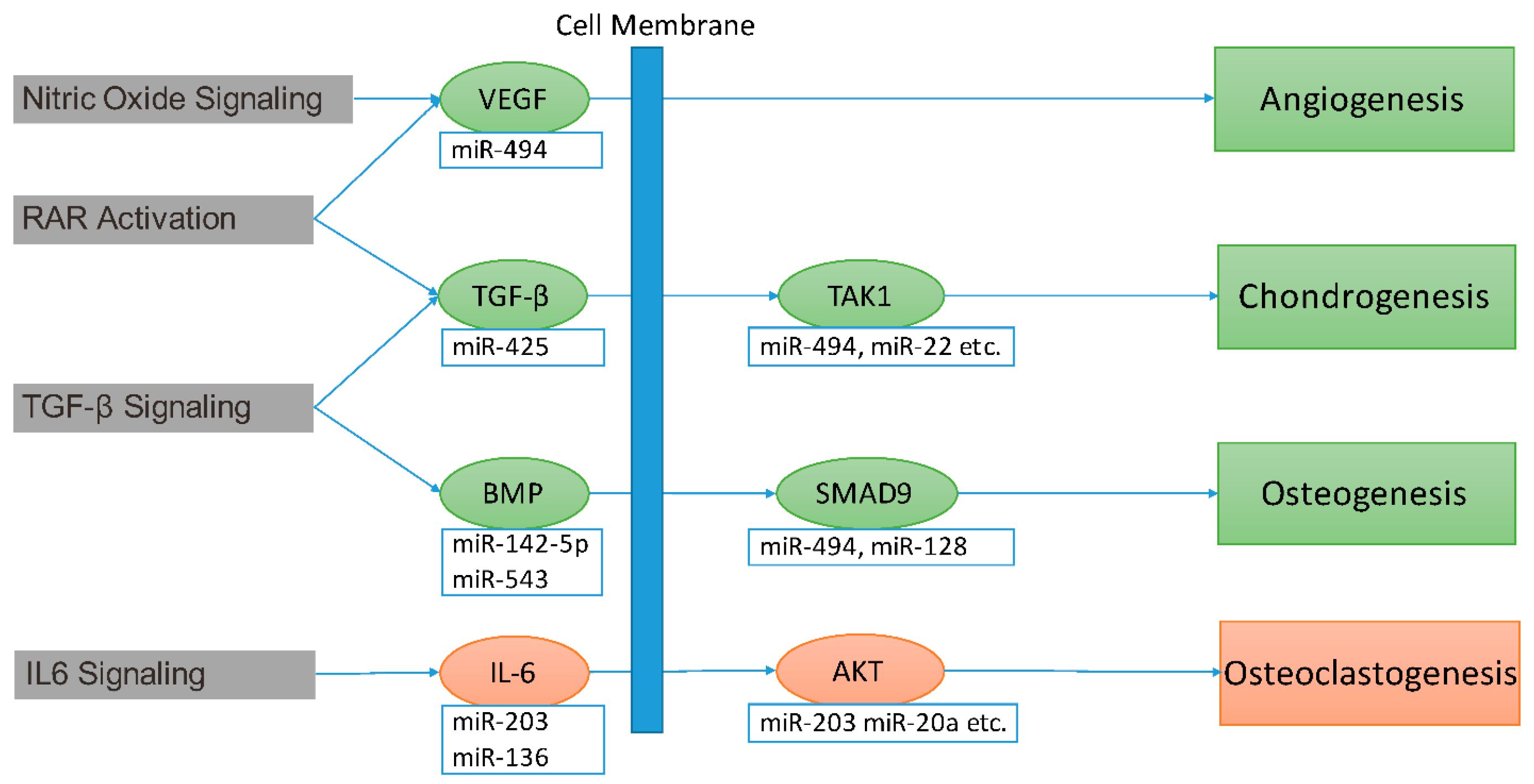
2.4. miRNA Contribution to Impaired Fracture Healing (RCIFH) and Pathway Enrichment Analysis
2.5. The miR-494 Inhibits Chondrogenic Differentiation in Vitro
3. Discussion
4. Materials and Methods
4.1. Animal Model and Micro Computed Tomography (Micro-CT) Analysis
4.2. Tissue Sample and RNA Isolation
4.3. MicroRNAs (miRNAs) Microarray Labeling and Hybridization
4.4. Differentially Expressed miRNAs
4.5. Gene Microarray Data
4.6. Construction of Protein-Protein Interaction (PPI) Networks
4.7. Prediction of miRNA-Target Interactions
4.8. miRNA Contribution to Fracture Healing Impairment
- is the average value of miRNA i expression in adult female mice at 2-/4-week after fracture.
- is the average value of miRNA i expression in adult female mice at 0-week after fracture.
- is the average value of miRNA i expression in aged female mice at 2-/4-week after fracture.
- is the average value of miRNA i expression in aged female mice at 0-week after fracture.
- is the power of miRNA i in the molecular network of mice.
4.9. Pathway Enrichment Analysis
4.10. Cell Culture and Transfection
4.11. Chondrogenic Differentiation Assay
4.12. Quantitative RT-PCR Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gruber, R.; Koch, H.; Doll, B.A.; Tegtmeier, F.; Einhorn, T.A.; Hollinger, J.O. Fracture healing in the elderly patient. Exp. Gerontol. 2006, 41, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Virk, M.S.; Lieberman, J.R. Biologic adjuvants for fracture healing. Arthritis Res. Ther. 2012, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Center, J.R.; Eisman, J.A. Osteoporosis: Underrated, underdiagnosed and undertreated. Med. J. Aust. 2004, 180, S18–S22. [Google Scholar] [PubMed]
- Kanis, J.A.; Johnell, O.; Oden, A.; Sembo, I.; Redlund-Johnell, I.; Dawson, A.; de Laet, C.; Jonsson, B. Long-term risk of osteoporotic fracture in Malmo. Osteoporos. Int. 2000, 11, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Bliuc, D.; Nguyen, N.D.; Milch, V.E.; Nguyen, T.V.; Eisman, J.A.; Center, J.R. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 2009, 301, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Castronuovo, E.; Pezzotti, P.; Franzo, A.; Di Lallo, D.; Guasticchi, G. Early and late mortality in elderly patients after hip fracture: A cohort study using administrative health databases in the Lazio region, Italy. BMC Geriatr. 2011, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007, 22, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Blume, S.W.; Curtis, J.R. Medical costs of osteoporosis in the elderly medicare population. Osteoporos. Int. 2011, 22, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Kong, Y.W.; Ferland-McCollough, D.; Jackson, T.J.; Bushell, M. MicroRNAs in cancer management. Lancet Oncol. 2012, 13, e249–e258. [Google Scholar] [CrossRef]
- Murata, K.; Ito, H.; Yoshitomi, H.; Yamamoto, K.; Fukuda, A.; Yoshikawa, J.; Furu, M.; Ishikawa, M.; Shibuya, H.; Matsuda, S. Inhibition of miR-92a enhances fracture healing via promoting angiogenesis in a model of stabilized fracture in young mice. J. Bone Miner. Res. 2014, 29, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Pourrajab, F.; Vakili Zarch, A.; Hekmatimoghaddam, S.; Zare-Khormizi, M.R. MicroRNAs; easy and potent targets in optimizing therapeutic methods in reparative angiogenesis. J. Cell. Mol. Med. 2015, 19, 2702–2714. [Google Scholar] [CrossRef] [PubMed]
- Gurusinghe, S.; Strappe, P. Gene modification of mesenchymal stem cells and articular chondrocytes to enhance chondrogenesis. BioMed Res. Int. 2014, 2014, 369528. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Yang, B.; Guo, H.; Kang, F. MicroRNAs regulate osteogenesis and chondrogenesis. Biochem. Biophys. Res. Commun. 2012, 418, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, B.; Li, Q.; Peng, J.; Yang, Z.; Wang, A.; Li, D.; Hou, Z.; Lv, K.; Kan, G.; et al. miR-214 targets atf4 to inhibit bone formation. Nat. Med. 2013, 19, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Van Wijnen, A.J.; van de Peppel, J.; van Leeuwen, J.P.; Lian, J.B.; Stein, G.S.; Westendorf, J.J.; Oursler, M.J.; Im, H.J.; Taipaleenmaki, H.; Hesse, E.; et al. MicroRNA functions in osteogenesis and dysfunctions in osteoporosis. Curr. Osteoporos. Rep. 2013, 11, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, C.; Karpinski, K.; Haug, A.T.; Vester, H.; Schmitt, A.; Bauer, J.S.; van Griensven, M. Five freely circulating miRNAs and bone tissue miRNAs are associated with osteoporotic fractures. J. Bone Miner. Res. 2014, 29, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, L.; Huang, S.; Hou, Y.; Liu, Y.; Chan, K.M.; Pan, X.H.; Li, G. miR-21 overexpressing mesenchymal stem cells accelerate fracture healing in a rat closed femur fracture model. BioMed Res. Int. 2015, 2015, 412327. [Google Scholar] [CrossRef] [PubMed]
- Schindeler, A.; McDonald, M.M.; Bokko, P.; Little, D.G. Bone remodeling during fracture repair: The cellular picture. Semin. Cell Dev. Biol. 2008, 19, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Saran, U.; Gemini Piperni, S.; Chatterjee, S. Role of angiogenesis in bone repair. Arch. Biochem. Biophys. 2014, 561, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, F.; Ogasawara, A.; Goto, K.; Moriya, H.; Ninomiya, Y.; Einhorn, T.A.; Yamazaki, M. Spatial and temporal gene expression in chondrogenesis during fracture healing and the effects of basic fibroblast growth factor. J. Orthop. Res. 2001, 19, 935–944. [Google Scholar] [CrossRef]
- Bahney, C.S.; Hu, D.P.; Miclau, T., 3rd; Marcucio, R.S. The multifaceted role of the vasculature in endochondral fracture repair. Front. Endocrinol. 2015, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Minkwitz, S.; Fassbender, M.; Kronbach, Z.; Wildemann, B. Longitudinal analysis of osteogenic and angiogenic signaling factors in healing models mimicking atrophic and hypertrophic non-unions in rats. PLoS ONE 2015, 10, e0124217. [Google Scholar] [CrossRef] [PubMed]
- Hadjiargyrou, M.; Zhi, J.; Komatsu, D.E. Identification of the microRNA transcriptome during the early phases of mammalian fracture repair. Bone 2016, 87, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, Y.; Guo, X.; Xu, H.; Xu, Z.; Duan, D.; Wang, K. Identification of differentially expressed microRNAs involved in non-traumatic osteonecrosis through microRNA expression profiling. Gene 2015, 565, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Tang, J.; He, H.; Cheng, P.; Chen, C. miR-142–5p promotes bone repair by maintaining osteoblast activity. J. Bone Miner. Metab. 2016. [Google Scholar] [CrossRef] [PubMed]
- Waki, T.; Lee, S.Y.; Niikura, T.; Iwakura, T.; Dogaki, Y.; Okumachi, E.; Kuroda, R.; Kurosaka, M. Profiling microRNA expression in fracture nonunions: Potential role of microRNAs in nonunion formation studied in a rat model. Bone Jt. J. 2015, 97-B, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Weilner, S.; Skalicky, S.; Salzer, B.; Keider, V.; Wagner, M.; Hildner, F.; Gabriel, C.; Dovjak, P.; Pietschmann, P.; Grillari-Voglauer, R.; et al. Differentially circulating miRNAs after recent osteoporotic fractures can influence osteogenic differentiation. Bone 2015, 79, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Garcia, M.M.; Rodriguez-Fernandez, J.B.; Puga-Sarmiento, E.; Charle-Crespo, M.A.; Gomes-Carvalho, C.S.; Prejigueiro-Santas, A. [Incidence of hip fractures due to osteoporosis in relation to the prescription of drugs for their prevention and treatment in Galicia, Spain]. Aten. Primaria 2011, 43, 82–88. [Google Scholar] [PubMed]
- Almeida, M.I.; Silva, A.M.; Vasconcelos, D.M.; Almeida, C.R.; Caires, H.; Pinto, M.T.; Calin, G.A.; Santos, S.G.; Barbosa, M.A. miR-195 in human primary mesenchymal stromal/stem cells regulates proliferation, osteogenesis and paracrine effect on angiogenesis. Oncotarget 2016, 7, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, G.; Miao, H.; Tang, R.; Song, Y.; Hu, Y.; Wang, Z.; Hou, Y. MicroRNA-494 inhibits the growth and angiogenesis-regulating potential of mesenchymal stem cells. FEBS Lett. 2015, 589, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Welten, S.M.; Bastiaansen, A.J.; de Jong, R.C.; de Vries, M.R.; Peters, E.A.; Boonstra, M.C.; Sheikh, S.P.; La Monica, N.; Kandimalla, E.R.; Quax, P.H.; et al. Inhibition of 14q32 microRNAs miR-329, miR-487b, miR-494, and miR-495 increases neovascularization and blood flow recovery after ischemia. Circ. Res. 2014, 115, 696–708. [Google Scholar] [CrossRef] [PubMed]
- Street, J.; Bao, M.; deGuzman, L.; Bunting, S.; Peale, F.V., Jr.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef] [PubMed]
- Hing, K.A. Bone repair in the twenty-first century: Biology, chemistry or engineering? Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2821–2850. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.A.; Xie, C.; Zuscik, M.J.; Kingsley, P.; Schwarz, E.M.; Awad, H.; Guldberg, R.; Drissi, H.; Puzas, J.E.; Boyce, B.; et al. Reduced COX-2 expression in aged mice is associated with impaired fracture healing. J. Bone Miner. Res. 2009, 24, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.F.; Von Roemeling, C.; Gao, H.D.; Zhang, J.; Guo, C.A.; Yan, Z.Q. Analysis of altered microRNA expression profile in the reparative interface of the femoral head with osteonecrosis. Exp. Mol. Pathol. 2015, 98, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Jevon, M.; Sabokbar, A.; Fujikawa, Y.; Hirayama, T.; Neale, S.D.; Wass, J.; Athanasou, N.A. Gender- and age-related differences in osteoclast formation from circulating precursors. J. Endocrinol. 2002, 172, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Plummer, P.N.; Freeman, R.; Taft, R.J.; Vider, J.; Sax, M.; Umer, B.A.; Gao, D.; Johns, C.; Mattick, J.S.; Wilton, S.D.; et al. MicroRNAs regulate tumor angiogenesis modulated by endothelial progenitor cells. Cancer Res. 2013, 73, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Yang, T.; Zeng, H.C.; Campeau, P.M.; Chen, Y.; Bertin, T.; Dawson, B.C.; Munivez, E.; Tao, J.; Lee, B.H. miRNA-34c regulates notch signaling during bone development. Hum. Mol. Genet. 2012, 21, 2991–3000. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhang, Z.; Chen, W.; Huang, G.; He, A.; Hou, C.; Long, Y.; Yang, Z.; Zhang, Z.; Liao, W. MicroRNA-320 regulates matrix metalloproteinase-13 expression in chondrogenesis and interleukin-1β-induced chondrocyte responses. Osteoarthr. Cartil. 2016, 24, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Underhill, T.M.; Sampaio, A.V.; Weston, A.D. Retinoid signalling and skeletal development. Novartis Found. Symp. 2001, 232, 171–185. [Google Scholar] [PubMed]
- Cash, D.E.; Bock, C.B.; Schughart, K.; Linney, E.; Underhill, T.M. Retinoic acid receptor α function in vertebrate limb skeletogenesis: A modulator of chondrogenesis. J. Cell Biol. 1997, 136, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.-P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Peruzzi, B.; Cappariello, A.; Del Fattore, A.; Rucci, N.; de Benedetti, F.; Teti, A. c-Src and IL-6 inhibit osteoblast differentiation and integrate IGFBP5 signalling. Nat. Commun. 2012, 3, 630. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Udagawa, N.; Takahashi, N.; Miyaura, C.; Tanaka, S.; Yamada, Y.; Koishihara, Y.; Ohsugi, Y.; Kumaki, K.; Taga, T.; et al. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc. Natl. Acad. Sci. USA 1993, 90, 11924–11928. [Google Scholar] [CrossRef] [PubMed]
- Axmann, R.; Bohm, C.; Kronke, G.; Zwerina, J.; Smolen, J.; Schett, G. Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum. 2009, 60, 2747–2756. [Google Scholar] [CrossRef] [PubMed]
- Kudo, O.; Sabokbar, A.; Pocock, A.; Itonaga, I.; Fujikawa, Y.; Athanasou, N.A. Interleukin-6 and interleukin-11 support human osteoclast formation by a rankl-independent mechanism. Bone 2003, 32, 1–7. [Google Scholar] [CrossRef]
- Kaneshiro, S.; Ebina, K.; Shi, K.; Higuchi, C.; Hirao, M.; Okamoto, M.; Koizumi, K.; Morimoto, T.; Yoshikawa, H.; Hashimoto, J. IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J. Bone Miner. Metab. 2014, 32, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Hughes, F.J.; Howells, G.L. Interleukin-6 inhibits bone formation in vitro. Bone Miner. 1993, 21, 21–28. [Google Scholar] [CrossRef]
- Kwan Tat, S.; Padrines, M.; Theoleyre, S.; Heymann, D.; Fortun, Y. IL-6, RANKL, TNF-α/IL-1: Interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004, 15, 49–60. [Google Scholar] [PubMed]
- Bonnarens, F.; Einhorn, T.A. Production of a standard closed fracture in laboratory animal bone. J. Orthop. Res. 1984, 2, 97–101. [Google Scholar] [CrossRef] [PubMed]
- He, Y.X.; Liu, Z.; Pan, X.H.; Tang, T.; Guo, B.S.; Zheng, L.Z.; Xie, X.H.; Wang, X.L.; Lee, K.M.; Li, G.; et al. Deletion of estrogen receptor β accelerates early stage of bone healing in a mouse osteotomy model. Osteoporos. Int. 2012, 23, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.K. Limma: Linear models for microarray data. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor; Springer: Berlin, Germany, 2005; pp. 397–420. [Google Scholar]
- Oughtred, R.; Chatr-Aryamontri, A.; Breitkreutz, B.J.; Chang, C.S.; Rust, J.M.; Theesfeld, C.L.; Heinicke, S.; Breitkreutz, A.; Chen, D.; Hirschman, J.; et al. Use of the biogrid database for analysis of yeast protein and genetic interactions. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.H.; Chang, N.W.; Shrestha, S.; Hsu, S.D.; Lin, Y.L.; Lee, W.H.; Yang, C.D.; Hong, H.C.; Wei, T.Y.; Tu, S.J.; et al. Mirtarbase 2016: Updates to the experimentally validated miRNA-target interactions database. Nucleic Acids Res. 2016, 44, D239–D247. [Google Scholar] [CrossRef] [PubMed]
- Reczko, M.; Maragkakis, M.; Alexiou, P.; Grosse, I.; Hatzigeorgiou, A.G. Functional microRNA targets in protein coding sequences. Bioinformatics 2012, 28, 771–776. [Google Scholar] [CrossRef] [PubMed]
- John, B.; Enright, A.J.; Aravin, A.; Tuschl, T.; Sander, C.; Marks, D.S. Human microRNA targets. PLoS Biol. 2004, 2, e363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, N.; Wang, X. miRDB: An online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 2015, 43, D146–D152. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Green, J.; Pollard, J.; Tugendreich, S. Causal analysis approaches in ingenuity pathway analysis (IPA). Bioinformatic 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]

| 2-Week | 4-Week | ||
|---|---|---|---|
| miRNA | RCIFH | miRNA | RCIFH |
| miR-494 | −1692.98 | miR-494 | −2038.91 |
| miR-139-5p | −428.294 | miR-125a-3p | −492.18 |
| miR-142-5p | −278.332 | miR-24 | 326.6378 |
| miR-206 | 269.8789 | miR-144 | −271.429 |
| miR-181b | 222.3468 | miR-497 | 259.1864 |
| miR-199a-5p | 203.6746 | miR-195 | 246.0054 |
| miR-223 | −192.034 | miR-15b | 230.7585 |
| miR-144 | −182.219 | miR-23b | 168.3962 |
| miR-125a-5p | 180.9605 | let-7e | 143.6061 |
| miR-22 | −151.984 | miR-223 | 140.6847 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, B.; Zhang, Z.-K.; Liu, J.; He, Y.-X.; Tang, T.; Li, J.; Guo, B.-S.; Lu, A.-P.; Zhang, B.-T.; Zhang, G. Bioinformatics and Microarray Analysis of miRNAs in Aged Female Mice Model Implied New Molecular Mechanisms for Impaired Fracture Healing. Int. J. Mol. Sci. 2016, 17, 1260. https://doi.org/10.3390/ijms17081260
He B, Zhang Z-K, Liu J, He Y-X, Tang T, Li J, Guo B-S, Lu A-P, Zhang B-T, Zhang G. Bioinformatics and Microarray Analysis of miRNAs in Aged Female Mice Model Implied New Molecular Mechanisms for Impaired Fracture Healing. International Journal of Molecular Sciences. 2016; 17(8):1260. https://doi.org/10.3390/ijms17081260
Chicago/Turabian StyleHe, Bing, Zong-Kang Zhang, Jin Liu, Yi-Xin He, Tao Tang, Jie Li, Bao-Sheng Guo, Ai-Ping Lu, Bao-Ting Zhang, and Ge Zhang. 2016. "Bioinformatics and Microarray Analysis of miRNAs in Aged Female Mice Model Implied New Molecular Mechanisms for Impaired Fracture Healing" International Journal of Molecular Sciences 17, no. 8: 1260. https://doi.org/10.3390/ijms17081260
APA StyleHe, B., Zhang, Z.-K., Liu, J., He, Y.-X., Tang, T., Li, J., Guo, B.-S., Lu, A.-P., Zhang, B.-T., & Zhang, G. (2016). Bioinformatics and Microarray Analysis of miRNAs in Aged Female Mice Model Implied New Molecular Mechanisms for Impaired Fracture Healing. International Journal of Molecular Sciences, 17(8), 1260. https://doi.org/10.3390/ijms17081260







