Full-Length cDNA Cloning, Molecular Characterization and Differential Expression Analysis of Lysophospholipase I from Ovis aries
Abstract
:1. Introduction
2. Results
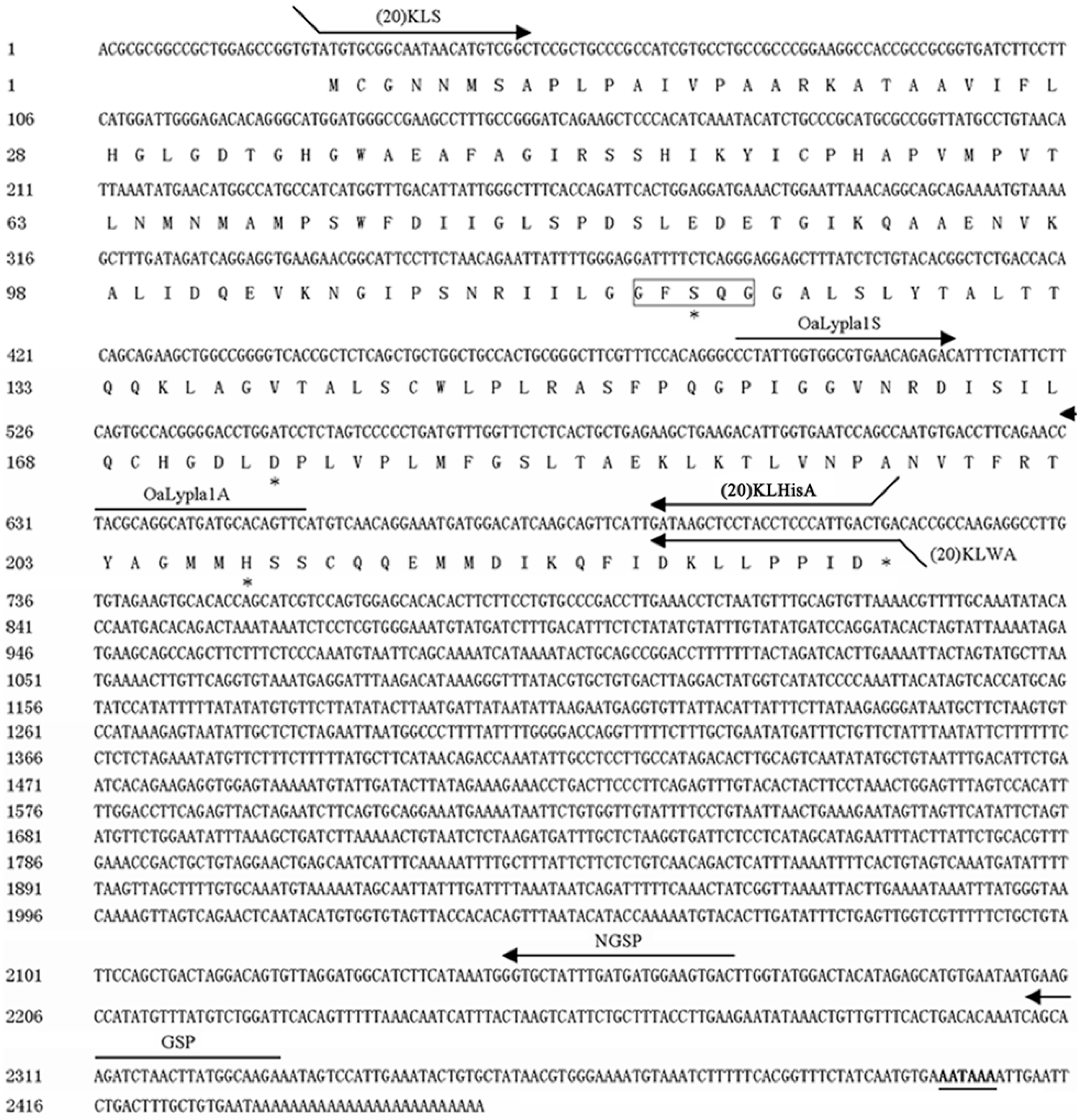
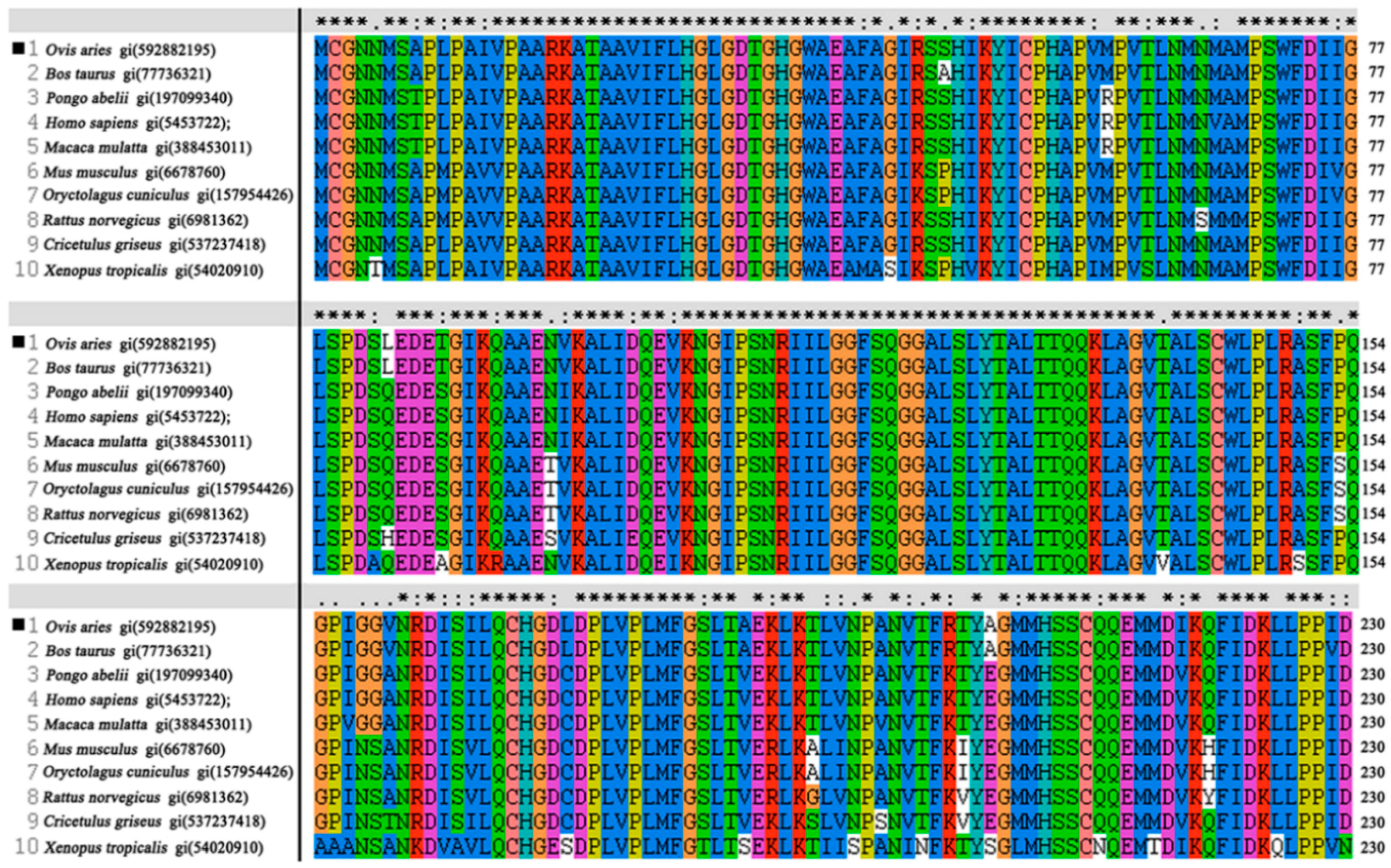
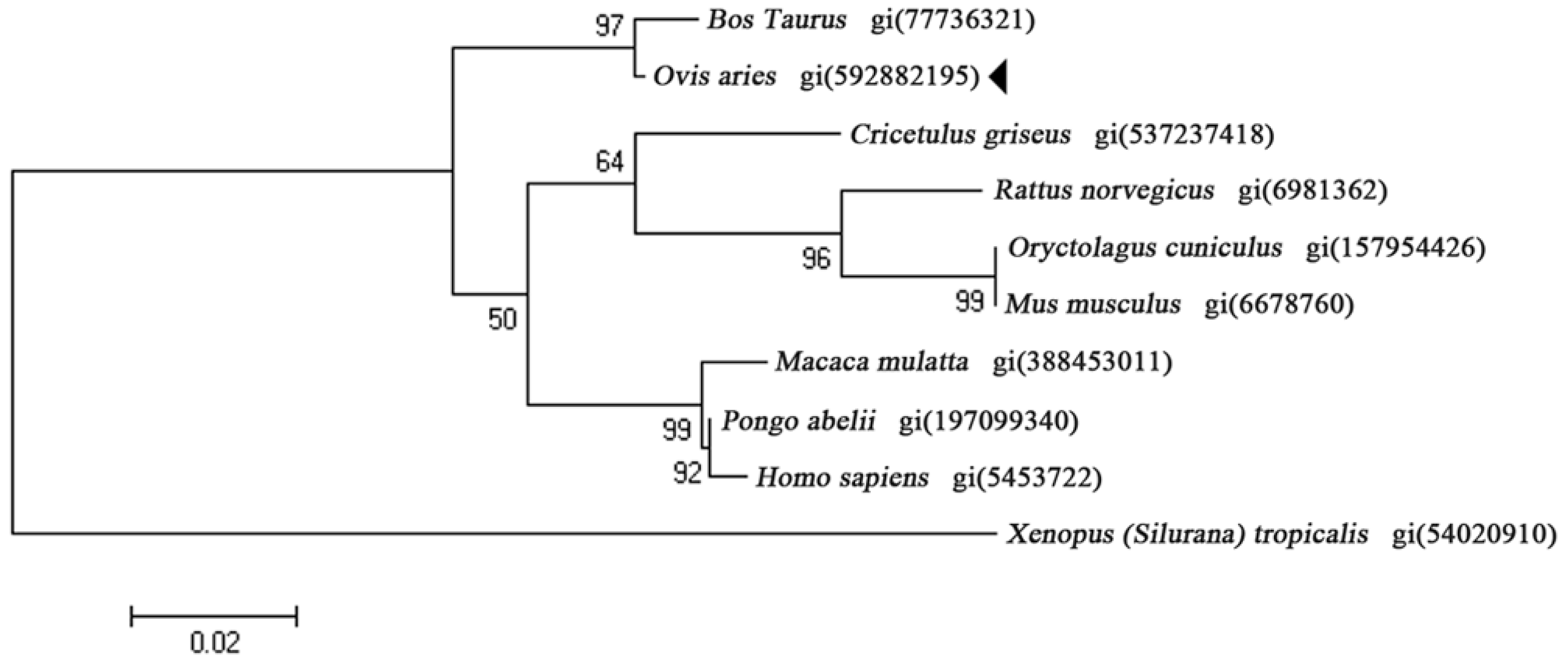
2.1. Characterization of OaLypla1 cDNA
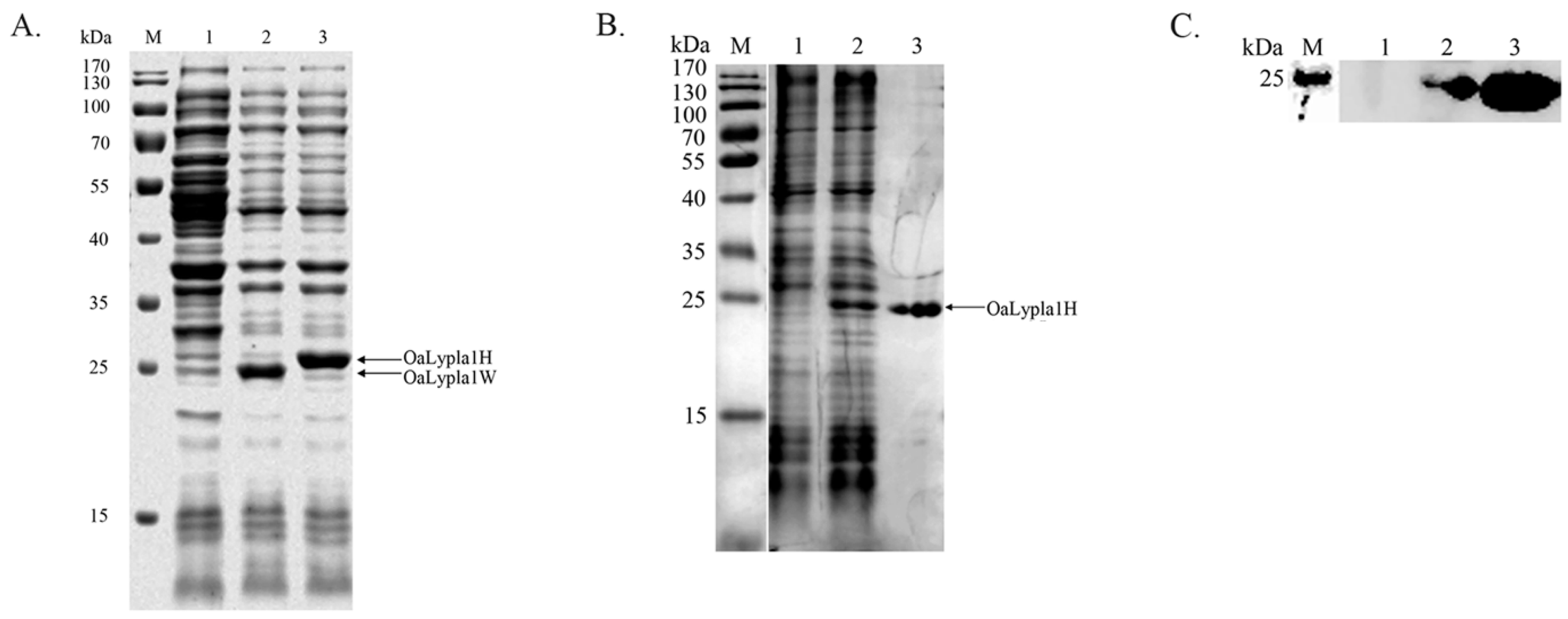
2.2. Protein Expression
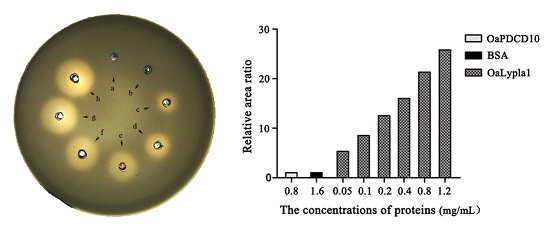
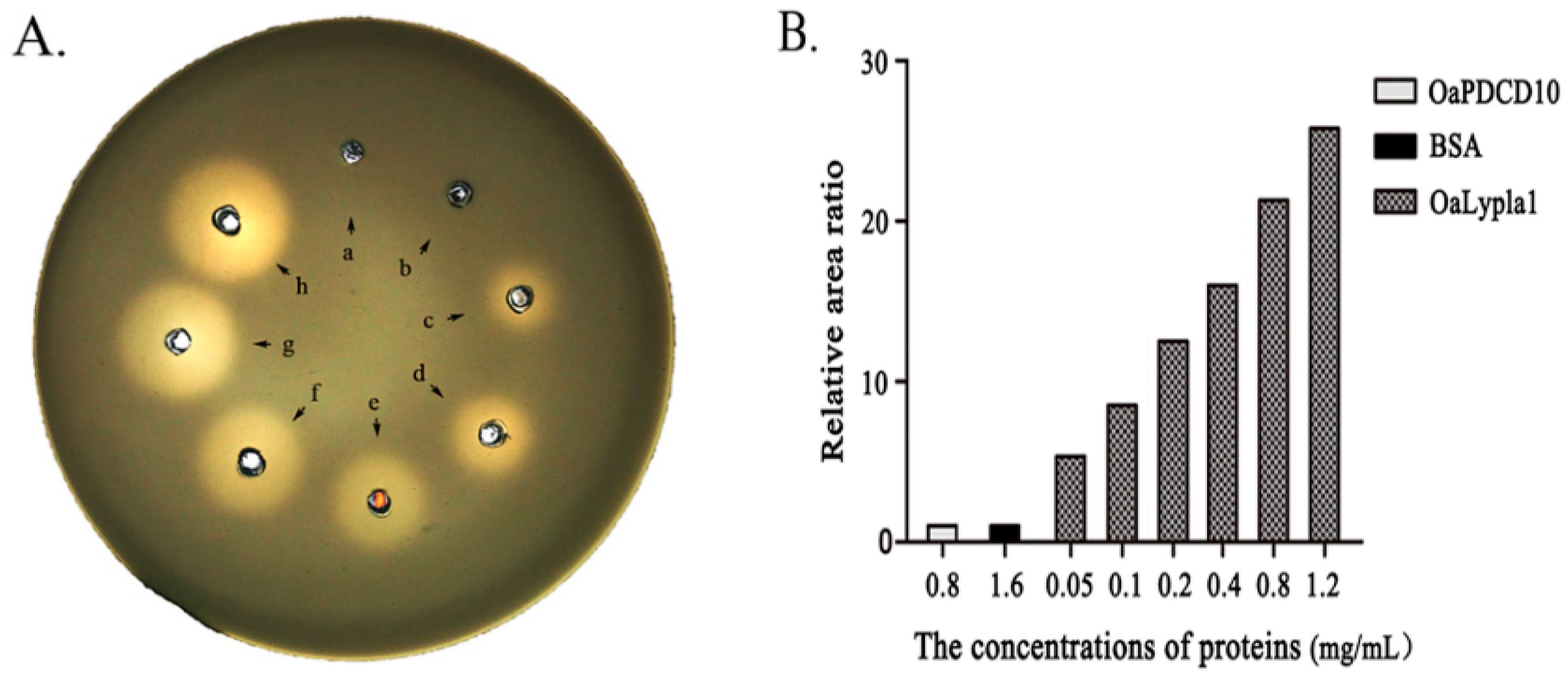
2.3. Activity Assay
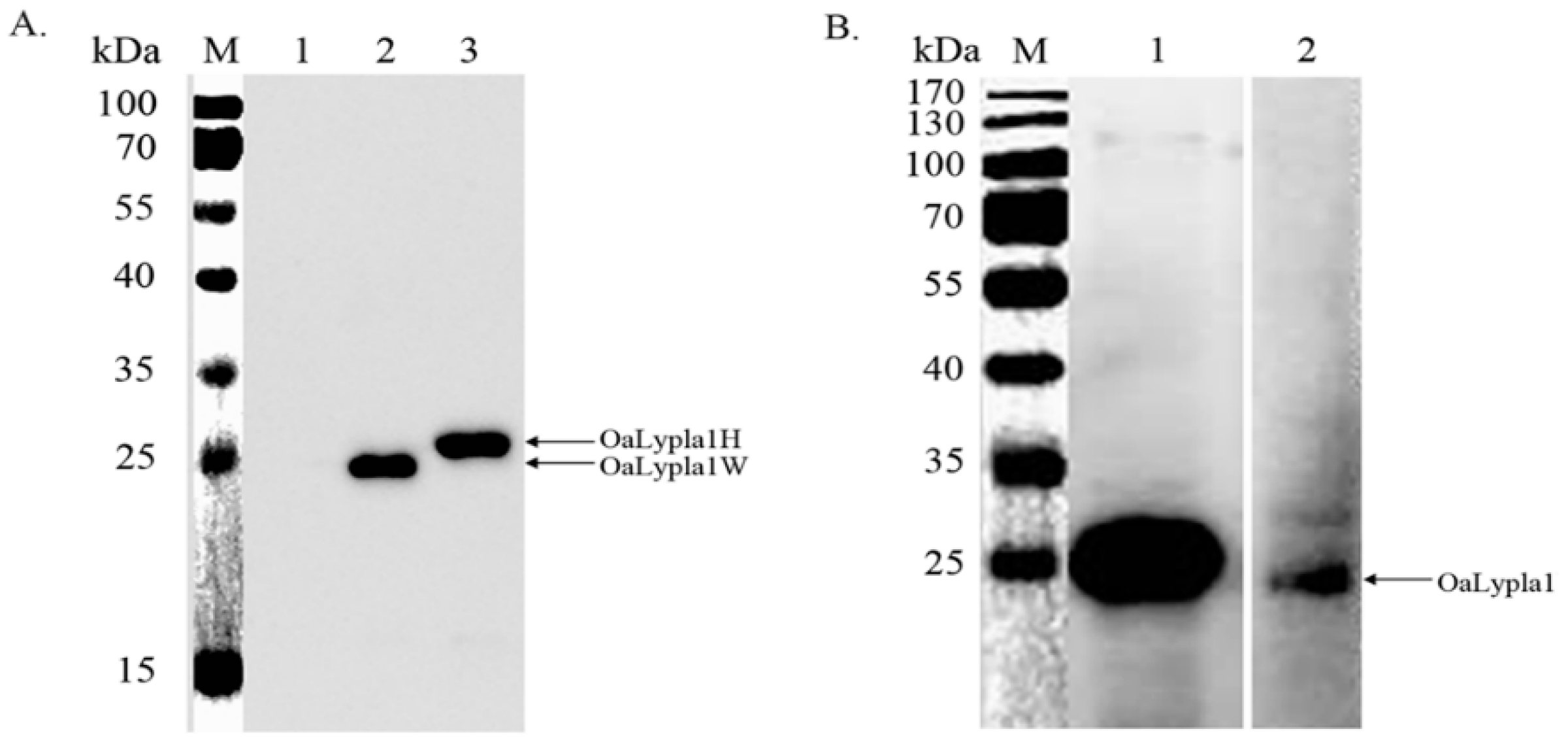
2.4. Specificity of the Monoclonal Antibody (mAb)
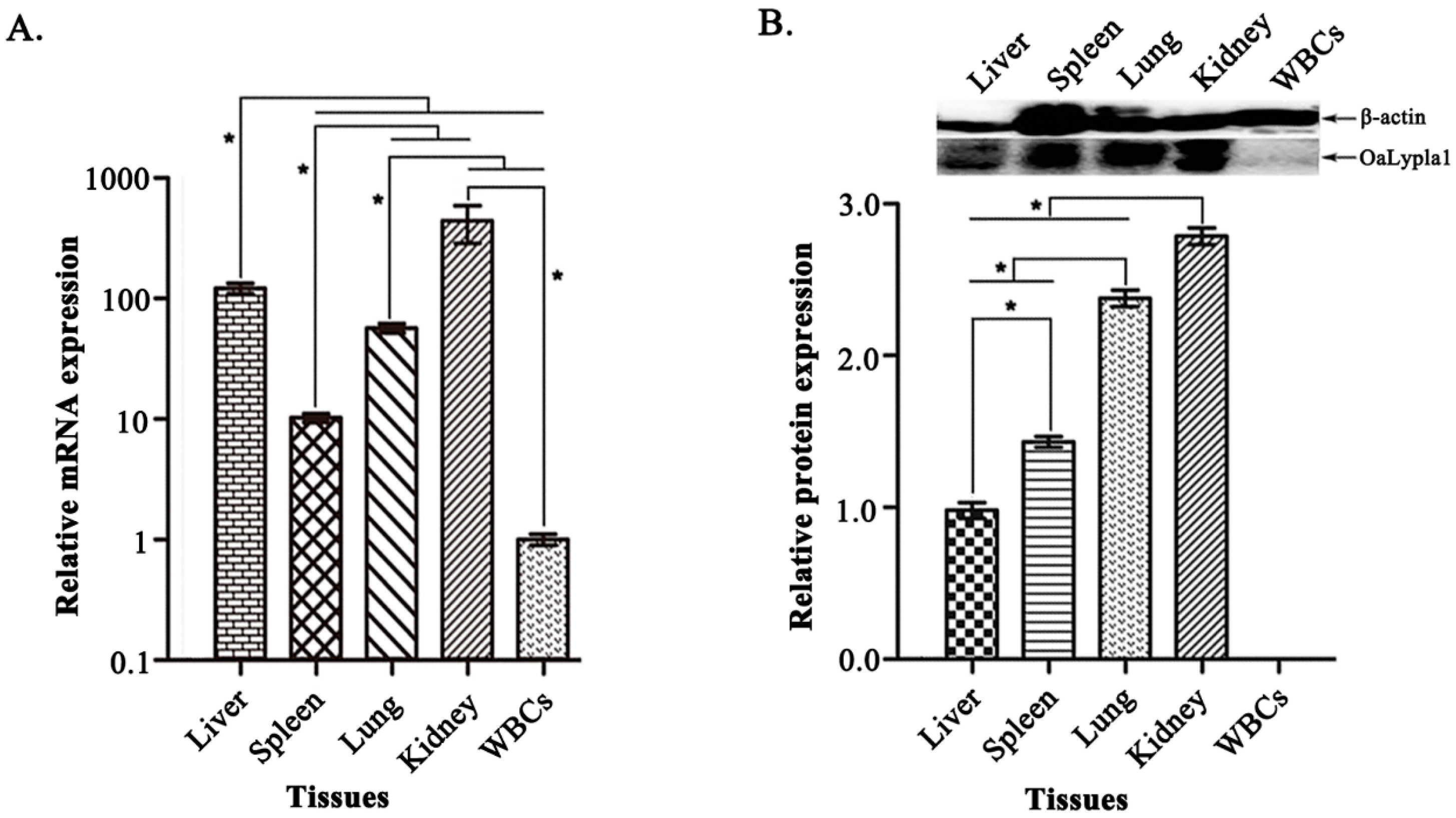
2.5. Tissue Distribution of OaLypla1
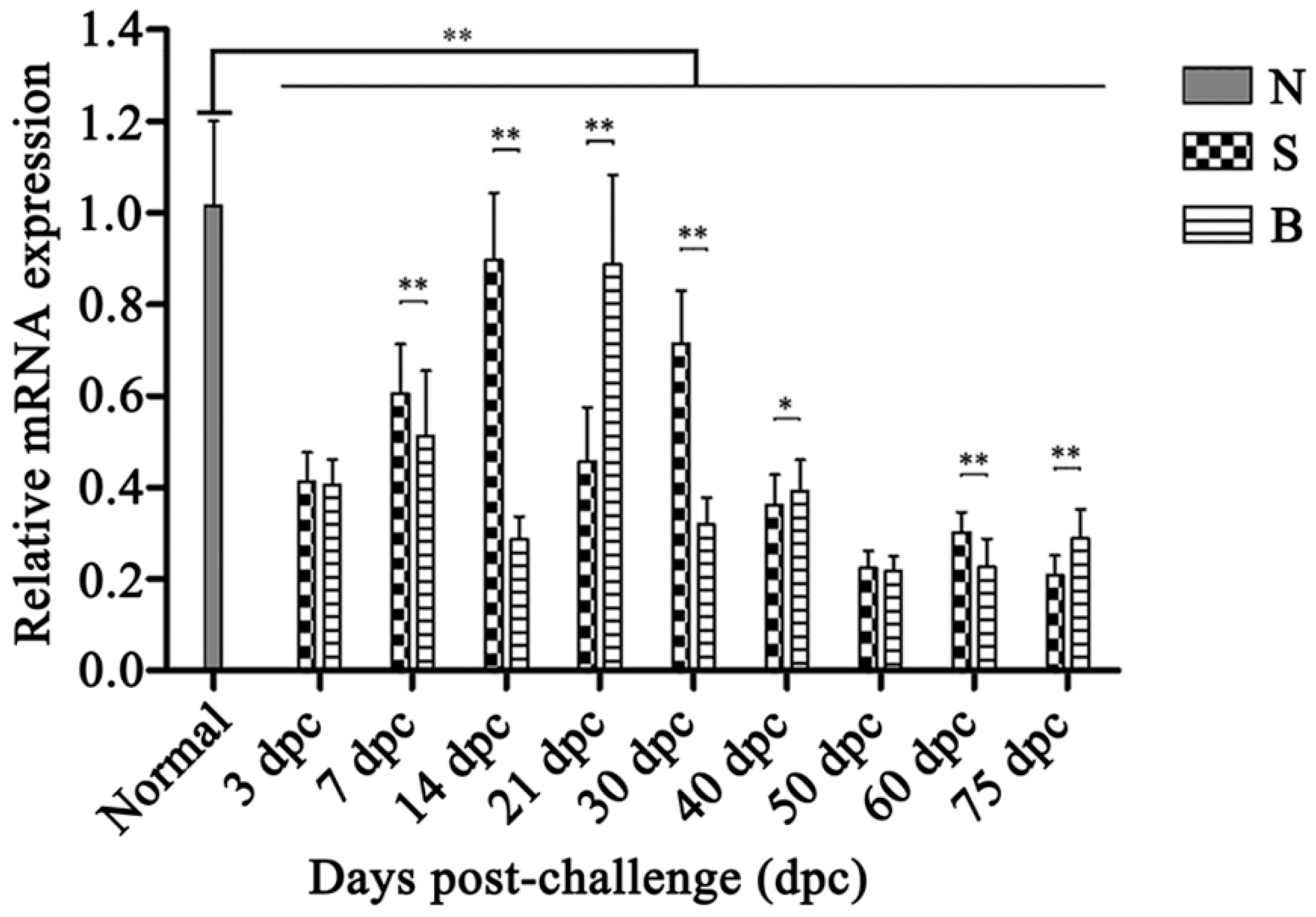
2.6. OaLypla1 Expression Profiles after Challenge with Virulent and Avirulent Brucella Strains
3. Discussion
4. Materials and Methods
4.1. Animals and Cells
4.2. Total RNA Isolation
4.3. 5′-RACE and Sequence Assembly
4.4. Sequence Verification and Analysis
4.5. Cloning of OaLypla1
4.6. Expression and Purification of the Recombinant OaLypla1 Protein
4.7. Phospholipase Activity Assay
4.8. Preparation of the mAb against the OaLypla1 Protein
4.9. Specificity Analysis
4.10. Tissue Distribution
4.11. Relative Transcript Level Analysis of OaLypla1 in Buffy Coats after Challenge with Virulent and Avirulent Brucella Strains
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Portilla, D.; Crew, M.D.; Grant, D.; Serrero, G.; Bates, L.M.; Dai, G.; Sasner, M.; Cheng, J.; Buonanno, A. cDNA cloning and expression of a novel family of enzymes with calcium-independent phospholipase A2 and lysophospholipase activities. J. Am. Soc. Nephrol. 1998, 9, 1178–1186. [Google Scholar] [PubMed]
- Wang, A.; Yang, H.C.; Friedman, P.; Johnson, C.A.; Dennis, E.A. A specific human lysophospholipase: cDNA cloning, tissue distribution and kinetic characterization. Biochim. Biophys. Acta 1999, 1437, 157–169. [Google Scholar] [CrossRef]
- Duncan, J.A.; Gilman, A.G. A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein alpha subunits and p21RAS. J. Biol. Chem. 1998, 273, 15830–15837. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Hayashi, H.; Yamashita, S. Purification, cDNA cloning, and regulation of lysophospholipase from rat liver. J. Biol. Chem. 1996, 271, 7705–7711. [Google Scholar] [PubMed]
- Blaskovic, S.; Adibekian, A.; Blanc, M.; van der Goot, G.F. Mechanistic effects of protein palmitoylation and the cellular consequences thereof. Chem. Phys. Lipids 2014, 180, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Dennis, E.A. Mammalian lysophospholipases. Biochim. Biophys. Acta 1999, 1439, 1–16. [Google Scholar] [CrossRef]
- Moolenaar, W.H.; Hla, T. SnapShot: Bioactive lysophospholipids. Cell 2012, 148, 378–378.e2. [Google Scholar] [CrossRef] [PubMed]
- Sevastou, I.; Kaffe, E.; Mouratis, M.A.; Aidinis, V. Lysoglycerophospholipids in chronic inflammatory disorders: The PLA2/LPC and ATX/LPA axes. Biochim. Biophys. Acta 2013, 1831, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Miyazaki, A.; Hakamata, H.; Kobori, S.; Shichiri, M.; Horiuchi, S. Endocytic uptake of lysophosphatidylcholine mediated by macrophage scavenger receptor plays a major role in oxidized low density lipoprotein-induced macrophage growth. J. Atheroscler Thromb. 1996, 2, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Miyazaki, A.; Hakamata, H.; Sasaki, T.; Yui, S.; Yamazaki, M.; Shichiri, M.; Horiuchi, S. Lysophosphatidylcholine plays an essential role in the mitogenic effect of oxidized low density lipoprotein on murine macrophages. J. Biol. Chem. 1994, 269, 31430–31435. [Google Scholar] [PubMed]
- Ngwenya, B.Z.; Yamamoto, N. Activation of peritoneal macrophages by lysophosphatidylcholine. Biochim. Biophys. Acta 1985, 839, 9–15. [Google Scholar] [CrossRef]
- Burdzy, K.; Munder, P.G.; Fischer, H.; Westphal, O. Increase in the Phagocytosis of Peritoneal Macrophages by Lysolecithin. Z. Naturforschung B 1964, 19, 1118–1120. [Google Scholar]
- Asaoka, Y.; Oka, M.; Yoshida, K.; Sasaki, Y.; Nishizuka, Y. Role of lysophosphatidylcholine in T-lymphocyte activation: Involvement of phospholipase A2 in signal transduction through protein kinase C. Proc. Natl. Acad. Sci. USA 1992, 89, 6447–6451. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, Y.; Oka, M.; Yoshida, K.; Nishizuka, Y. Lysophosphatidylcholine as a possible second messenger synergistic to diacylglycerol and calcium ion for T-lymphocyte activation. Biochem. Biophys. Res. Commun. 1991, 178, 1378–1385. [Google Scholar] [CrossRef]
- Liu-Wu, Y.; Hurt-Camejo, E.; Wiklund, O. Lysophosphatidylcholine induces the production of IL-1 β by human monocytes. Atherosclerosis 1998, 137, 351–357. [Google Scholar] [CrossRef]
- Wang, A.; Johnson, C.A.; Jones, Y.; Ellisman, M.H.; Dennis, E.A. Subcellular localization and PKC-dependent regulation of the human lysophospholipase A/acyl-protein thioesterase in WISH cells. Biochim. Biophys. Acta 2000, 1484, 207–214. [Google Scholar] [CrossRef]
- Aicart-Ramos, C.; Valero, R.A.; Rodriguez-Crespo, I. Protein palmitoylation and subcellular trafficking. Biochim. Biophys. Acta 2011, 1808, 2981–2994. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, T.; Tsutsumi, R.; Noritake, J.; Fukata, Y.; Fukata, M. Dynamic protein palmitoylation in cellular signaling. Prog. Lipid Res. 2009, 48, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, R.; Fukata, Y.; Noritake, J.; Iwanaga, T.; Perez, F.; Fukata, M. Identification of G protein α subunit-palmitoylating enzyme. Mol. Cell. Biol. 2009, 29, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Frenal, K.; Kemp, L.E.; Soldati-Favre, D. Emerging roles for protein S-palmitoylation in Toxoplasma biology. Int. J. Parasitol. 2014, 44, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Kishi, M.; Sugimoto, H.; Taguchi, R.; Obinata, H.; Ohshima, N.; Tatei, K.; Izumi, T. Thioesterase activity and subcellular localization of acylprotein thioesterase 1/lysophospholipase 1. Biochim. Biophys. Acta 2009, 1791, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Rocks, O.; Gerauer, M.; Vartak, N.; Koch, S.; Huang, Z.P.; Pechlivanis, M.; Kuhlmann, J.; Brunsveld, L.; Chandra, A.; Ellinger, B.; et al. The palmitoylation machinery is a spatially organizing system for peripheral membrane proteins. Cell 2010, 141, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Vartak, N.; Papke, B.; Grecco, H.E.; Rossmannek, L.; Waldmann, H.; Hedberg, C.; Bastiaens, P.I. The autodepalmitoylating activity of APT maintains the spatial organization of palmitoylated membrane proteins. Biophys. J. 2014, 106, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Verkruyse, L.A.; Hofmann, S.L. Lysosomal targeting of palmitoyl-protein thioesterase. J. Biol. Chem. 1996, 271, 15831–15836. [Google Scholar] [PubMed]
- Boschiroli, M.L.; Foulongne, V.; O’Callaghan, D. Brucellosis: A worldwide zoonosis. Curr. Opin. Microbiol. 2001, 4, 58–64. [Google Scholar] [CrossRef]
- Lin, Y.; Xiang, Z.; He, Y. Ontology-based representation and analysis of host-Brucella interactions. J. Biomed. Semant. 2015, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Roop, R.M.; Bellaire, B.H.; Valderas, M.W.; Cardelli, J.A. Adaptation of the brucella to their intracellular niche. Mol. Microbiol. 2004, 52, 621–630. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Reichow, S.; Ramamoorthy, S.; Ding, X.; Lathigra, R.; Craig, J.C.; Sobral, B.W.; Schurig, G.G.; Sriranganathan, N.; Boyle, S.M. Brucella melitensis triggers time-dependent modulation of apoptosis and down-regulation of mitochondrion-associated gene expression in mouse macrophages. Infect. Immun. 2006, 74, 5035–5046. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.; de Chastellier, C.; Franchini, D.M.; Pizarro-Cerda, J.; Moreno, E.; Gorvel, J.P. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 2003, 198, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Wu, Q.; Kahl-McDonagh, M.; Ficht, T.A. Cytotoxicity in macrophages infected with rough Brucella mutants is type IV secretion system dependent. Infect. Immun. 2008, 76, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Prada, C.M.; Zelazowska, E.B.; Nikolich, M.; Hadfield, T.L.; Roop, R.M., II; Robertson, G.L.; Hoover, D.L. Interactions between Brucella melitensis and human phagocytes: Bacterial surface O-Polysaccharide inhibits phagocytosis, bacterial killing, and subsequent host cell apoptosis. Infect. Immun. 2003, 71, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Terraza, A.; Ouahrani-Bettache, S.; Liautard, J.P.; Dornand, J. In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells. Infect. Immun. 2000, 68, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Buchmeier, N.A.; Heffron, F. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect. Immun. 1991, 59, 2232–2238. [Google Scholar] [PubMed]
- Eskra, L.; Mathison, A.; Splitter, G. Microarray analysis of mRNA levels from RAW264.7 macrophages infected with Brucella abortus. Infect. Immun. 2003, 71, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, C.A.; Drake, K.L.; Adams, L.G. Transcriptome analysis of HeLa cells response to Brucella melitensis infection: A molecular approach to understand the role of the mucosal epithelium in the onset of the Brucella pathogenesis. Microbes Infect. 2012, 14, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Satou, M.; Nishi, Y.; Yoh, J.; Hattori, Y.; Sugimoto, H. Identification and characterization of acyl-protein thioesterase 1/lysophospholipase I as a ghrelin deacylation/lysophospholipid hydrolyzing enzyme in fetal bovine serum and conditioned medium. Endocrinology 2010, 151, 4765–4775. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Deems, R.A.; Dennis, E.A. Cloning, expression, and catalytic mechanism of murine lysophospholipase I. J. Biol. Chem. 1997, 272, 12723–12729. [Google Scholar] [CrossRef] [PubMed]
- Habermann, E.; Hardt, K.L. A sensitive and specific plate test for the quantitation of phospholipases. Anal. Biochem. 1972, 50, 163–173. [Google Scholar] [CrossRef]
- Wang, A.; Loo, R.; Chen, Z.; Dennis, E.A. Regiospecificity and catalytic triad of lysophospholipase I. J. Biol. Chem. 1997, 272, 22030–22036. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Morales, L.; Diego-Garcia, E.; Segovia, L.; Gutierrez Mdel, C.; Possani, L.D. Venom from the centipede Scolopendra viridis Say: Purification, gene cloning and phylogenetic analysis of a phospholipase A2. Toxicon 2009, 54, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ponce, D.; Lopez-Vera, E.; Aguilar, M.B.; Sanchez-Rodriguez, J. Preliminary results of the in vivo and in vitro characterization of a tentacle venom fraction from the jellyfish Aurelia aurita. Toxins (Basel) 2013, 5, 2420–2433. [Google Scholar] [CrossRef] [PubMed]
- Fortes-Dias, C.L.; Ortolani, P.L.; Fernandes, C.A.; Lobo, K.R.; Amaral de Melo, L.; Borges, M.H.; Pazin, W.M.; Neto Mde, O.; Fernandez, R.M.; Fontes, M.R. Insights on the structure of native CNF, an endogenous phospholipase A2 inhibitor from Crotalus durissus terrificus, the South American rattlesnake. Biochim. Biophys. Acta 2014, 1844, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.L.; Andrich, F.; Fortes-Dias, C.L.; Perales, J.; Teixeira-Ferreira, A.; Vassallo, D.V.; Cruz, J.S.; Figueiredo, S.G. Molecular and biochemical characterization of a cytolysin from the Scorpaena plumieri (scorpionfish) venom: Evidence of pore formation on erythrocyte cell membrane. Toxicon 2013, 74, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Lino, R.C.; Martins, F.I.; Florentino, I.F.; Nascimento, M.V.; Galdino, P.M.; Andrade, C.H.; Rezende, K.R.; Menegatti, R.; Costa, E.A. Anti-inflammatory effect of (E)-4-(3,7-dimethylocta-2,6-dienylamino)phenol, a new derivative of 4-nerolidylcatechol. J. Pharm. Pharmacol. 2013, 65, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Wu, G.; Zhang, W.W. Correlation between mRNA and protein abundance in Desulfovibrio vulgaris: A multiple regression to identify sources of variations. Biochem. Biophys. Res. Commun. 2006, 339, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Stepaniants, S.B.; Mao, M.; Weng, L.; Feetham, M.C.; Doyle, M.J.; Yi, E.C.; Dai, H.; Thorsson, V.; Eng, J.; et al. Integrated genomic and proteomic analyses of gene expression in Mammalian cells. Mol. Cell. Proteom. 2004, 3, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Dorneles, E.M.; Teixeira-Carvalho, A.; Araujo, M.S.; Sriranganathan, N.; Lage, A.P. Immune response triggered by Brucella abortus following infection or vaccination. Vaccine 2015, 33, 3659–3666. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.N.; Liu, Z.S.; Lu, S.Y.; Hu, P.; Li, Y.S.; Feng, X.L.; Zhang, S.Y.; Wang, N.; Meng, Q.F.; Yang, Y.J.; et al. Full-length cDNA cloning, molecular characterization and differential expression analysis of peroxiredoxin 6 from Ovis aries. Vet. Immunol. Immunopathol. 2015, 164, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Ruebsaamen, K. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis 2010, 208, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Graler, M.H.; Goetzl, E.J. Lysophospholipids and their G protein-coupled receptors in inflammation and immunity. Biochim. Biophys. Acta 2002, 1582, 168–174. [Google Scholar] [CrossRef]
- Feng, X.L.; Lu, S.Y.; Liu, D.; Li, L.; Wu, X.Z.; Song, J.; Hu, P.; Li, Y.S.; Tang, F.; Li, Z.H.; et al. Direct competitive immunosorbent assay for detection of MEHP in human urine. Chemosphere 2013, 92, 150–155. [Google Scholar] [CrossRef] [PubMed]
- NCBI. Available online: http://www.ncbi.nlm.nih.gov (accessed on 30 March 2016).
- Compute pI/Mw tool. Available online: http://web.expasy.org/compute_pi/ (accessed on 30 March 2016).
- Motif Scan. Available online: http://hits.isb-sib.ch/cgi-bin/PFSCAN (accessed on 30 March 2016).
- Lobo de Araujo, A.; Radvanyi, F. Determination of phospholipase A2 activity by a colorimetric assay using a pH indicator. Toxicon 1987, 25, 1181–1188. [Google Scholar] [CrossRef]
- Ben Bacha, A.; Al-Daihan, S.K.; Mejdoub, H. Purification, characterization and bactericidal activities of phospholipase A2 from the dromedary intestine. Int. J. Biol. Macromol. 2013, 57, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Abousalham, A.; Verger, R. Egg yolk lipoproteins as substrates for lipases. Biochim. Biophys. Acta 2000, 1485, 56–62. [Google Scholar] [CrossRef]
- Yang, Y.J.; Liu, Z.S.; Lu, S.Y.; Li, C.; Hu, P.; Li, Y.S.; Liu, N.N.; Tang, F.; Xu, Y.M.; Zhang, J.H.; et al. Molecular cloning, expression and characterization of programmed cell death 10 from sheep (Ovis aries). Gene 2015, 558, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Y.Y.; Shen, Q.F.; Lu, S.Y.; Ren, H.L.; Li, Y.S.; Liu, Z.S.; Pan, F.G.; Meng, X.M.; Zhang, J.H. Development of a novel antibody probe useful for domoic acid detection. Biosens. Bioelectron. 2009, 24, 3159–3163. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tang, F.; Ren, H.L.; Xu, Y.M.; Zou, D.Y.; Liu, N.N.; Li, Y.S.; Zhou, Y.; Song, J.; Li, Z.H.; Zhang, Y.Y.; et al. Kinetics and cross-reactivity of the antibody in sheep inoculated with virulent and avirulent Brucella. J. Anim. Vet. Adv. 2012, 11, 1564–1569. [Google Scholar] [CrossRef]
| Primer | Object | Sequence (5′-3′) |
|---|---|---|
| GSP | 5′-RACE | 5′-TCTTGCCATAAGTTAGATCTTGCTG-3′ |
| NGSP | 5′-RACE | 5′-GTCACTTCCATCATCAAATAGCACC-3′ |
| (20)KLS | ORF amplification | 5′-CATATGTGCGGCAATAACATGTCGGC-3′ |
| (20)KLHisA | ORF amplification | 5′-CTCGAGGTCAATGGGAGGTAGGAGCTTATC-3′ |
| (20)KLWA | ORF amplification | 5′-CTCGAGTCAGTCAATGGGAGGTAGGAGCTTATC-3′ |
| β-actin-S | qPCR | 5′-CCCAAGGCCAACCGTGAGAAGATGA-3′ |
| β-actin-A | qPCR | 5′-CGAAGTCCAGGGCCACGTAGCAGAG-3′ |
| OaLypla1S | qPCR | 5′-CCTATTGGTGGCGTGAACAGAGAC-3′ |
| OaLypla1A | qPCR | 5′-GAACTGTGCATCATGCCTGCGTAG-3′ |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.-N.; Liu, Z.-S.; Hu, P.; Zhang, Y.; Lu, S.-Y.; Li, Y.-S.; Yang, Y.-J.; Zhang, D.-S.; Zhou, Y.; Ren, H.-L. Full-Length cDNA Cloning, Molecular Characterization and Differential Expression Analysis of Lysophospholipase I from Ovis aries. Int. J. Mol. Sci. 2016, 17, 1206. https://doi.org/10.3390/ijms17081206
Liu N-N, Liu Z-S, Hu P, Zhang Y, Lu S-Y, Li Y-S, Yang Y-J, Zhang D-S, Zhou Y, Ren H-L. Full-Length cDNA Cloning, Molecular Characterization and Differential Expression Analysis of Lysophospholipase I from Ovis aries. International Journal of Molecular Sciences. 2016; 17(8):1206. https://doi.org/10.3390/ijms17081206
Chicago/Turabian StyleLiu, Nan-Nan, Zeng-Shan Liu, Pan Hu, Ying Zhang, Shi-Ying Lu, Yan-Song Li, Yong-Jie Yang, Dong-Song Zhang, Yu Zhou, and Hong-Lin Ren. 2016. "Full-Length cDNA Cloning, Molecular Characterization and Differential Expression Analysis of Lysophospholipase I from Ovis aries" International Journal of Molecular Sciences 17, no. 8: 1206. https://doi.org/10.3390/ijms17081206
APA StyleLiu, N.-N., Liu, Z.-S., Hu, P., Zhang, Y., Lu, S.-Y., Li, Y.-S., Yang, Y.-J., Zhang, D.-S., Zhou, Y., & Ren, H.-L. (2016). Full-Length cDNA Cloning, Molecular Characterization and Differential Expression Analysis of Lysophospholipase I from Ovis aries. International Journal of Molecular Sciences, 17(8), 1206. https://doi.org/10.3390/ijms17081206