Neuroprotective Effect of Salvianolic Acids against Cerebral Ischemia/Reperfusion Injury
Abstract
:1. Introduction
2. Results
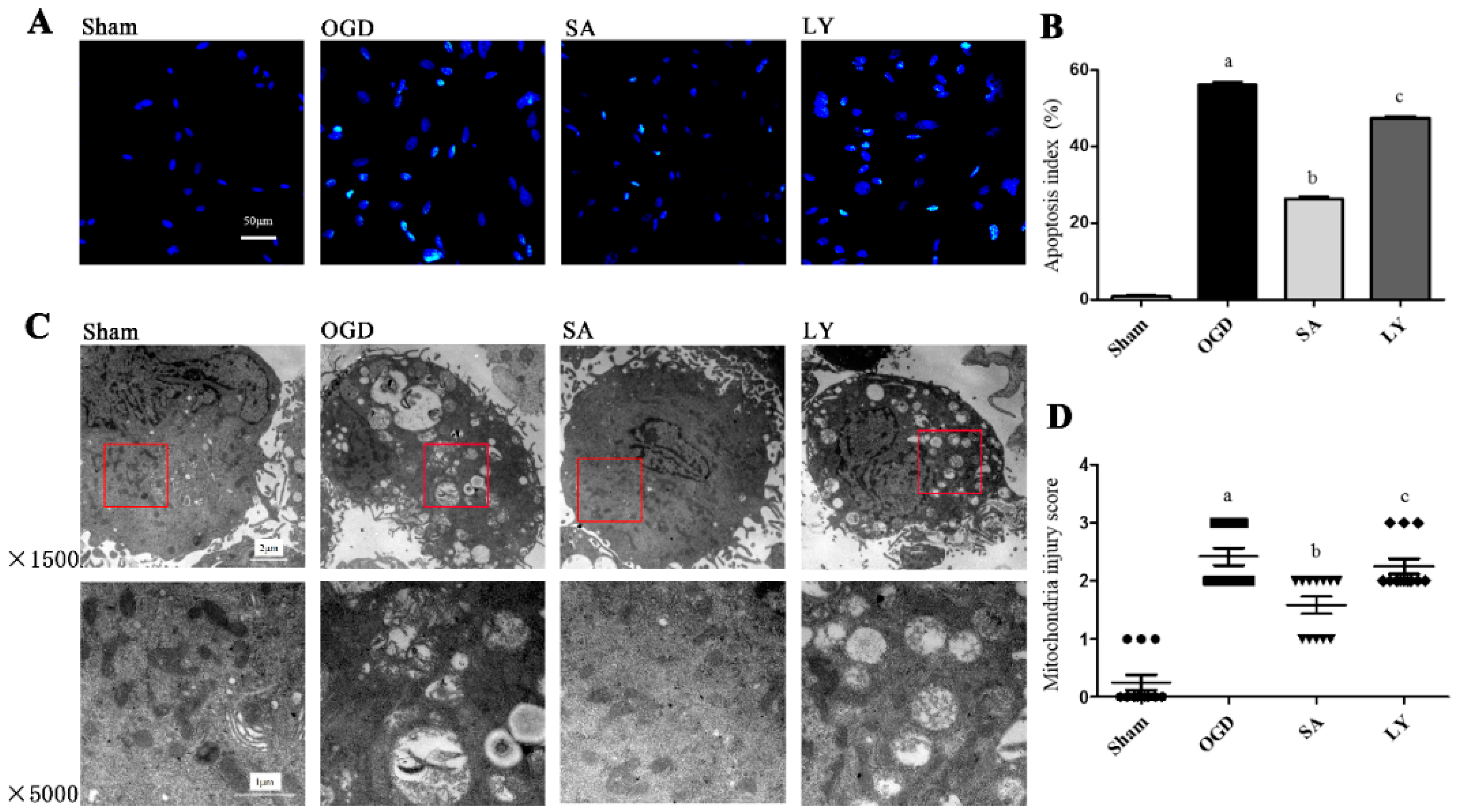
2.1. Effect of SA on Astrocytic Apoptosis Following Oxygen-Glucose Deprivation (OGD) Injury
2.2. Effect of SA on Ultrastructural Damage of Astrocyte Mitochondria
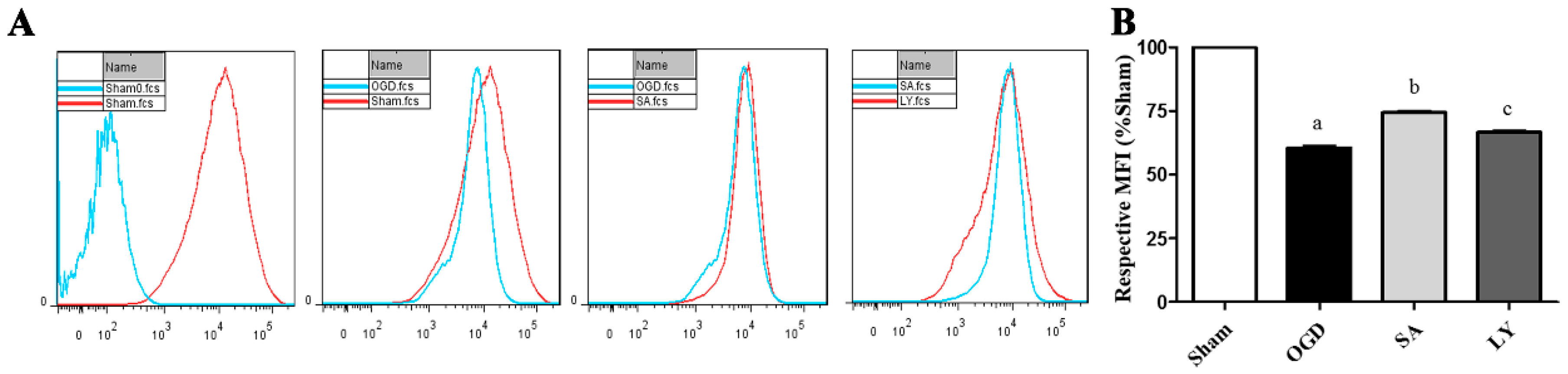
2.3. Effect of SA on Astrocyte Mitochondrial Membrane Potential (MMP)
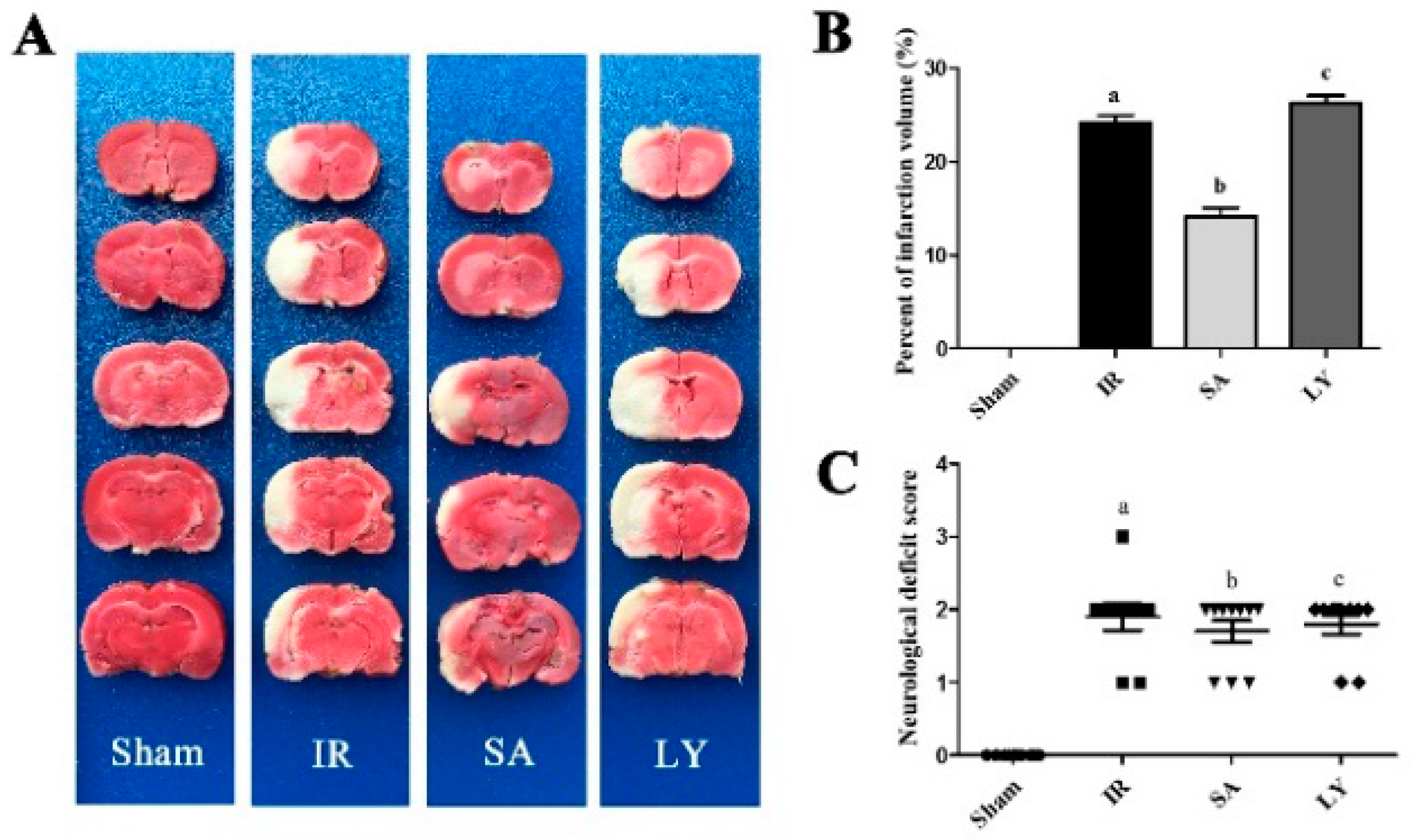
2.4. Infarct Volume and Neurological Deficit Scores after Middle Cerebral Artery Occlusion (MCAO)
2.5. Effect of SA on Superoxide Dismutase (SOD) Activity and Malondialdehyde (MDA) Content
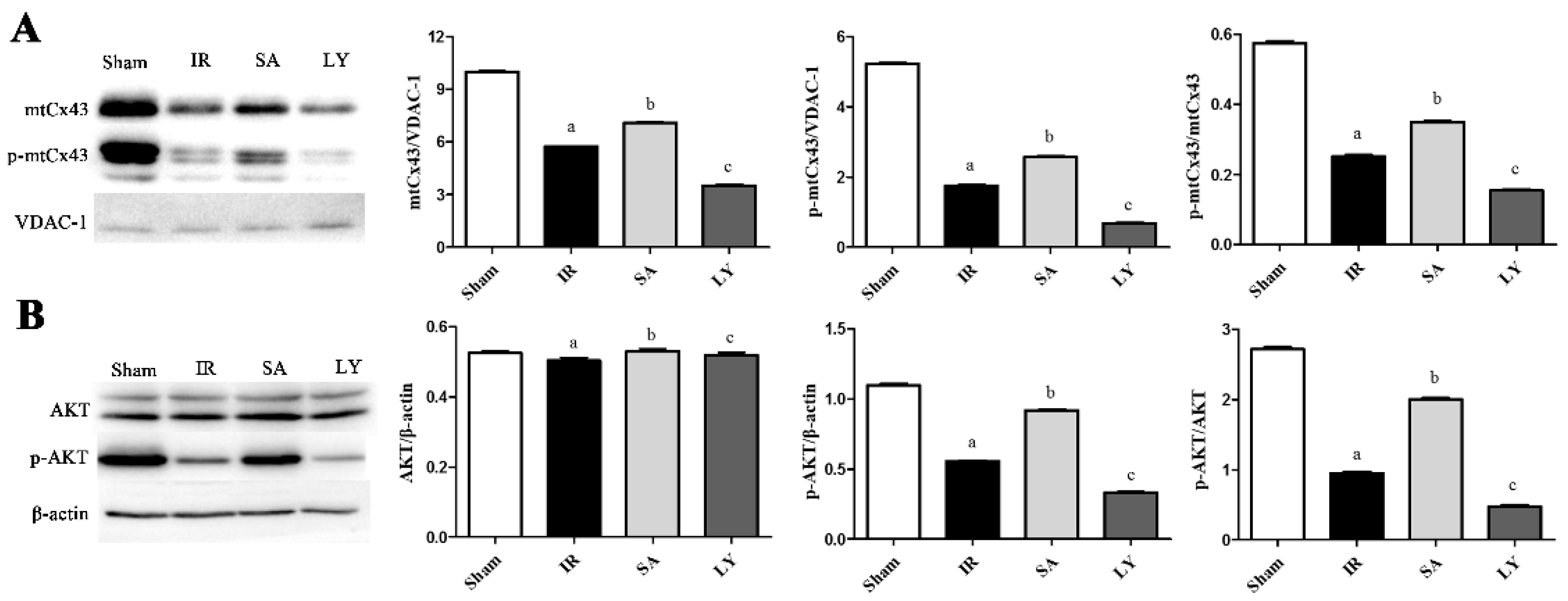
2.6. Effect of SA on mtCx43, p-mtCx43, AKT, and p-AKT Expression
3. Discussion
4. Materials and Methods
4.1. Animals and Drugs
4.2. Isolation and Culture of Rat Astrocytes
4.3. OGD Injury to Astrocytes
4.4. Analysis of Astrocyte Mitochondria Ultrastructure by Transmission Electron Microscopy
4.5. TUNEL Staining to Measure Astrocyte Apoptosis
4.6. Detection of Mitochondrial Membrane Potential
4.7. Middle Cerebral Artery Occlusion Model
4.8. Neurological Evaluation
4.9. TTC Staining
4.10. Mitochondria Isolation
4.11. Detection of SOD Activity and MDA Content
4.12. Western Blot Analysis
4.13. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| I/R | ischemia-reperfusion |
| Cx43 | connexin 43 |
| mtCx43 | mitochondrial connexin 43 |
| MCAO | middle cerebral artery occlusion |
| PI3K/AKT | phosphatidylinositol 3-kinase/protein kinase B |
| SA | salvianolic acids |
| SalB | salvianolic acid B |
| SalA | salvianolic acid A |
| LY | LY 294002 |
| OGD | oxygen glucose deprivation |
| TTC | 2,3,5-triphenyltetrazolium chloride |
| DAPI | 4,6-diamidino-2-phenylindole |
| TUNEL | terminal deoxynucleotidyl transferase dUTP nick end labeling |
| MMP | mitochondrial membrane potential |
| Rh 123 | Rhodamine 123 |
| p- | phosphorylated |
| VDAC-1 | voltage-dependent anion channel |
| SOD | superoxide dismutase |
| MDA | malondialdehyde |
| TEM | transmission electron microscopy |
References
- Broussalis, E.; Killer, M.; McCoy, M.; Harrer, A.; Trinka, E.; Kraus, J. Current therapies in ischemic stroke. Part A. Recent developments in acute stroke treatment and in stroke prevention. Drug Discov. Today 2012, 17, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L. Time is brain—Quantified. Stroke J. Cereb. Circ. 2006, 37, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hu, Y.; Zhai, X.; Lin, M.; Chen, Z.; Tian, X.; Zhang, F.; Gao, D.; Ma, X.; Lv, L.; et al. Salvianolic acid a preconditioning confers protection against concanavalin a-induced liver injury through sirt1-mediated repression of p66shc in mice. Toxicol. Appl. Pharmacol. 2013, 273, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Shen, D.P.; Wang, Q.L.; Tao, Y.Y.; Liu, C.H. Investigation of the absorbed and metabolized components of danshen from fuzheng huayu recipe and study on the anti-hepatic fibrosis effects of these components. J. Ethnopharmacol. 2013, 148, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zhang, X.; Zhang, Q.; Zhao, G.; Wei, J.; Ma, S.; Zhu, W.; Wei, M. Protective effects of salvianolate on microvascular flow in a porcine model of myocardial ischaemia and reperfusion. Arch. Cardiovasc. Dis. 2011, 104, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Tsuchida, M.; Lampe, P.D.; Murakami, M. Cardiomyocyte fgf signaling is required for cx43 phosphorylation and cardiac gap junction maintenance. Exp. Cell Res. 2013, 319, 2152–2165. [Google Scholar] [CrossRef] [PubMed]
- Kalvelyte, A.; Imbrasaite, A.; Bukauskiene, A.; Verselis, V.K.; Bukauskas, F.F. Connexins and apoptotic transformation. Biochem. Pharmacol. 2003, 66, 1661–1672. [Google Scholar] [CrossRef]
- Azarashvili, T.; Baburina, Y.; Grachev, D.; Krestinina, O.; Evtodienko, Y.; Stricker, R.; Reiser, G. Calcium-induced permeability transition in rat brain mitochondria is promoted by carbenoxolone through targeting connexin43. Am. J. Physiol. Cell Physiol. 2011, 300, C707–C720. [Google Scholar] [CrossRef] [PubMed]
- Orellana, J.A.; Froger, N.; Ezan, P.; Jiang, J.X.; Bennett, M.V.; Naus, C.C.; Giaume, C.; Saez, J.C. Atp and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J. Neurochem. 2011, 118, 826–840. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Ungefug, E.; Heusch, G.; Leybaert, L.; Schulz, R. Connexin 43 impacts on mitochondrial potassium uptake. Front. Pharmacol. 2013, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Srisakuldee, W.; Makazan, Z.; Nickel, B.E.; Zhang, F.; Thliveris, J.A.; Pasumarthi, K.B.; Kardami, E. The fgf-2-triggered protection of cardiac subsarcolemmal mitochondria from calcium overload is mitochondrial connexin 43-dependent. Cardiovasc. Res. 2014, 103, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Falkowska, A.; Gutowska, I.; Goschorska, M.; Nowacki, P.; Chlubek, D.; Baranowska-Bosiacka, I. Energy metabolism of the brain, including the cooperation between astrocytes and neurons, especially in the context of glycogen metabolism. Int. J. Mol. Sci. 2015, 16, 25959–25981. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Shin, M.S.; Lee, J.M.; Cho, H.S.; Kim, C.J.; Kim, Y.J.; Choi, H.R.; Jeon, J.W. Inhibitory effects of isoquinoline alkaloid berberine on ischemia-induced apoptosis via activation of phosphoinositide 3-kinase/protein kinase b signaling pathway. Int. Neurourol. J. 2014, 18, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Broughton, B.R.S.; Reutens, D.C.; Sobey, C.G. Apoptotic mechanisms after cerebral ischemia. Stroke J. Cereb. Circ. 2009, 40, e331–e339. [Google Scholar] [CrossRef] [PubMed]
- Bian, B.; Yu, X.; Wang, Q.; Teng, T.; Nie, J. Atorvastatin protects myocardium against ischemia-reperfusion arrhythmia by increasing connexin 43 expression: A rat model. Eur. J. Pharmacol. 2015, 768, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.T.; Guo, Q.M.; Wang, P.; Wang, Q. Salvianic acid a inhibits lipopolysaccharide-induced apoptosis through regulating glutathione peroxidase activity and malondialdehyde level in vascular endothelial cells. Chin. J. Nat. Med. 2012, 10, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.R.; Zhang, H.M.; Ye, T.X.; Xiang, Z.J.; Yuan, Y.J.; Guo, Z.X.; Zhao, L.B. Characterization of the radical scavenging and antioxidant activities of danshensu and salvianolic acid b. Food Chem. Toxicol. 2008, 46, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.J.; Li, D.Y.; Fang, F.; Chen, D.; Qi, L.L.; Zhang, R.Q.; Xu, T.D.; Sun, H. Salvianolic acid a demonstrates cardioprotective effects in rat hearts and cardiomyocytes after ischemia/reperfusion injury. J. Cardiovasc. Pharm. 2011, 58, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Guan, Y.; Duan, J.L.; Wei, G.; Zhu, Y.R.; Quan, W.; Guo, C.; Zhou, D.; Wang, Y.H.; Xi, M.M.; et al. Cardioprotective effect of danshensu against myocardial ischemia/reperfusion injury and inhibits apoptosis of h9c2 cardiomyocytes via akt and erk1/2 phosphorylation. Eur. J. Pharmacol. 2013, 699, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Lin, S.J.; Ku, H.H.; Shiao, M.S.; Lin, F.Y.; Chen, J.W.; Chen, Y.L. Salvianolic acid b attenuates vcam-1 and icam-1 expression in tnf-alpha-treated human aortic endothelial cells. J. Cell. Biochem. 2001, 82, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, M.; Le, X.; Meng, J.; Huang, L.; Yu, P.; Chen, J.; Wu, P. Antioxidant and cardioprotective effects of danshensu (3-(3,4-dihydroxyphenyl)-2-hydroxy-propanoic acid from salvia miltiorrhiza) on isoproterenol-induced myocardial hypertrophy in rats. Phytomed. Int. J. Phytother. Phytopharmacol. 2011, 18, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Lo, E.H.; Dalkara, T.; Moskowitz, M.A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003, 4, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Franke, T.F.; Kaplan, D.R.; Cantley, L.C. Pi3k: Downstream aktion blocks apoptosis. Cell 1997, 88, 435–437. [Google Scholar] [CrossRef]
- Calleja, V.; Alcor, D.; Laguerre, M.; Park, J.; Vojnovic, B.; Hemmings, B.A.; Downward, J.; Parker, P.J.; Larijani, B. Intramolecular and intermolecular interactions of protein kinase b define its activation in vivo. PLoS Biol. 2007, 5, e95. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.A.; Lampe, P.D. Injury-triggered akt phosphorylation of cx43: A zo-1-driven molecular switch that regulates gap junction size. J. Cell Sci. 2014, 127, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Deli, M.A.; Kawaguchi, H.; Shimizudani, T.; Shimono, T.; Kittel, A.; Tanaka, K.; Niwa, M. A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem. Int. 2009, 54, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Flameng, W.; Borgers, M.; Daenen, W.; Stalpaert, G. Ultrastructural and cytochemical correlates of myocardial protection by cardiac hypothermia in man. J. Thorac. Cardiovasc. Surg. 1980, 79, 413–424. [Google Scholar] [PubMed]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke J. Cereb. Circ. 1989, 20, 84–91. [Google Scholar] [CrossRef]
- Hou, S.; Shen, P.P.; Zhao, M.M.; Liu, X.P.; Xie, H.Y.; Deng, F.; Feng, J.C. Mechanism of Mitochondrial Connexin43’s Protection of the Neurovascular Unit under Acute Cerebral Ischemia-Reperfusion Injury. Int. J. Mol. Sci. 2016, 17, 679. [Google Scholar] [CrossRef] [PubMed]
| Group (n = 6 in Each) | SOD Activity (U/mg Protein) | MDA Content (mmol/mg Protein) |
|---|---|---|
| Sham | 118.9 ± 2.3 | 6.1 ± 0.3 |
| IR | 91.0 ± 2.5 a | 10.8 ± 0.6 a |
| SA | 137.6 ± 1.9 b | 8.3 ± 0.3 b |
| LY | 96.3 ± 2.4 c | 9.6 ± 0.4 c |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, S.; Zhao, M.-M.; Shen, P.-P.; Liu, X.-P.; Sun, Y.; Feng, J.-C. Neuroprotective Effect of Salvianolic Acids against Cerebral Ischemia/Reperfusion Injury. Int. J. Mol. Sci. 2016, 17, 1190. https://doi.org/10.3390/ijms17071190
Hou S, Zhao M-M, Shen P-P, Liu X-P, Sun Y, Feng J-C. Neuroprotective Effect of Salvianolic Acids against Cerebral Ischemia/Reperfusion Injury. International Journal of Molecular Sciences. 2016; 17(7):1190. https://doi.org/10.3390/ijms17071190
Chicago/Turabian StyleHou, Shuai, Ming-Ming Zhao, Ping-Ping Shen, Xiu-Ping Liu, Yuan Sun, and Jia-Chun Feng. 2016. "Neuroprotective Effect of Salvianolic Acids against Cerebral Ischemia/Reperfusion Injury" International Journal of Molecular Sciences 17, no. 7: 1190. https://doi.org/10.3390/ijms17071190
APA StyleHou, S., Zhao, M.-M., Shen, P.-P., Liu, X.-P., Sun, Y., & Feng, J.-C. (2016). Neuroprotective Effect of Salvianolic Acids against Cerebral Ischemia/Reperfusion Injury. International Journal of Molecular Sciences, 17(7), 1190. https://doi.org/10.3390/ijms17071190






