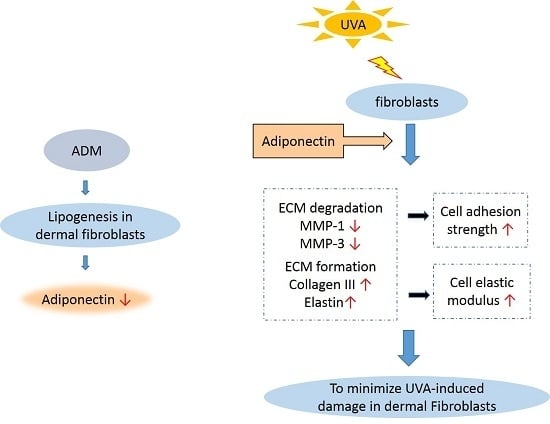
Dermal Lipogenesis Inhibits Adiponectin Production in Human Dermal Fibroblasts while Exogenous Adiponectin Administration Prevents against UVA-Induced Dermal Matrix Degradation in Human Skin
Abstract
:1. Introduction
2. Results
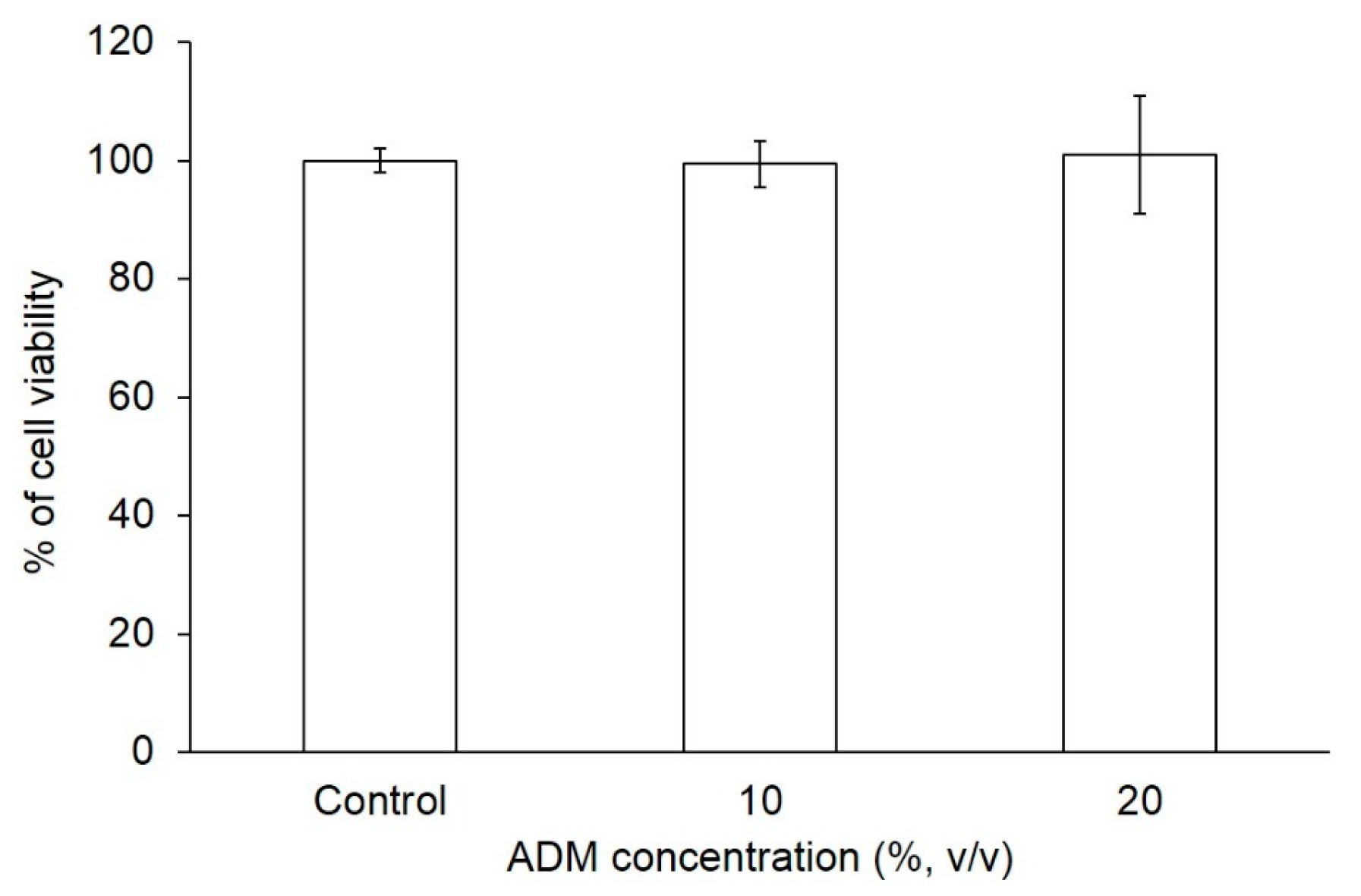
2.1. The Effect of ADM on Cell Viability of Hs68 Fibroblasts
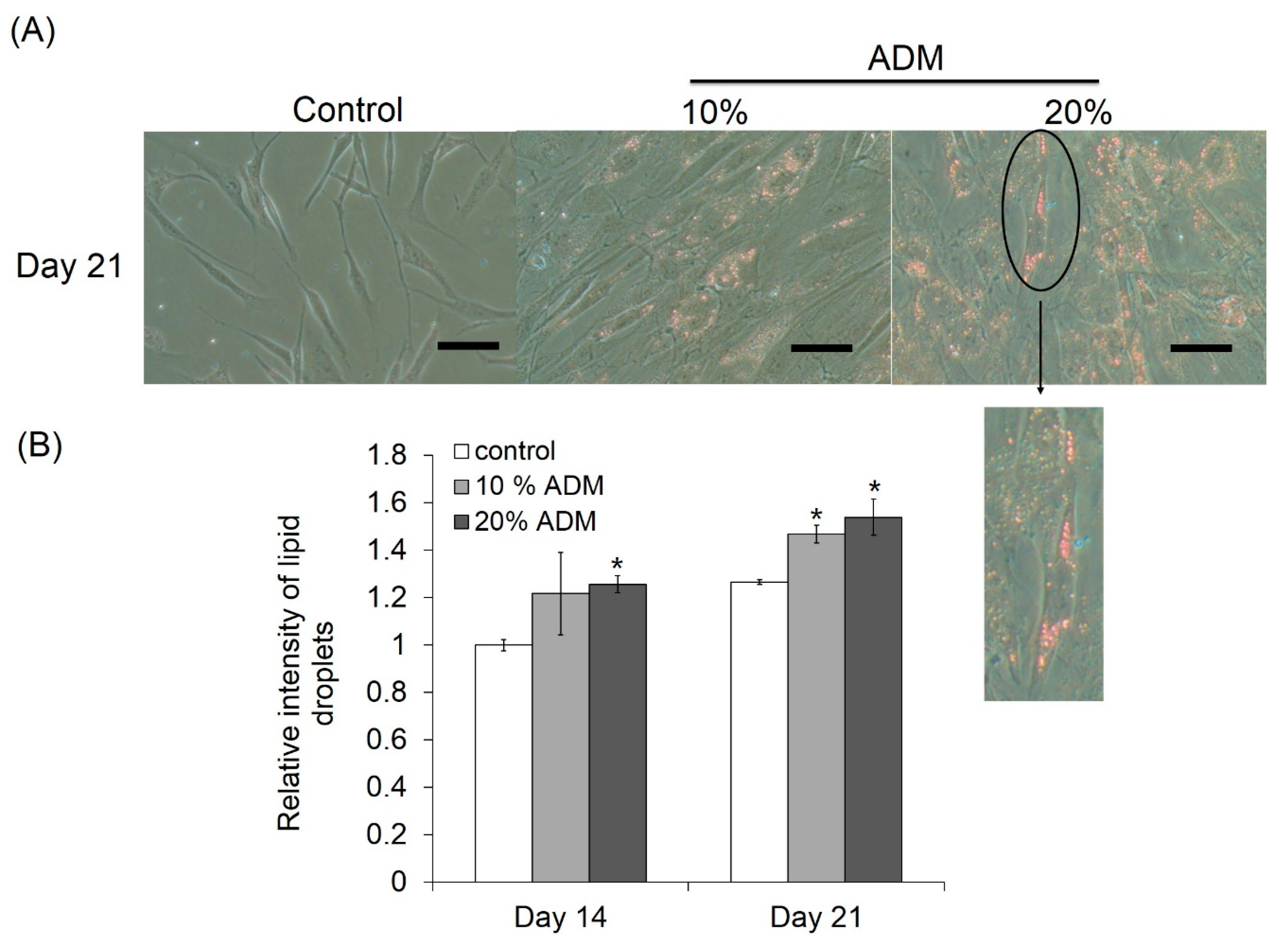
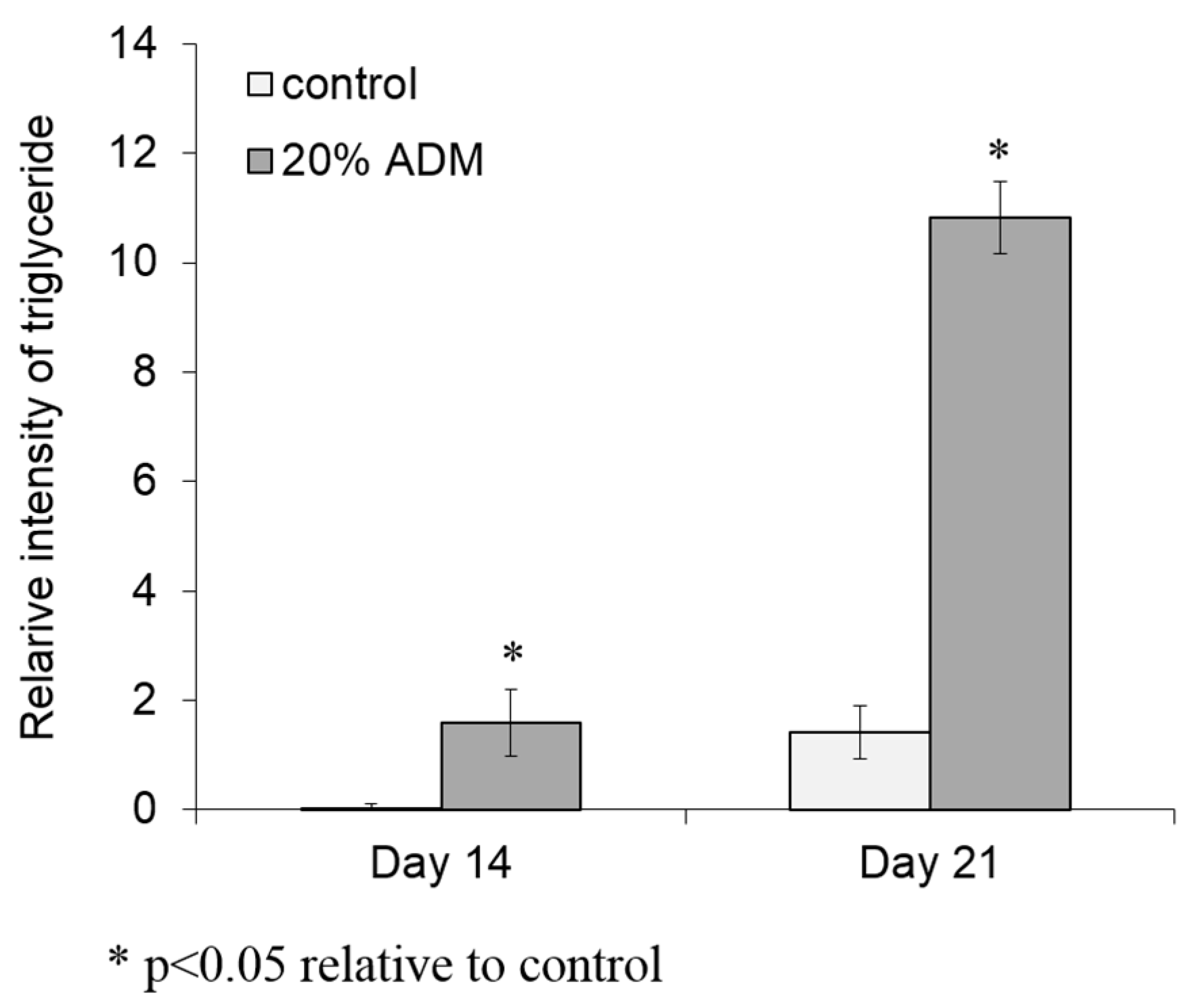
2.2. ADM Stimulated Lipid Accumulation in Hs68 Fibroblasts
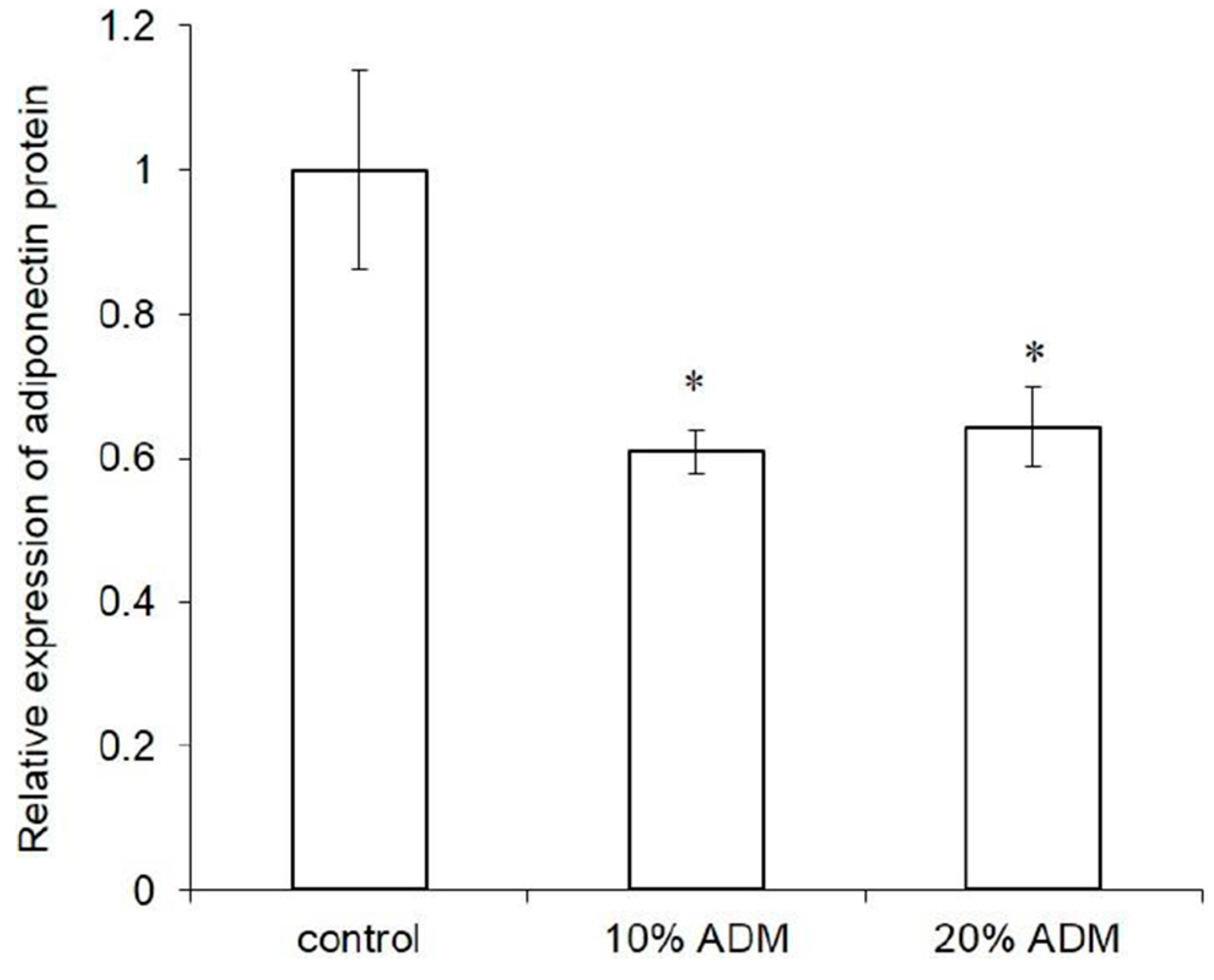
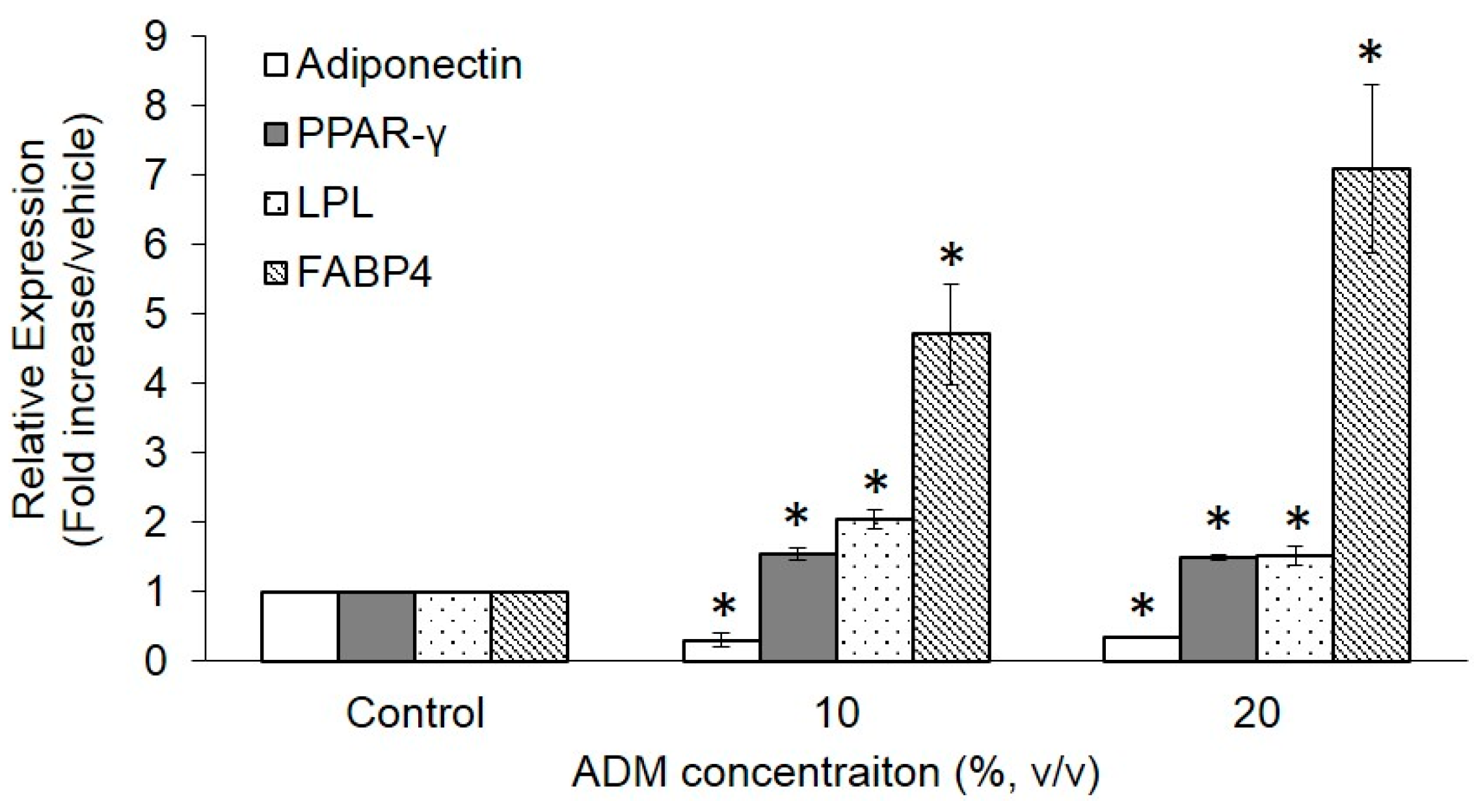
2.3. Expression of Adipogenesis Markers in ADM-Induced Hs68 Fibroblasts
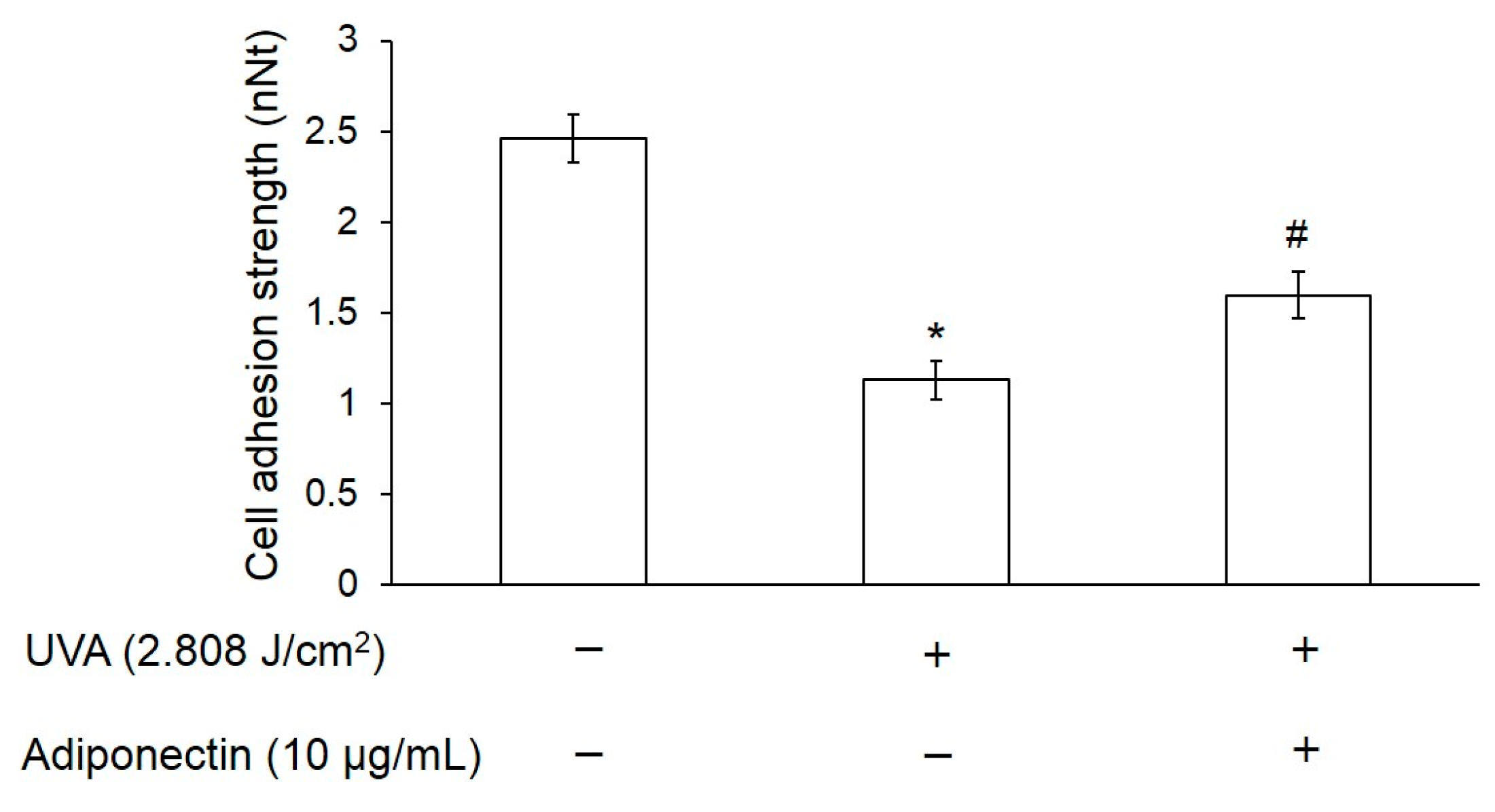
2.4. Adiponectin Can Increase Cell Adhesion Strength of UVA-Irradiated Hs68 Fibroblasts
2.5. Adiponectin Can Increase Elastic Modulus of UVA-Irradiated Hs68 Fibroblasts
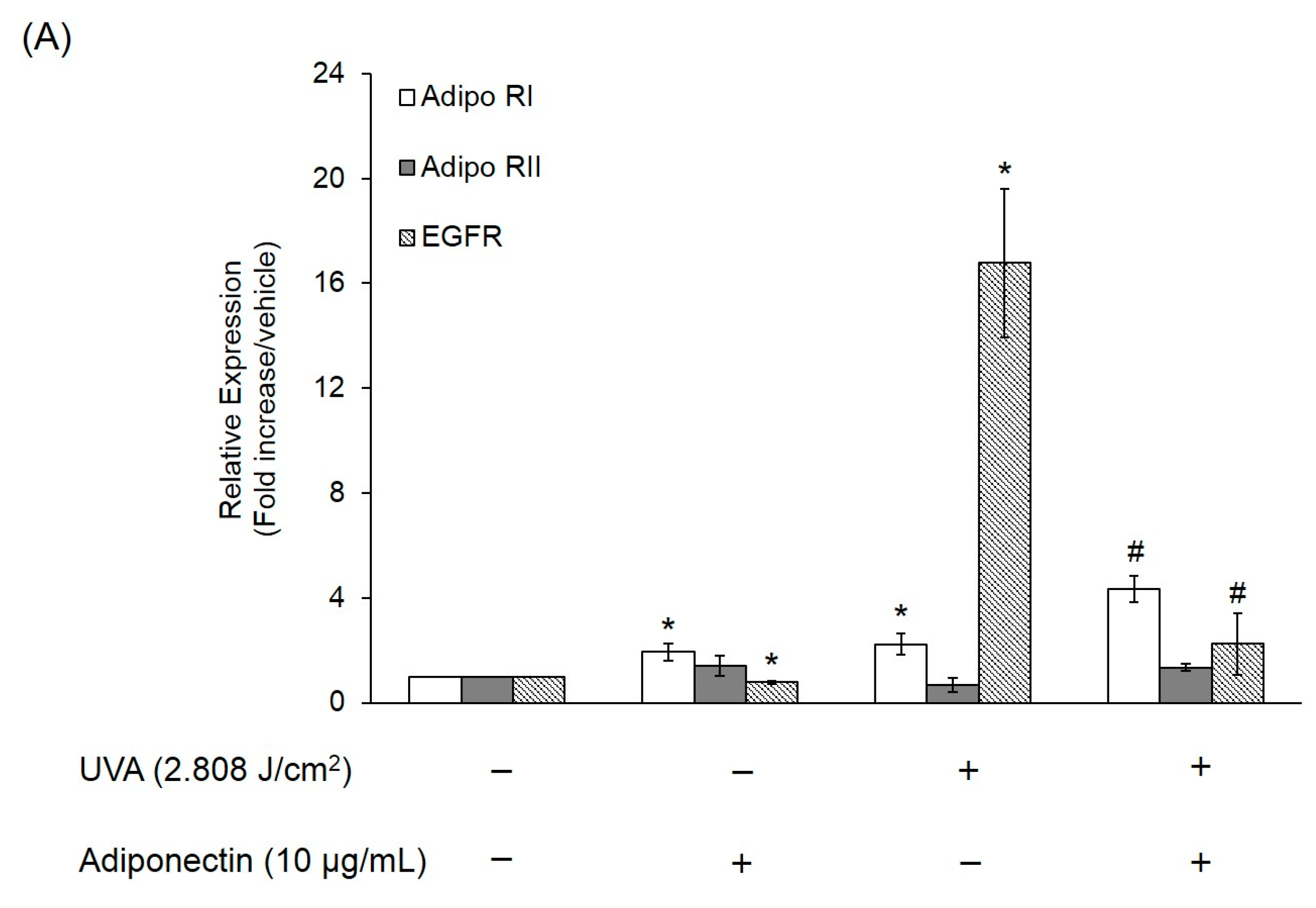
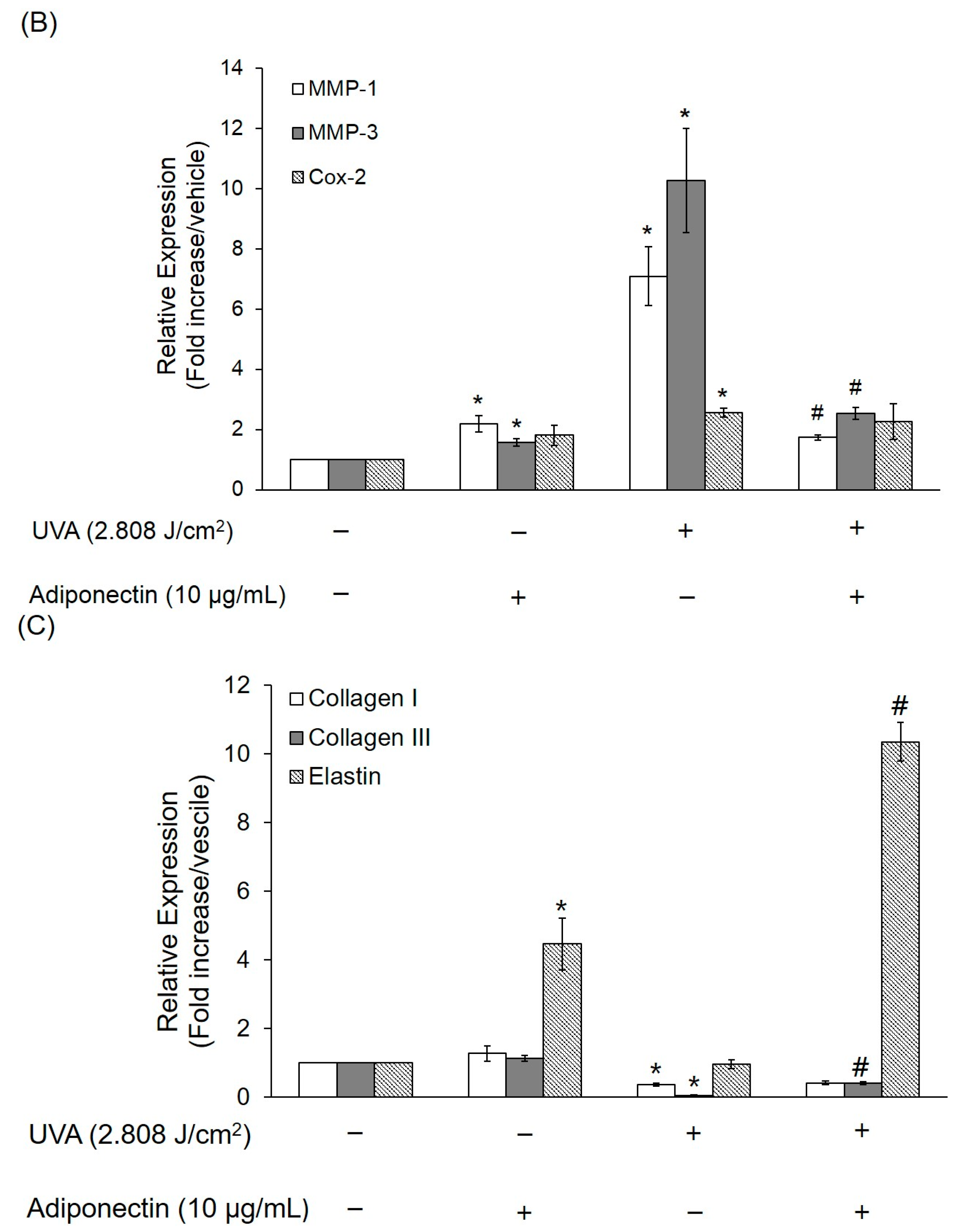
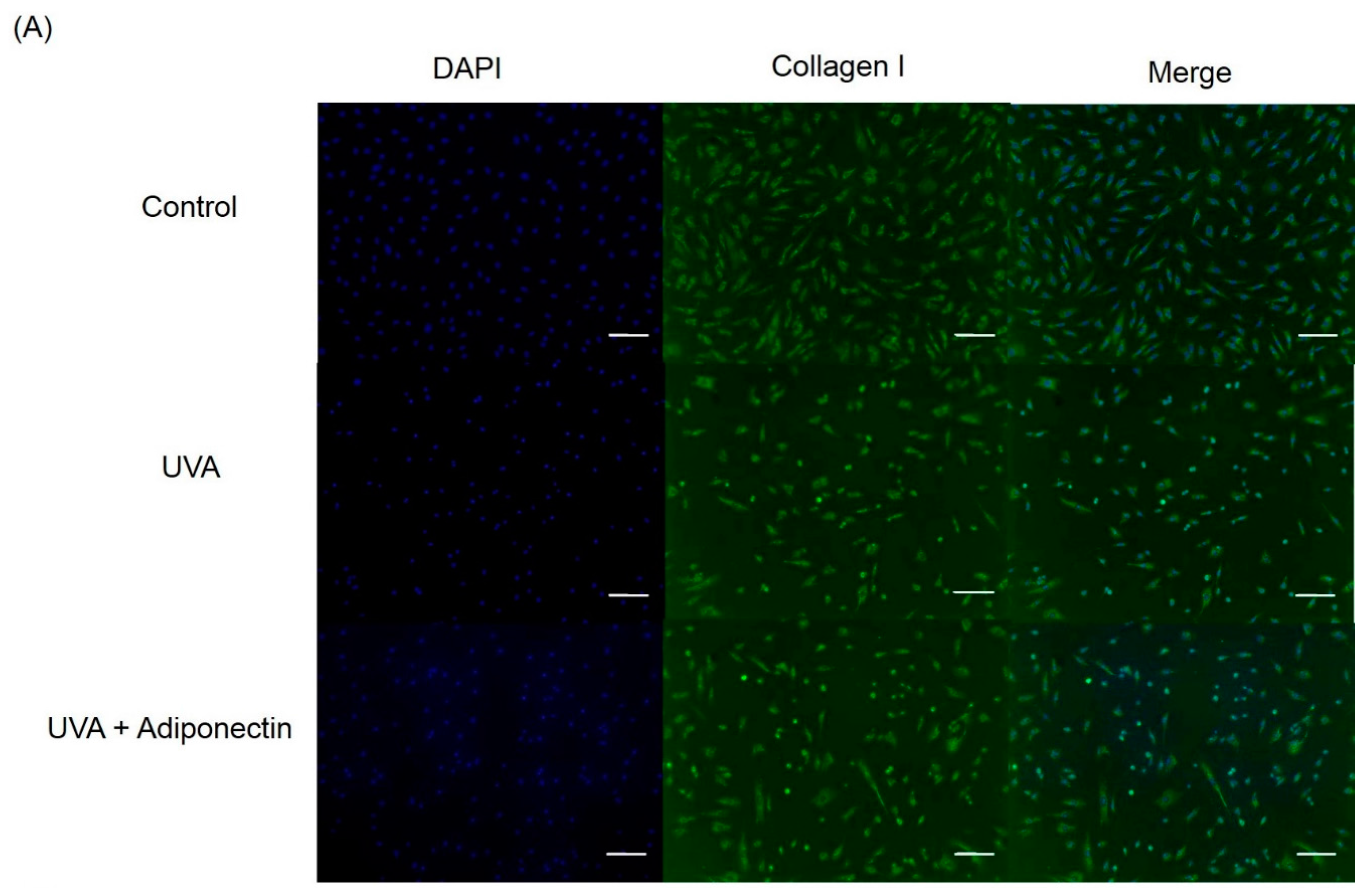
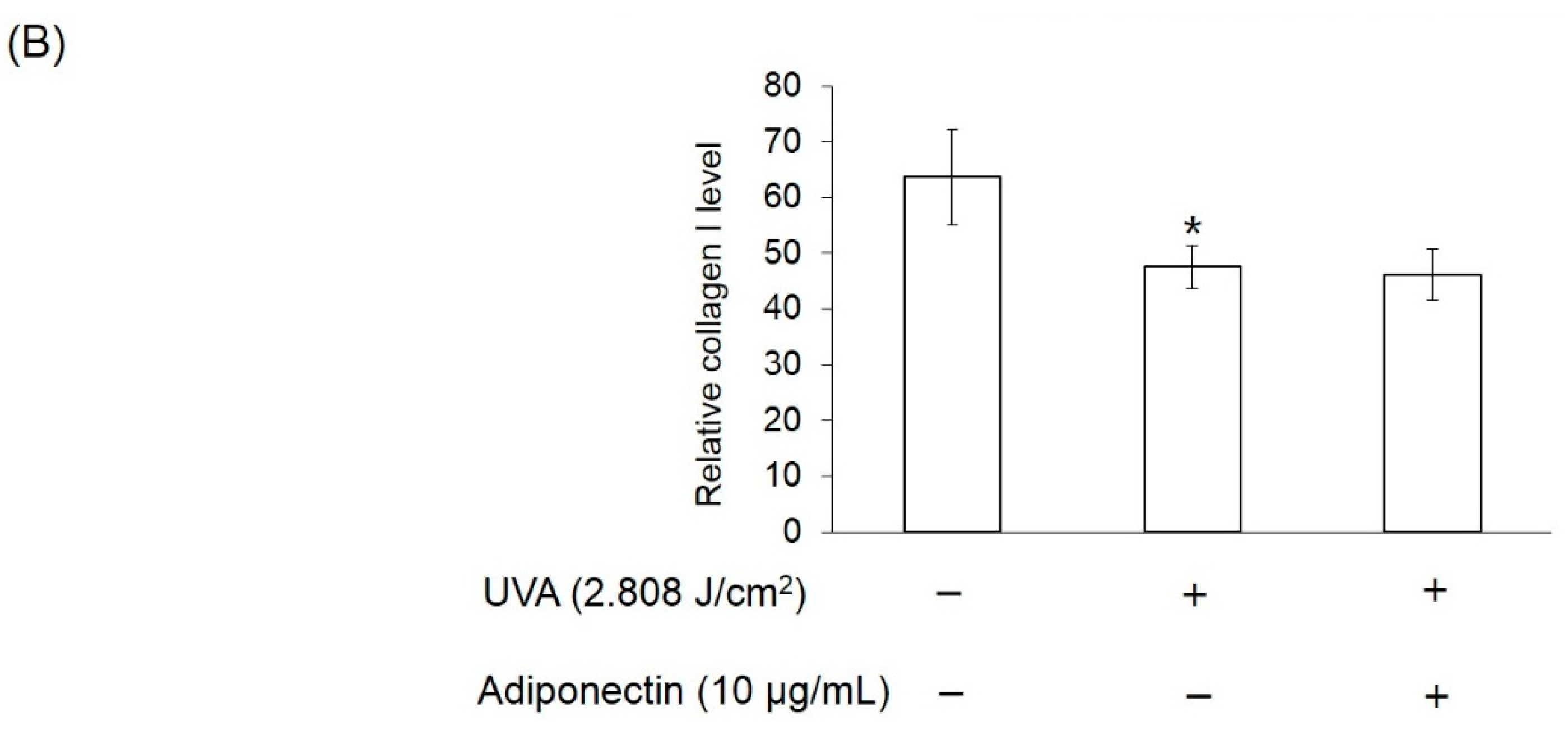
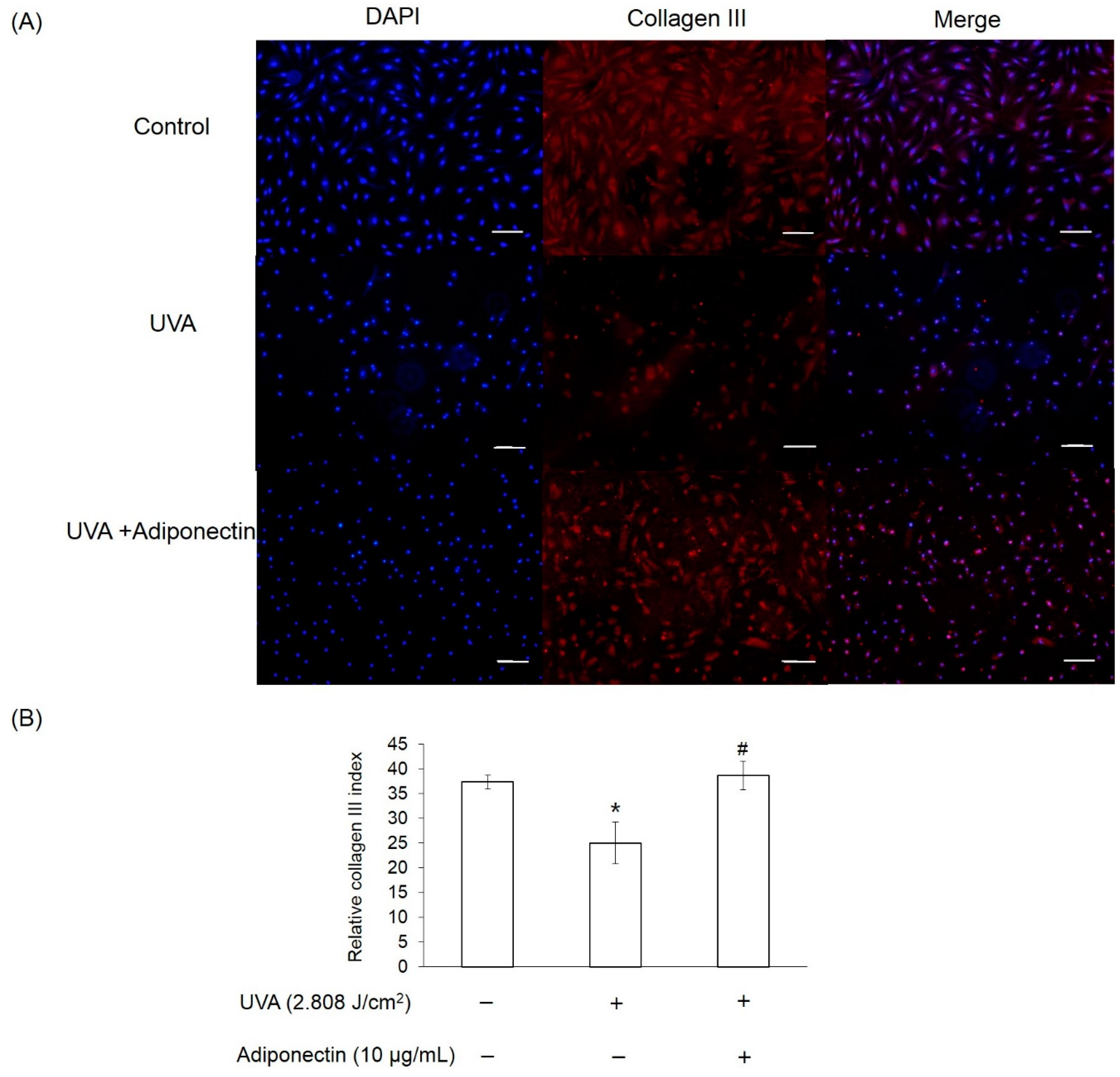
2.6. The Effect of Adiponectin on Gene and Protein Expression of UVA-Irradiated Human Dermal Fibroblasts
3. Discussion
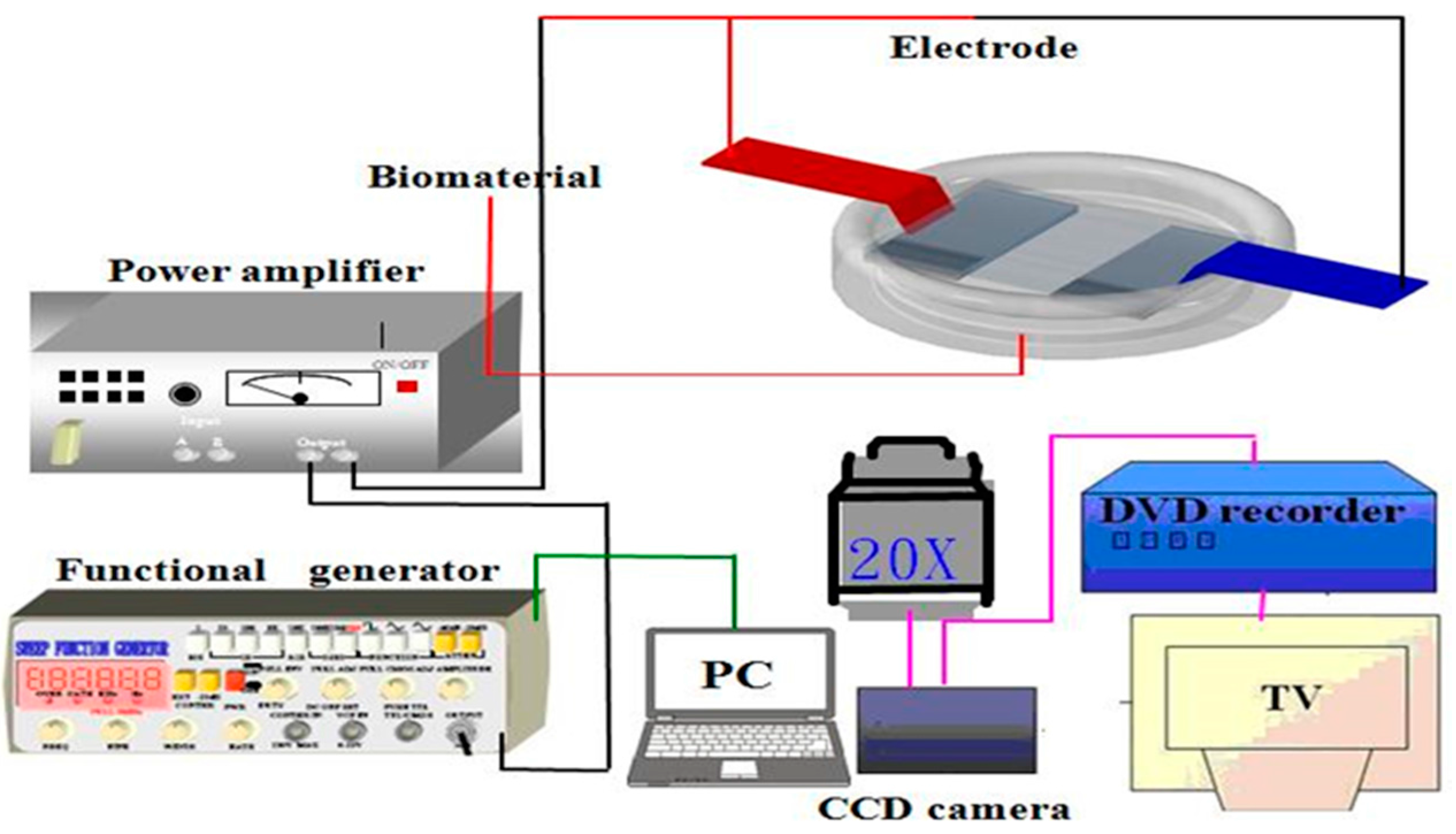
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. UVA Irradiation and Treatment
4.3. Cell Viability
4.4. Oil Red O Staining
4.5. Triglyceride Assay
4.6. Measurement of Cell Adhesion Strength
4.7. The Young’s Modulus Analysis
4.8. Quantitative Real-Time PCR
4.9. Immunofluorescence Staining
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Ezure, T.; Amano, S. Increased subcutaneous adipose tissue impairs dermal function in diet-induced obese mice. Exp. Dermatol. 2010, 19, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, H.; Toda, S.; Yonemitsu, N.; Watanabe, K. Effects of fat cells on keratinocytes and fibroblasts in a reconstructed rat skin model using collagen gel matrix culture. Br. J. Dermatol. 2001, 144, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.E.; Scherer, P.E. Adiponectin—Journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J. Intern. Med. 2005, 257, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Tada, Y.; Hau, C.S.; Mitsui, A.; Kamata, M.; Asano, Y.; Sugaya, M.; Kadono, T.; Masamoto, Y.; Kurokawa, M.; et al. Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from γδ-T cells. Nat. Commun. 2015, 6, 7687. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Jaszewska, E.; Soin, M.; Filipek, A.; Naruszewicz, M. UVA-induced ROS generation inhibition by Oenothera paradoxa defatted seeds extract and subsequent cell death in human dermal fibroblasts. J. Photochem. Photobiol. B 2013, 126, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.E.; Kim, M.S.; Jeon, W.K.; Seo, Y.K.; Kim, B.C.; Hahn, J.H.; Park, C.S. A nuclear factor kappa B-derived inhibitor tripeptide inhibits UVB-induced photoaging process. J. Dermatol. Sci. 2014, 76, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Shin, J.C.; Park, S.M.; Kim, N.R.; Kwak, W.J.; Choi, B.H. Pinus densiflora extract protects human skin fibroblasts against UVB-induced photoaging by inhibiting the expression of MMPs and increasing type I procollagen expression. Toxicol. Rep. 2014, 1, 658–666. [Google Scholar] [CrossRef]
- Ouchi, N.; Walsh, K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta 2007, 380, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.A.; Nguyen, V.T.; Levi, B.; James, A.W. Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev. 2011, 20, 1793–1804. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kadowaki, T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013, 17, 185–196. [Google Scholar] [CrossRef] [PubMed]
- El-Abaseri, T.B.; Hammiller, B.; Repertinger, S.K.; Hansen, L.A. The epidermal growth factor receptor increases cytokine production and cutaneous inflammation in response to ultraviolet irradiation. ISRN Dermatol. 2013, 2013, 848705. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Xuan, W.; Zhao, J.; Bin, J.; Zhao, H.; Asakura, M.; Funahashi, T.; Takashima, S.; Kitakaze, M. Antihypertrophic effects of adiponectin on cardiomyocytes are associated with the inhibition of heparin-binding epidermal growth factor signaling. Biochem. Biophys. Res. Commun. 2010, 393, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Datta, S.; Wang, Z.; Li, X.Y.; Quan, T.; Chung, J.H.; Kang, S.; Voorhees, J.J. c-Jun-Dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J. Clin. Investig. 2000, 106, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Rakar, J.; Lönnqvist, S.; Sommar, P.; Junker, J.; Kratz, G. Interpreted gene expression of human dermal fibroblasts after adipo-, chondro- and osteogenic phenotype shifts. Differentiation 2012, 84, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Camp, H.S.; Ren, D.; Leff, T. Adipogenesis and fat-cell function in obesity and diabetes. Trends Mol. Med. 2002, 8, 442–427. [Google Scholar] [CrossRef]
- Bouraoui, L.; Cruz-Garcia, L.; Gutiérrez, J.; Capilla, E.; Navarro, I. Regulation of lipoprotein lipase gene expression by insulin and troglitazone in rainbow trout (Oncorhynchus mykiss) adipocyte cells in culture. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012, 161, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Liu, W.; Kuang, S. Fatty acid binding protein 4 expression marks a population of adipocyte progenitors in white and brown adipose tissues. FASEB J. 2013, 27, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461S–465S. [Google Scholar] [PubMed]
- Shibata, S.; Tada, Y.; Asano, Y.; Hau, C.S.; Kato, T.; Saeki, H.; Yamauchi, T.; Kubota, N.; Kadowaki, T.; Sato, S. Adiponectin regulates cutaneous wound healing by promoting keratinocyte proliferation and migration via the ERK signaling pathway. J. Immunol. 2012, 189, 3231–3241. [Google Scholar] [CrossRef] [PubMed]
- Christ, K.V.; Williamson, K.B.; Masters, K.S.; Turner, K.T. Measurement of single-cell adhesion strength using a microfluidic assay. Biomed. Microdevices 2010, 12, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bi, B.; Zeng, J.; Zhou, Y.; Yang, P.; Guo, Y.; Zhu, J.; Yang, Q.; Zhu, N.; Liu, T. Rosiglitazone ameliorates senescence-like phenotypes in a cellular photoaging model. J. Dermatol. Sci. 2015, 77, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Oyle, M.; Wong, C.; Rocha, M.; Jones, D.L. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell 2007, 1, 470–478. [Google Scholar]
- Chen, S.; Lewallen, M.; Xie, T. Adhesion in the stem cell niche: Biological roles and regulation. Development 2013, 140, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.S.; Lee, G.Y.; Ong, C.N.; Lim, C.T. AFM indentation study of breast cancer cells. Biochem. Biophys. Res. Commun. 2008, 374, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-H.; Lin, Y.-H.; Lin, S.; Tsai-Wu, J.-J.; Herbert-Wu, C.H.; Jiang, C.-C. Surface ultrastructure and mechanical property of human chondrocyte revealed by atomic force microscopy. Osteoarthr. Cartil. 2008, 16, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Rong, Y.H.; Ning, F.G.; Zhang, G.A. The content and ratio of type I and III collagen in skin differ with age and injury. Afr. J. Biotechnol. 2011, 10, 2524–2529. [Google Scholar]
- Wenk, J.; Brenneisen, P.; Meewes, C.; Wlaschek, M.; Peters, T.; Blaudschun, R.; Ma, W.; Kuhr, L.; Schneider, L.; Scharffetter-Kochanek, K. UV-induced oxidative stress and photoaging. Curr. Probl. Dermatol. 2001, 29, 83–94. [Google Scholar] [PubMed]
- Carbone, F.; La Rocca, C.; Matarese, G. Immunological functions of leptin and adiponectin. Biochimie 2012, 94, 2082–2088. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, Y.K.; Kim, M.K.; Kim, S.; Kim, J.Y.; Lee, D.H.; Chung, J.H. UV-induced inhibition of adipokine production in subcutaneous fat aggravates dermal matrix degradation in human skin. Sci. Rep. 2016, 6, 25616. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Han, J.; Lee, C.S.; Soo, B.H.; Lim, K.M.; Ha, H. Carnosic acid, a phenolic diterpene from rosemary, prevents UV-induced expression of matrix metalloproteinases in human skin fibroblasts and keratinocytes. Exp. Dermatol. 2013, 22, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Lorch, J.H.; Thomas, T.O.; Schmoll, H.J. Bortezomib inhibits cell-cell adhesion and cell migration and enhances epidermal growth factor receptor inhibitor-induced cell death in squamous cell cancer. Cancer Res. 2007, 67, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, G.; Brown, G.; Lauffenburger, D.A.; Wells, A.; Griffith, L.G. Cell adhesion and motility depend on nanoscale RGD clustering. J. Cell Sci. 2000, 113, 1677–1686. [Google Scholar] [PubMed]
- Koskinen, A.; Juslin, S.; Nieminen, R.; Moilanen, T.; Vuolteenaho, K.; Moilanen, E. Adiponectin associates with markers of cartilage degradation in osteoarthritis and induces production of proinflammatory and catabolic factors through mitogen-activated protein kinase pathways. Arthritis Res. Ther. 2011, 13, R184. [Google Scholar] [CrossRef] [PubMed]
- Dadson, K.; Turdi, S.; Boo, S.; Hinz, B.; Sweeney, G. Temporal and Molecular analyses of cardiac extracellular matrix remodeling following pressure overload in adiponectin deficient mice. PLoS ONE 2015, 10, e0121049. [Google Scholar] [CrossRef] [PubMed]
- Vorkapic, E.; Wågsäter, D.; Stijn, C.V.; Kim, J.; Eriksson, P.; Tangirala, R. Adiponectin inhibits angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 2013, 33, A243. [Google Scholar]
- Ay, C.; Yeh, C.C.; Hsu, M.C.; Hurng, H.Y.; Kwok, P.C.L.; Chang, H.I. Evaluation of the Correlation between Focal Adhesion Kinase Phosphorylation and Cell Adhesion Force Using “DEP” Technology. Sensors 2012, 12, 5951–5965. [Google Scholar] [CrossRef] [PubMed]
- Lien, H.C.; Wu, M.C.; Yeh, M.K.; Chang, H.I. Using atomic force microscope to investigate the influence of UVB radiation on cell behaviour and elasticity of dermal fibroblasts. Int. J. Number Theory 2013, 10, 973–983. [Google Scholar] [CrossRef]


| Primer | Sequence (Forward) | Sequence (Reverse) |
|---|---|---|
| GADPH | CATGAGAAGTATGACAACAGCCT | AGTCCTTCCACGATACCAAAGT |
| PPAR-γ | AGCCTGCGAAAGCCTTTTGGTG | GGCTTCACATTCAGCAAACCTGG |
| LPL | GGACTTGGAGATGTGGACCA | TGCTGCTTCTTTTGGCTCTG |
| FABP4 | AAAGTCAAGAGCACCATAACC | TTCAATGCGAACTTCAGTCC |
| Adiponectin | TATCCCCAACATGCCCATTCG | TAGGCAAAGTAGTACAGCCCA |
| EGFR | TGGCATCTTTAAGGGCTCCA | TGGCTAGTCGGTGTAAACGT |
| Adipo-RI | ACACCCTTCTCTGAGCCTTC | GGTGTGAAAGTGGGCTGAAG |
| Adipo-RII | TGGAGCCCATTTTAGAGGCA | CACCGACCTTCCCATACCTT |
| MMP-1 | CTGGCCACAACTGCCAAATG | CTGTCCCTGAACAGCCCAGTACTT |
| MMP-3 | ATTCCATGGAGCCAGGCTTTC | CATTTGGGTCAAACTCCAACTGTG |
| COX-2 | GCCCTTCACGTTATTGCAGATG | ATATGTTCTCCTGCCTACTGGAA |
| Collagen-I | AAAAGGAAGCTTGGTCCACT | GTGTGGAGAAAGGAGCAGAA |
| Collagen-III | GAAGGGCAGGGAACAACTTG | TTTGGCATGGTTCTGGCTTC |
| Elastin | GGCCATTCCTGGTGGAGTTCC | AACTGGCTTAAGAGGTTTGCCTCCA |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, C.-L.; Huang, L.-H.; Tsai, H.-Y.; Chang, H.-I. Dermal Lipogenesis Inhibits Adiponectin Production in Human Dermal Fibroblasts while Exogenous Adiponectin Administration Prevents against UVA-Induced Dermal Matrix Degradation in Human Skin. Int. J. Mol. Sci. 2016, 17, 1129. https://doi.org/10.3390/ijms17071129
Fang C-L, Huang L-H, Tsai H-Y, Chang H-I. Dermal Lipogenesis Inhibits Adiponectin Production in Human Dermal Fibroblasts while Exogenous Adiponectin Administration Prevents against UVA-Induced Dermal Matrix Degradation in Human Skin. International Journal of Molecular Sciences. 2016; 17(7):1129. https://doi.org/10.3390/ijms17071129
Chicago/Turabian StyleFang, Chien-Liang, Ling-Hung Huang, Hung-Yueh Tsai, and Hsin-I Chang. 2016. "Dermal Lipogenesis Inhibits Adiponectin Production in Human Dermal Fibroblasts while Exogenous Adiponectin Administration Prevents against UVA-Induced Dermal Matrix Degradation in Human Skin" International Journal of Molecular Sciences 17, no. 7: 1129. https://doi.org/10.3390/ijms17071129
APA StyleFang, C.-L., Huang, L.-H., Tsai, H.-Y., & Chang, H.-I. (2016). Dermal Lipogenesis Inhibits Adiponectin Production in Human Dermal Fibroblasts while Exogenous Adiponectin Administration Prevents against UVA-Induced Dermal Matrix Degradation in Human Skin. International Journal of Molecular Sciences, 17(7), 1129. https://doi.org/10.3390/ijms17071129