Mechanisms Underlying the Immune Response Generated by an Oral Vibrio cholerae Vaccine
Abstract
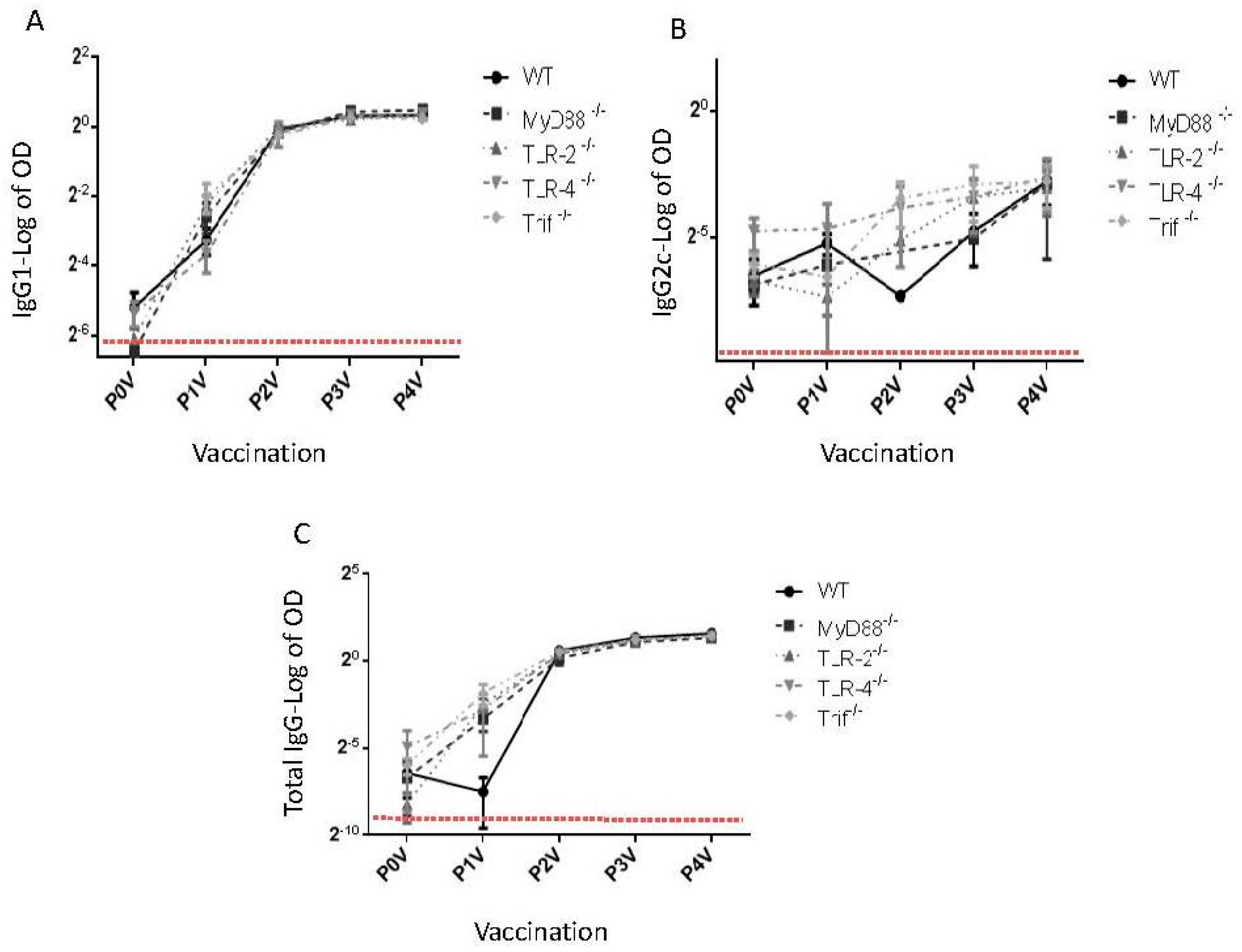
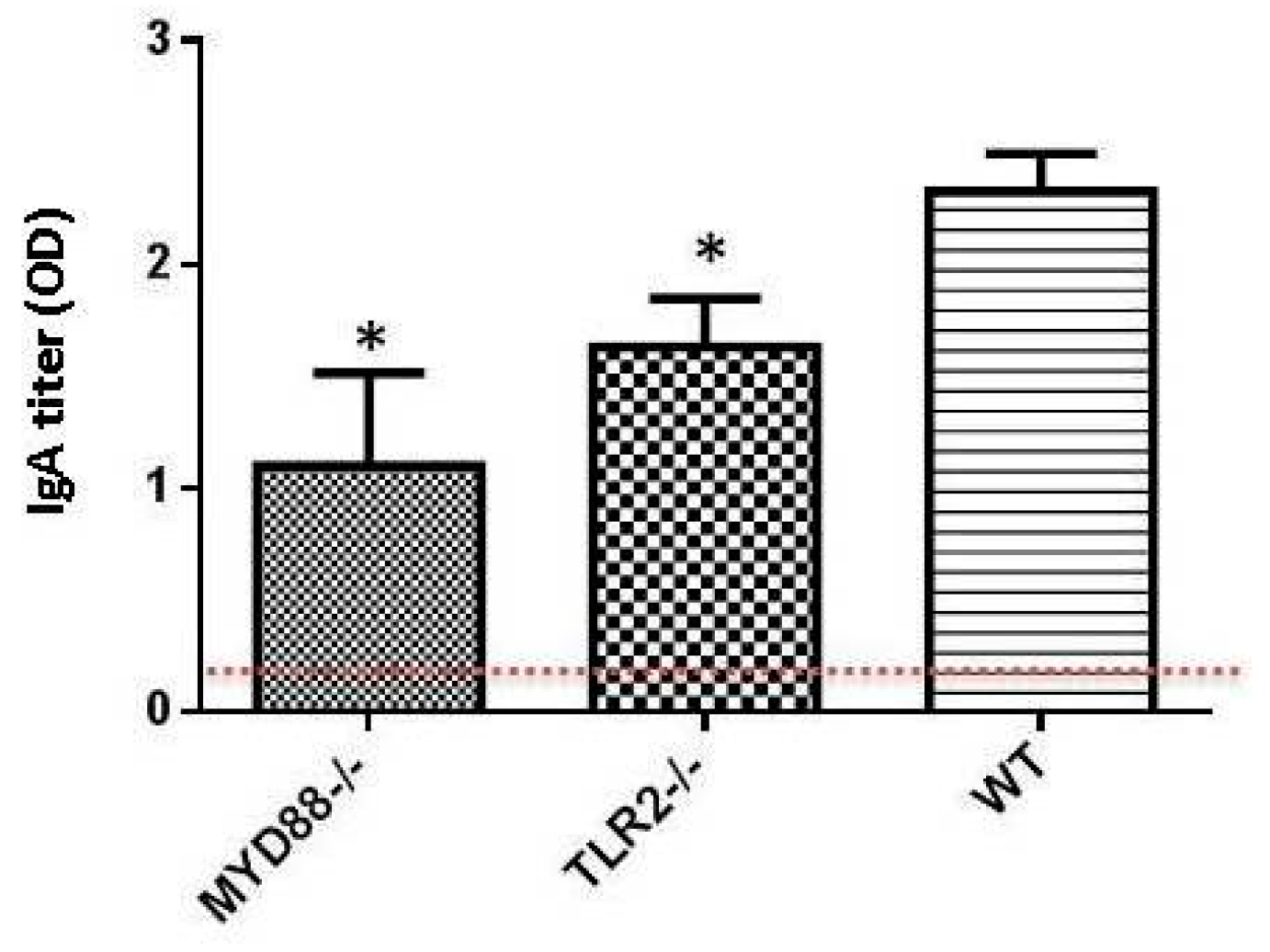
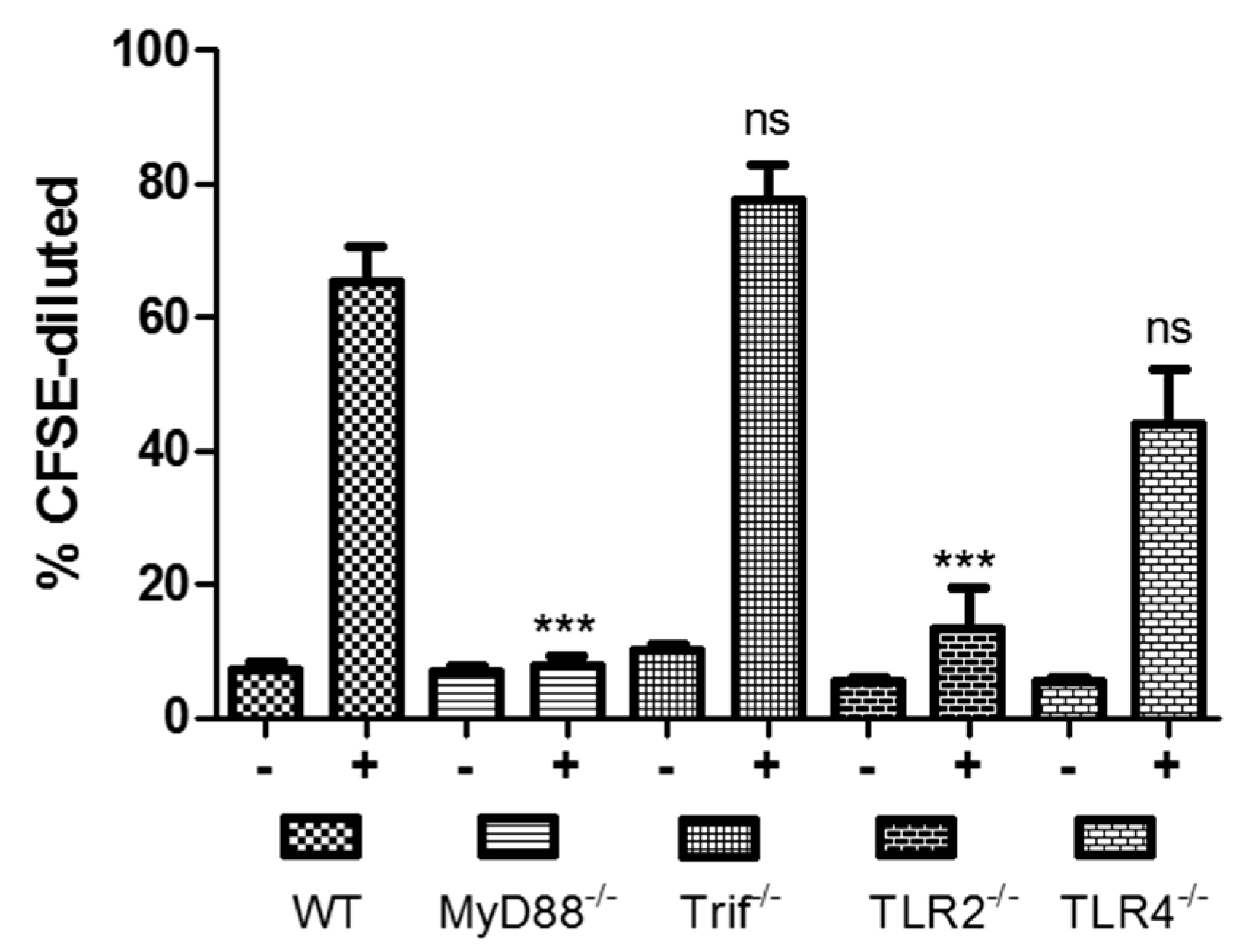
:1. Toll-Like Receptors and Dukoral Vaccine
2. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| CTB | Cholera toxin B subunit |
| TLR | Toll-Like Receptors |
| ETEC | Enterotoxigenic Escherichia coli |
| V. cholera | Vibrio cholera |
References
- Azizi, A.; Kumar, A.; Diaz-Mitoma, F.; Mestecky, J. Enhancing oral vaccine potency by targeting intestinal M cells. PLoS Pathog. 2010, 6, e1001147. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.; Ghunaim, H.; Diaz-Mitoma, F.; Mestecky, J. Mucosal HIV vaccines: A holy grail or a dud? Vaccine 2010, 28, 4015–4026. [Google Scholar] [CrossRef] [PubMed]
- Newsted, D.; Fallahi, F.; Golshani, A.; Azizi, A. Advances and challenges in mucosal adjuvant technology. Vaccine 2015, 33, 2399–2405. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.; Edamura, K.N.; Leung, G.; Gisonni-Lex, L.; Mallet, L. Short communication: Evaluating the level of expressed HIV type 1 gp120 and gag proteins in the vCP1521 vector by two immunoplaque methods. AIDS Res. Hum. Retrovir. 2013, 29, 397–399. [Google Scholar] [PubMed]
- Jelinek, T.; Kollaritsch, H. Vaccination with Dukoral against travelers’ diarrhea (ETEC) and cholera. Expert Rev. Vaccines 2008, 7, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Manicassamy, S.; Pulendran, B. Modulation of adaptive immunity with Toll-like receptors. Semin. Immunol. 2009, 21, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Muzio, M.; Polntarutti, N.; Bosisio, D.; Prahladan, M.K.; Mantovani, A. Toll like receptor family (TLT) and signalling pathway. Eur. Cytokine Netw. 2000, 11, 489–490. [Google Scholar] [PubMed]
- Muzio, M.; Bosisio, D.; Polentarutti, N.; D’amico, G.; Stoppacciaro, A.; Mancinelli, R.; van’t Veer, C.; Penton-Rol, G.; Ruco, L.P.; Allavena, P.; et al. Differential expression and regulation of Toll-like receptors (TLR) in human leukocytes: Selective expression of TLR3 in dendritic cells. J. Immunol. 2000, 164, 5998–6004. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B. Variegation of the immune response with dendritic cells and pathogen recognition receptors. J. Immunol. 2005, 174, 2457–2465. [Google Scholar] [CrossRef] [PubMed]
- Shortman, K.; Liu, Y.J. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2002, 2, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Gururajan, M.; Jacob, J.; Pulendran, B. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS ONE 2007, 2, e863. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B. Tolls and beyond—Many roads to vaccine immunity. N. Engl. J. Med. 2007, 356, 1776–1778. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Fujii, K.; Nagata, N.; Takeuchi, O.; Akira, S.; Oshiumi, H.; Matsumoto, M.; Seya, T.; Koike, S. The Toll-like receptor 3-mediated antiviral response is important for protection against poliovirus infection in poliovirus receptor transgenic mice. J. Virol. 2012, 86, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, R.; Zanoni, I.; Granucci, F. Deciphering the complexity of Toll-like receptor signaling. Cell. Mol. Life Sci. 2010, 67, 4109–4134. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Takeuchi, O.; Fujita, T.; Inoue, J.; Muhlradt, P.F.; Sato, S.; Hoshino, K.; Akira, S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 2001, 167, 5887–5894. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Hoshino, K.; Kaisho, T.; Sanjo, H.; Takeuchi, O.; Sugiyama, M.; Okabe, M.; Takeda, K.; et al. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science 2003, 301, 640–643. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirskyj, D.; Kumar, A.; Azizi, A. Mechanisms Underlying the Immune Response Generated by an Oral Vibrio cholerae Vaccine. Int. J. Mol. Sci. 2016, 17, 1062. https://doi.org/10.3390/ijms17071062
Sirskyj D, Kumar A, Azizi A. Mechanisms Underlying the Immune Response Generated by an Oral Vibrio cholerae Vaccine. International Journal of Molecular Sciences. 2016; 17(7):1062. https://doi.org/10.3390/ijms17071062
Chicago/Turabian StyleSirskyj, Danylo, Ashok Kumar, and Ali Azizi. 2016. "Mechanisms Underlying the Immune Response Generated by an Oral Vibrio cholerae Vaccine" International Journal of Molecular Sciences 17, no. 7: 1062. https://doi.org/10.3390/ijms17071062
APA StyleSirskyj, D., Kumar, A., & Azizi, A. (2016). Mechanisms Underlying the Immune Response Generated by an Oral Vibrio cholerae Vaccine. International Journal of Molecular Sciences, 17(7), 1062. https://doi.org/10.3390/ijms17071062




