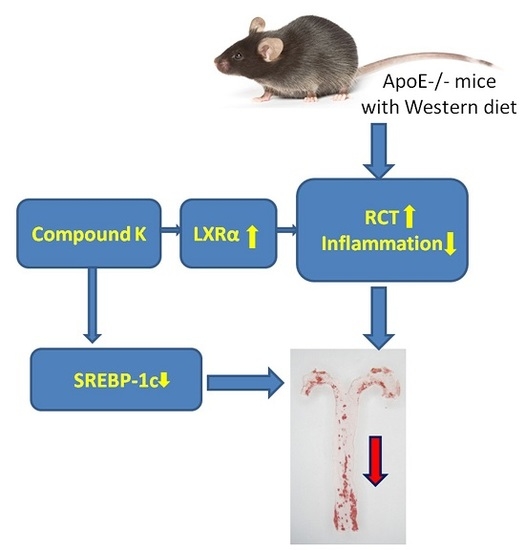
Compound K Attenuates the Development of Atherosclerosis in ApoE−/− Mice via LXRα Activation
Abstract
:1. Introduction
2. Results
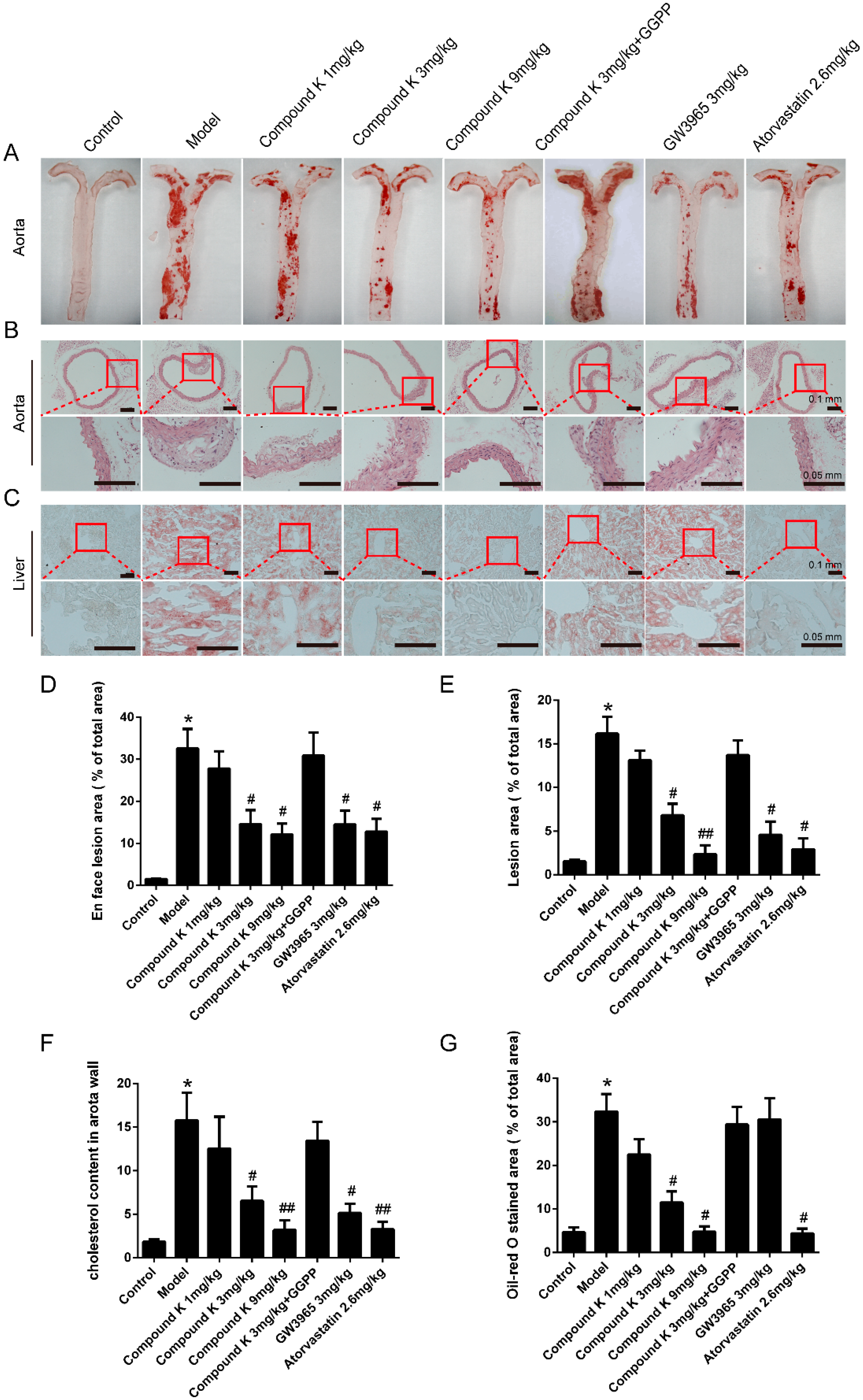
2.1. Compound K Attenuates Atherosclerotic Lesion and Fatty Liver in ApoE−/− Mice
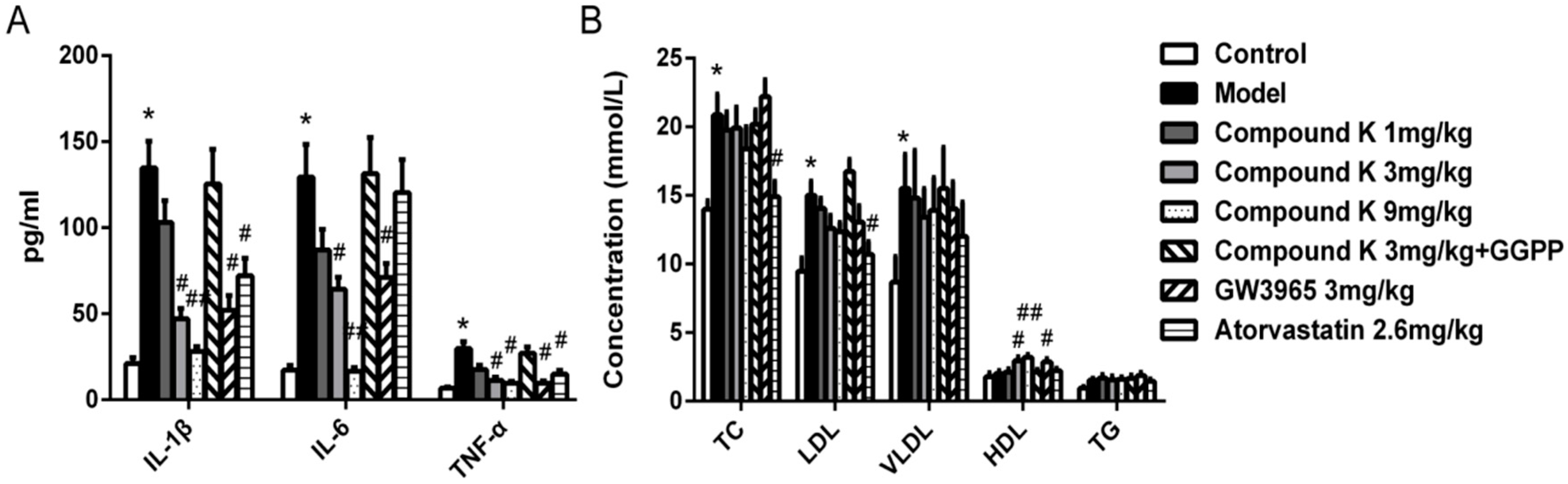
2.2. Compound K Decreases Serous Inflammatory Cytokines and Modulated Serum Lipid Profiles in ApoE−/− Mice
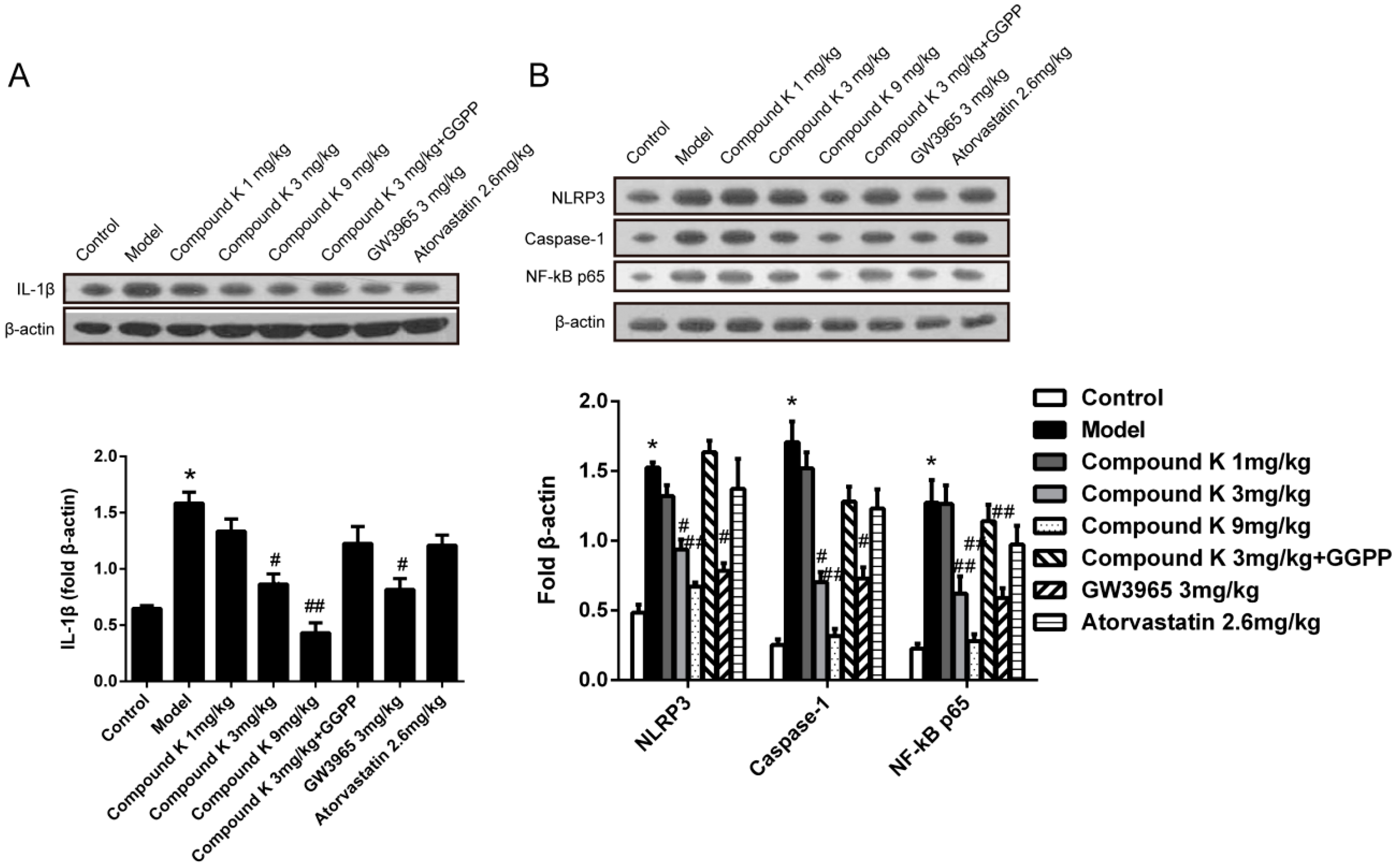
2.3. Compound K Reduces Inflammasome Activity in Atherosclerotic Lesion
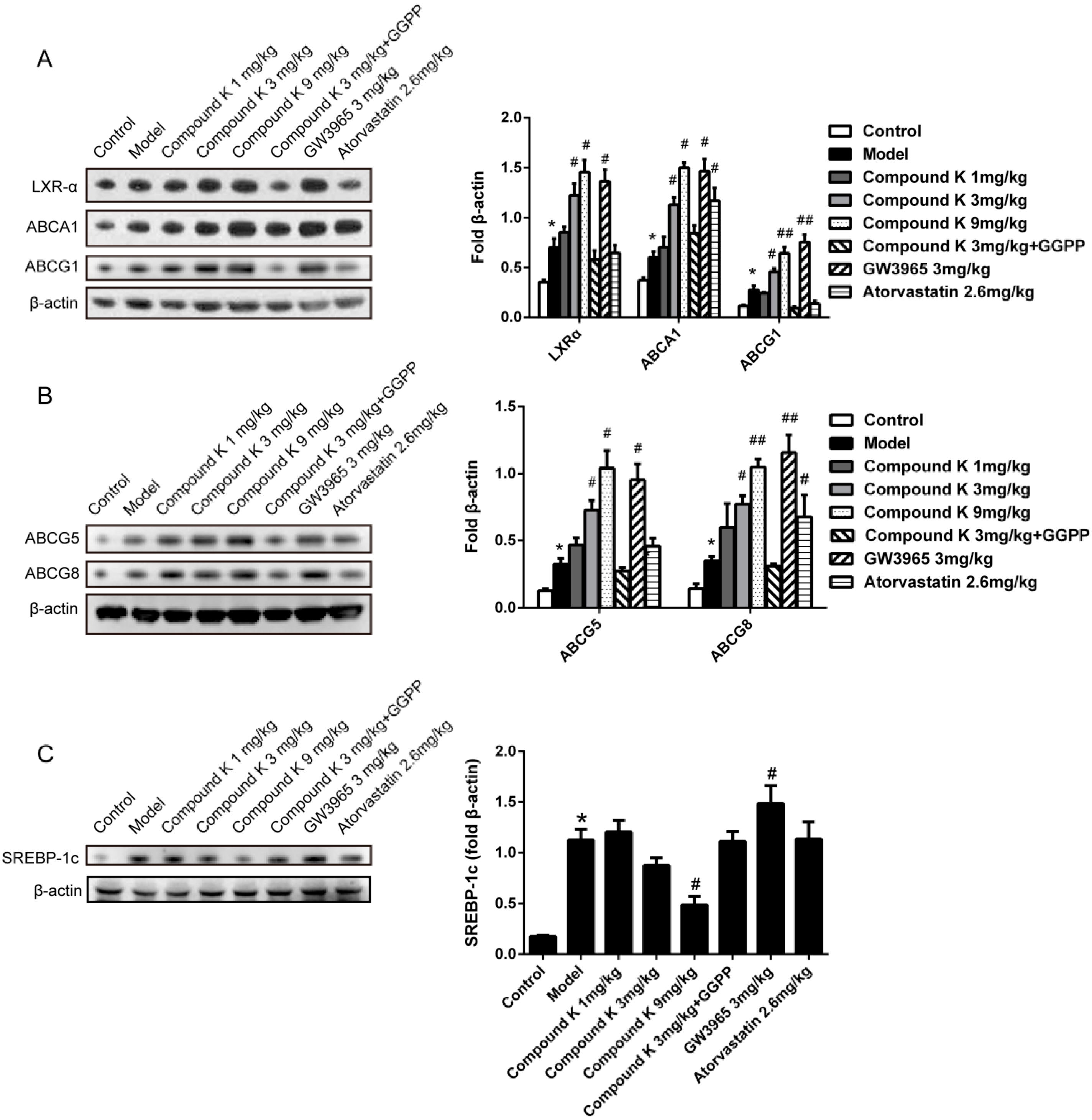
2.4. Compound K Promotes the Expression of Reverse Cholesterol Transport (RCT) Related Proteins
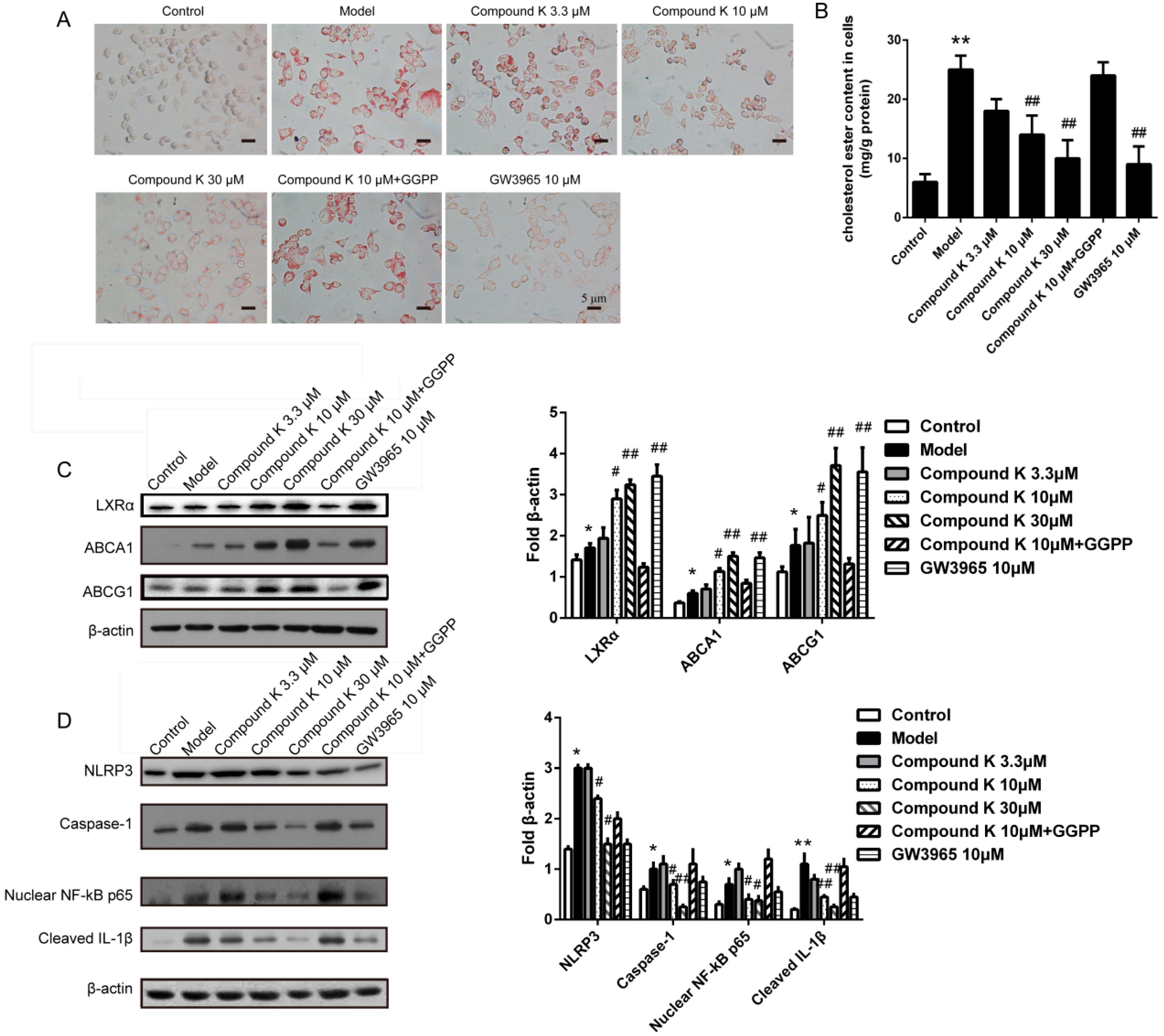
2.5. Compound K Inhibits the Formation of Foam Cell and the Activity of Inflammasome in Macrophages
3. Discussion
4. Materials and Methods
4.1. Mice and Treatments
4.2. Histology and Image Analysis
4.3. Determination of Serous Lipid and Cytokines
4.4. Cell Culture and Treatments
4.5. Immunoblotting
4.6. Analysis of Cholesterol Ester Content of Aorta and Foam Cells
4.7. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette transporter |
| GGPP | Geranylgeranyl pyrophosphate |
| HDL | high density lipoprotein |
| IL | interleukin |
| LDL | low density lipoprotein |
| LXRα | liver X receptor α |
| NF-κB | nuclear factor kappa B |
| NLRP3 | NOD-like receptor protein-3 |
| RCT | reverse cholesterol transport |
| SREBP | sterol regulatory element-binding protein |
| VLDL | very low density lipoprotein |
References
- Logue, J.; Murray, H.M.; Welsh, P.; Shepherd, J.; Packard, C.; Macfarlane, P.; Cobbe, S.; Ford, I.; Sattar, N. Obesity is associated with fatal coronary heart disease independently of traditional risk factors and deprivation. Heart 2011, 97, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Zhang, H.G.; Zhang, G.Y.; Fan, J.S.; Li, X.H.; Liu, Y.H.; Li, S.H.; Lian, X.M.; Tang, Z. Panax notoginseng saponins attenuate atherosclerosis in rats by regulating the blood lipid profile and an anti-inflammatory action. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Akao, T.; Kida, H.; Hatori, M.; Hattori, M.; Kobashi, K. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseg. J. Pharm. Pharmacol. 1998, 5, 1155–1160. [Google Scholar] [CrossRef]
- Cho, S.H.; Chung, K.S.; Choi, J.H.; Kim, D.H.; Lee, K.T. Compound K, a metabolite of ginseng saponin, induces apoptosis via caspase-8-dependent pathway in HL-60 human leukemia cells. BMC Cancer 2009, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Sung, J.H.; Lee, S.J.; Moon, C.K.; Lee, B.H. Antitumor activity of a novel ginseng saponin metabolite in human pulmonary adenocarcinoma cells resistant to cisplatin. Cancer Lett. 1999, 144, 39–43. [Google Scholar] [CrossRef]
- Hu, C.; Song, G.; Zhang, B.; Liu, Z.; Chen, R.; Zhang, H.; Hu, T. Intestinal metabolite compound K of panaxoside inhibits the growth of gastric carcinoma by augmenting apoptosis via bid-mediated mitochondrial pathway. J. Cell. Mol. Med. 2012, 16, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Ko, S.R.; Cho, B.G.; Shin, D.M.; Yuk, J.M.; Li, S.J.; Kim, J.M.; Evans, R.M.; Jung, J.S.; Song, D.K.; et al. The ginsenoside metabolite compound K, a novel agonist of glucocorticoid receptor, induces tolerance to endotoxin-induced lethal shock. J. Cell. Mol. Med. 2008, 12, 1739–1753. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, Z.Y.; Zhang, H.G.; Li, H.B.; Liu, Y.; Li, X.H. Panax notoginseng saponins decrease cholesterol ester via up-regulating ATP-binding cassette transporter A1 in foam cells. J. Ethnopharmacol. 2010, 132, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.S.; Liu, D.N.; Huang, G.; Xu, Z.Z.; Jia, Y.; Zhang, H.G.; Li, X.H.; He, F.T. Panax notoginseng saponins attenuate atherosclerosis via reciprocal regulation of lipid metabolism and inflammation by inducing liver X receptor alpha expression. J. Ethnopharmacol. 2012, 142, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, K.T.; Iseli, T.J.; Hoy, A.J.; George, J.; Grewal, T.; Roufogalis, B.D. Compound K modulates fatty acid-induced lipid droplet formation and expression of proteins involved in lipid metabolism in hepatocytes. Liver Int. 2013, 33, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Fruchart, J.C.; Sacks, F.M.; Hermans, M.P.; Assmann, G.; Brown, W.V.; Ceska, R.; Chapman, M.J.; Dodson, P.M.; Fioretto, P.; Ginsberg, H.N.; et al. The Residual risk reduction initiative: A call to action to reduce residual vascular risk in dyslipidaemic patient. Diabetes Vasc. Dis. Res. 2008, 5, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Z.; Ming, Y.L.; Chen, L.H.; Zheng, G.H.; Liu, S.S.; Chen, Q.X. Compound K-induced apoptosis of human hepatocellular carcinoma MHCC97-H cells in vitro. Oncol. Rep. 2014, 32, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.L.; Liu, Z.F.; Li, C.M.; Shen, J.Y.; Yin, H.X.; Li, G.S. Subchronic toxicity studies with ginsenoside compound K delivered to dogs via intravenous administration. Food Chem. Toxicol. 2011, 49, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Jonathan, E.F.; James, X.R.; Raanan, S.; Marie, S.; Yuliya, V.; Jianhua, L.; Katey, R.; Kathryn, M.; Michael, G.; Edward, A.F. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc. Natl. Acad. Sci. USA 2011, 108, 7166–7171. [Google Scholar]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Shin, Y.W.; Lee, H.U.; Kim, S.S.; Lee, Y.C.; Lee, B.Y.; Kim, D.H. Inhibitory effect of ginsenoside Rb1 and compound K on NO and prostaglandin E2 biosyntheses of RAW264.7 cells induced by lipopolysaccharide. Biol. Pharm. Bull. 2005, 28, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nuñez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, S.; Wang, X.; Dai, Y.; Khaidakov, M.; Deng, X.; Fan, Y.; Xiang, D.; Mehta, J.L. LOX-1, mtDNA damage, and NLRP3 inflammasome activation in macrophages: implications in atherogenesis. Cardiovasc. Res. 2014, 103, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Shin, J.A.; Jung, J.S.; Hyun, J.W.; Le, T.K.; Kim, D.H.; Park, E.M.; Kim, H.S. Anti-inflammatory mechanism of compound K in activated microglia and its neuroprotective effect on experimental stroke in mice. Biochem. Pharmacol. 2012, 341, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Im, S.S.; Osborne, T.F. Liver x receptors in atherosclerosis and inflammation. Circ. Res. 2011, 108, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.B.; McKilligin, E.; Pei, L.; Watson, M.A.; Collins, A.R.; Laffitte, B.A.; Chen, M.; Noh, G.; Goodman, J.; Hagger, G.N.; et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl. Acad. Sci. USA 2002, 99, 7604–7609. [Google Scholar] [CrossRef] [PubMed]
- Calpe-Berdiel, L.; Rotllan, N.; Fiévet, C.; Roig, R.; Blanco-Vaca, F.; Escolà-Gil, J.C. Liver X receptor-mediated activation of reverse cholesterol transport from macrophages to feces in vivo requires ABCG5/G8. J. Lipid Res. 2008, 49, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.B.; Laffitte, B.A.; Patel, P.H.; Watson, M.A.; Matsukuma, K.E.; Walczak, R.; Collins, J.L.; Osborne, T.F.; Tontonoz, P. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J. Biol. Chem. 2002, 277, 11019–11025. [Google Scholar] [CrossRef] [PubMed]
- Bełtowski, J. Liver X receptors (LXR) as therapeutic targets in dyslipidemia. Cardiovasc. Ther. 2008, 26, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Umetani, M.; Ghosh, P.; Ishikawa, T.; Umetani, J.; Ahmed, M.; Mineo, C.; Shaul, P.W. The cholesterol metabolite 27-hydroxycholesterol promotes atherosclerosis via proinflammatory processes mediated by estrogen receptor alpha. Cell Metab. 2014, 20, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Lee, K.P.; Jung, S.H.; Lee, D.Y.; Won, K.J.; Yun, Y.P.; Kim, B. Compound K, an intestinal metabolite of ginsenosides, inhibits PDGF-BB-induced VSMC proliferation and migration through G1 arrest and attenuates neointimal hyperplasia after arterial injury. Atherosclerosis 2013, 228, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kim do, Y.; Yuan, H.D.; Chung, I.K.; Chung, S.H. Compound K, intestinal metabolite of ginsenoside, attenuates hepatic lipid accumulation via AMPK activation in human hepatoma cells. J. Agric. Food Chem. 2009, 57, 1532–1537. [Google Scholar] [CrossRef] [PubMed]
- Zeini, M.; Través, P.G.; López-Fontal, R.; Pantoja, C.; Matheu, A.; Serrano, M.; Boscá, L.; Hortelano, S. Specific contribution of p19(ARF) to nitric oxide-dependent apoptosis. J. Immunol. 2006, 177, 3327–3336. [Google Scholar] [CrossRef] [PubMed]
- Rajamäki, K.; Lappalainen, J.; Oörni, K.; Välimäki, E.; Matikainen, S.; Kovanen, P.T.; Eklund, K.K. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: A novel link between cholesterol metabolism and inflammation. PLoS ONE 2010, 5, e11765. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Zheng, Y.; Li, Z.; Bao, L.; Dou, Y.; Tang, Y.; Zhang, J.; Zhou, J.; Liu, Y.; Jia, Y.; et al. Compound K Attenuates the Development of Atherosclerosis in ApoE−/− Mice via LXRα Activation. Int. J. Mol. Sci. 2016, 17, 1054. https://doi.org/10.3390/ijms17071054
Zhou L, Zheng Y, Li Z, Bao L, Dou Y, Tang Y, Zhang J, Zhou J, Liu Y, Jia Y, et al. Compound K Attenuates the Development of Atherosclerosis in ApoE−/− Mice via LXRα Activation. International Journal of Molecular Sciences. 2016; 17(7):1054. https://doi.org/10.3390/ijms17071054
Chicago/Turabian StyleZhou, Li, Yu Zheng, Zhuoying Li, Lingxia Bao, Yin Dou, Yuan Tang, Jianxiang Zhang, Jianzhi Zhou, Ya Liu, Yi Jia, and et al. 2016. "Compound K Attenuates the Development of Atherosclerosis in ApoE−/− Mice via LXRα Activation" International Journal of Molecular Sciences 17, no. 7: 1054. https://doi.org/10.3390/ijms17071054
APA StyleZhou, L., Zheng, Y., Li, Z., Bao, L., Dou, Y., Tang, Y., Zhang, J., Zhou, J., Liu, Y., Jia, Y., & Li, X. (2016). Compound K Attenuates the Development of Atherosclerosis in ApoE−/− Mice via LXRα Activation. International Journal of Molecular Sciences, 17(7), 1054. https://doi.org/10.3390/ijms17071054