In Situ Electron Microscopy of Lactomicroselenium Particles in Probiotic Bacteria
Abstract
:1. Introduction
2. Results
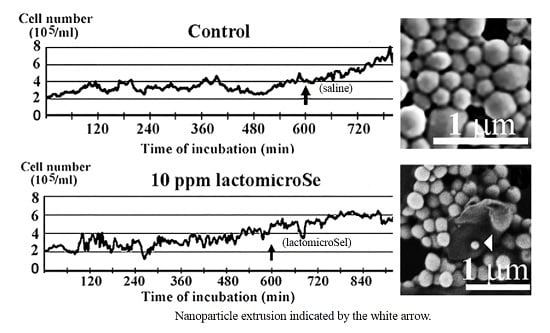
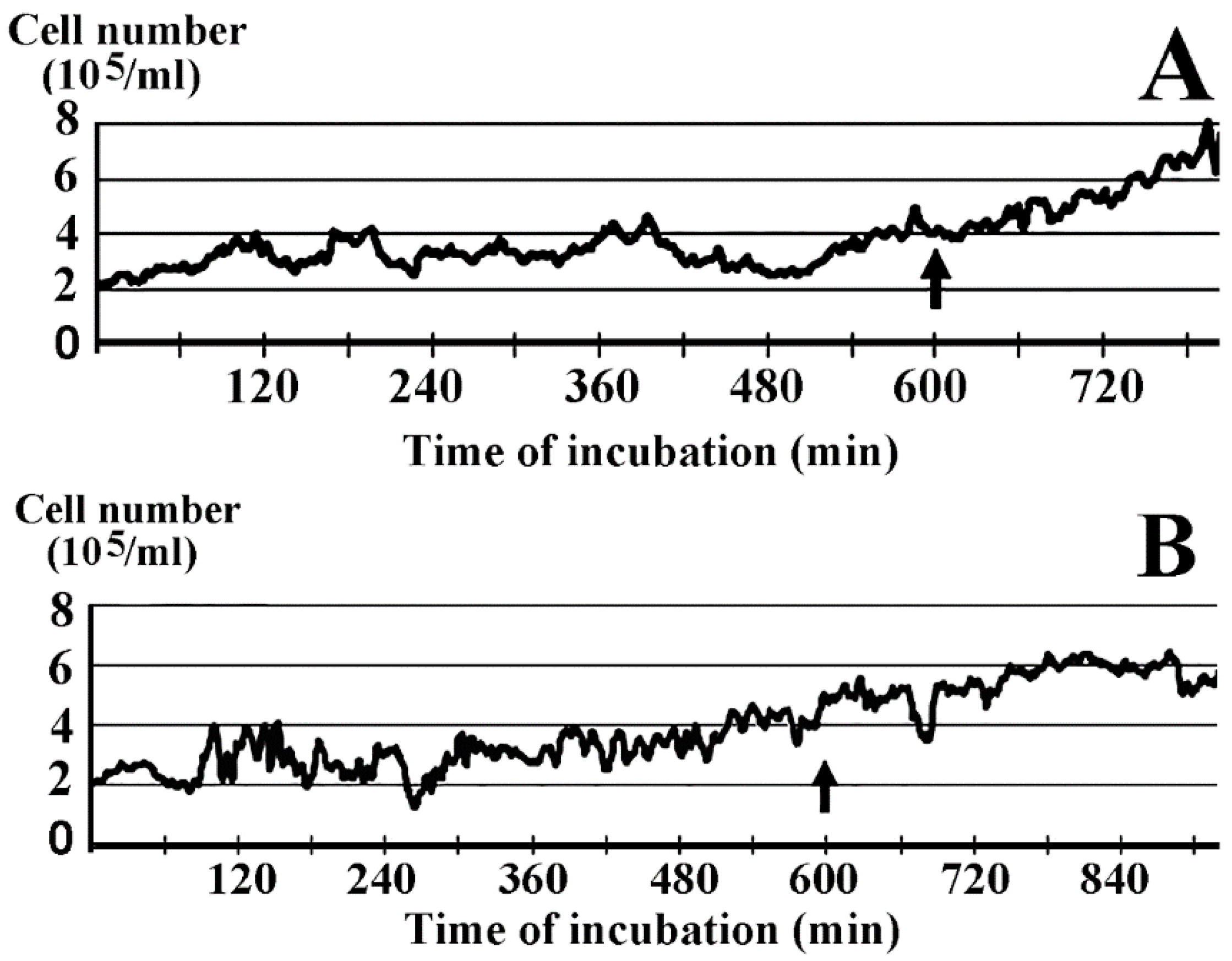
2.1. Inhibition of Cell Growth by Lactomicroselenium (LactomicroSel)
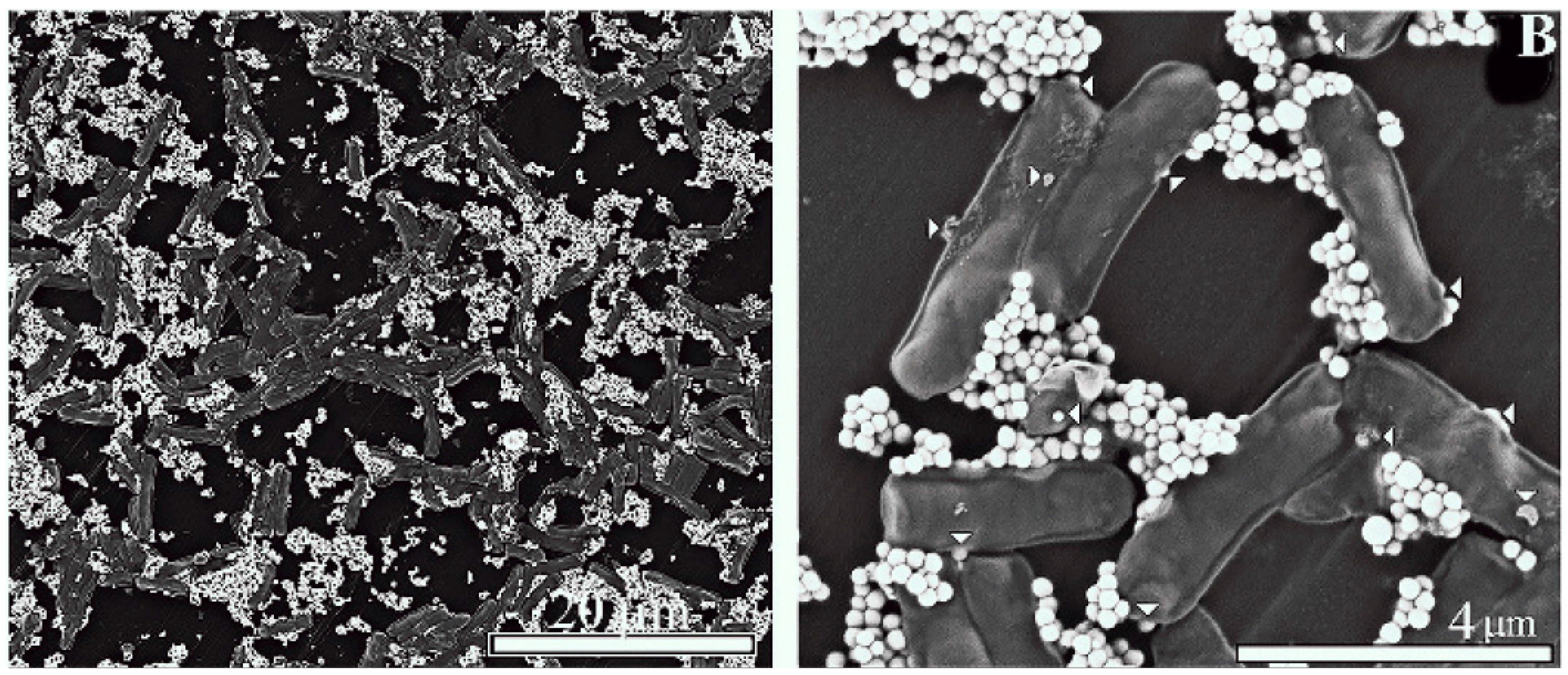
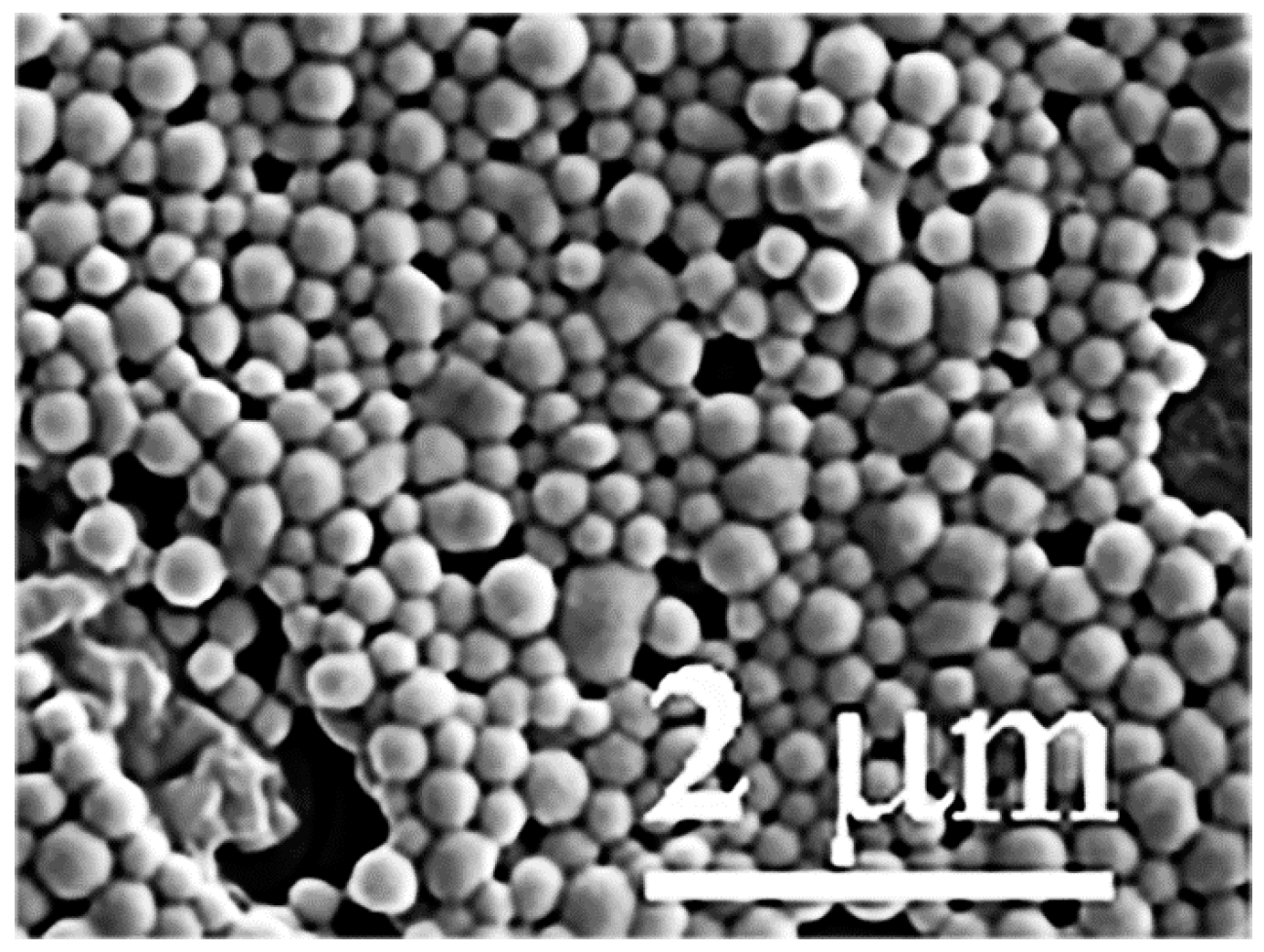
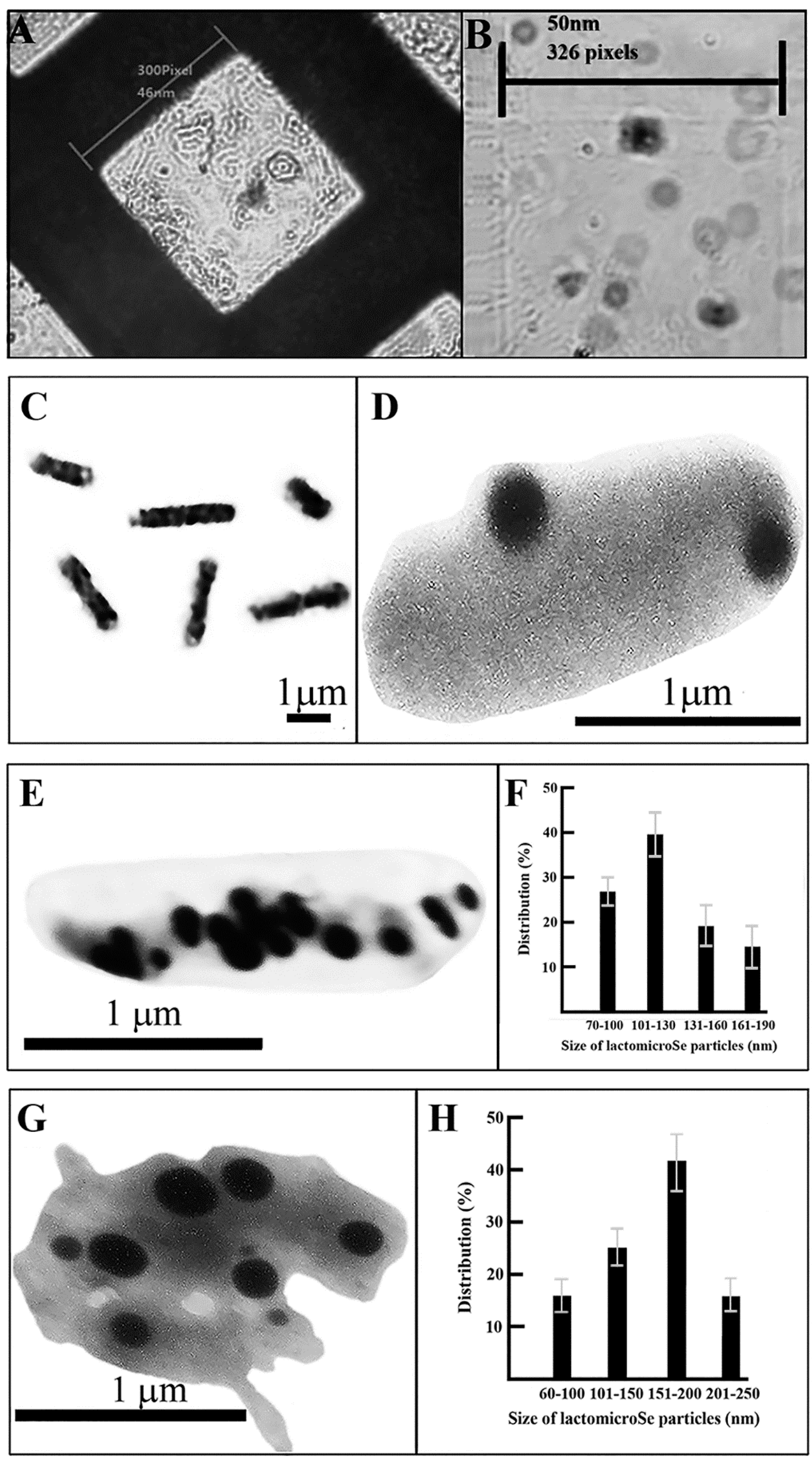
2.2. Scanning Electronmicroscopy of LactomicroSel Particles
2.3. Transmission Electron Microscopy
3. Discussion
4. Materials and Methods
4.1. Exposure of Cell Culture to LactomicroSel
4.2. Production of LactomicroSel
4.3. Determination of Lactomicroselenium Concentration
4.4. Transmission Electron Microscopy (TEM)
- (a)
- electric control rather than mechanical stage movement;
- (b)
- application of digitazing device; and
- (c)
- custom-built camera with Sony Exview-HAD CCD sensor (Sony Semiconductor Solutions Corporation, Tokyo, Japan).
4.5. Scanning Electron Microscopy (SEM)
4.6. Sofware
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eszenyi, P.; Sztrik, A.; Babka, B.; Prokisch, J. Elemental, nano-sized (100–500 nm) selenium production by probiotic lactic acid bacteria. Int. J. Biosci. Biochem. Bioinform. 2011, 1, 148–152. [Google Scholar] [CrossRef]
- Eszenyi, P.; Sztrik, A.; Babka, B.; Prokisch, J. Production of lactomicroSel® and nanosize selenium spheres by probiotic lactic acid bacteria. In International Conference on Food Engineering and Biotechnology IPCBBE; LACSIT Press: Singapore, 2011; Volume 9, pp. 97–101. [Google Scholar]
- Benko, I.; Nagy, G.; Tanczos, B.; Ungvari, E.; Sztrik, A.; Eszenyi, P.; Prokisch, J.; Banfalvi, G. Subacute toxicity of selenium sources in mice. Environ. Toxicol. Chem. 2012, 31, 2812–2820. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Benko, I.; Kiraly, G.; Voros, O.; Tanczos, B.; Sztrik, A.; Takács, T.; Pocsi, I.; Prokisch, J.; Banfalvi, G. Cellular and nephrotoxicity of selenium species. J. Trace Elem. Med. Biol. 2015, 30, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Pinczes, G.; Papai, G.; Kiraly, G.; Banfalvi, G. Teaching biology through remote access microscopy. In Current Microscopic Contribution to Advances in Science and Technology; Mendez-Villas, A., Ed.; Formatex Publisher: Badajoz, Spain, 2012; pp. 988–993. [Google Scholar]
- Oyetayo, V.O.; Adetuyi, F.C.; Akinyosoye, F.A. Safety and protective effect of Lactobacillus acidophilus and Lactobacillus casei used as probiotic agent in vivo. Afr. J. Biotechnol. 2003, 2, 448–452. [Google Scholar] [CrossRef]
- Kandler, O.; Weiss, N. In Bergey’s Manual of Systematic Bacteriology; Sneath, P.H.A., Mair, N.S., Sharpe, M.E., Holt, J.G., Eds.; Williams and Wilkins: Baltimore, MD, USA, 1986; Volume 2, pp. 1209–1234. [Google Scholar]
- Kokkinos, A.; Fasseas, C.; Eliopoulos, E.; Kalantzopoulos, G. Cell size of various lactic acid bacteria as determined by scanning electronmicroscope and image analysis. Dairy Sci. Technol. 1998, 78, 491–500. [Google Scholar] [CrossRef]
- Lewis, K. Programmed death in bacteria. Microbiol. Mol. Biol. Rev. 2000, 64, 3503–3514. [Google Scholar] [CrossRef]
- Engelberg-Kulka, H.; Amitai, S.; Kolodkin-Gal, I.; Hazan, R. Bacterial programmed cell death and multicellular behavior in bacteria. PLoS Genet. 2006, 2, e135. [Google Scholar] [CrossRef] [PubMed]
- Clementi, E.A.; Marks, L.R.; Duffey, M.E.; Hakansson, A.P. A novel initiation mechanism of death in Streptococcus pneumoniae induced by the human milk protein-lipid complex HAMLET and activated during physiological death. J. Biol. Chem. 2012, 287, 27168–27182. [Google Scholar] [CrossRef] [PubMed]
- Bayles, K.W. Bacterial programmed cell death: Making sense of a paradox. Nat. Rev. Microbiol. 2014, 12, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P.; Petrusevska, R.T.; Breitkreut, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P.; Stanbridge, E.J.; Foo, D.Y.; Cerutti, P.A.; Fusenig, N.E. c-Ha-ras oncogene expression in immortalized human keratinocytes (HaCaT) alters growth potential in vivo but lacks correlation with malignancy. Cancer Res. 1990, 50, 2840–2847. [Google Scholar] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, G.; Pinczes, G.; Pinter, G.; Pocsi, I.; Prokisch, J.; Banfalvi, G. In Situ Electron Microscopy of Lactomicroselenium Particles in Probiotic Bacteria. Int. J. Mol. Sci. 2016, 17, 1047. https://doi.org/10.3390/ijms17071047
Nagy G, Pinczes G, Pinter G, Pocsi I, Prokisch J, Banfalvi G. In Situ Electron Microscopy of Lactomicroselenium Particles in Probiotic Bacteria. International Journal of Molecular Sciences. 2016; 17(7):1047. https://doi.org/10.3390/ijms17071047
Chicago/Turabian StyleNagy, Gabor, Gyula Pinczes, Gabor Pinter, Istvan Pocsi, Jozsef Prokisch, and Gaspar Banfalvi. 2016. "In Situ Electron Microscopy of Lactomicroselenium Particles in Probiotic Bacteria" International Journal of Molecular Sciences 17, no. 7: 1047. https://doi.org/10.3390/ijms17071047
APA StyleNagy, G., Pinczes, G., Pinter, G., Pocsi, I., Prokisch, J., & Banfalvi, G. (2016). In Situ Electron Microscopy of Lactomicroselenium Particles in Probiotic Bacteria. International Journal of Molecular Sciences, 17(7), 1047. https://doi.org/10.3390/ijms17071047