Synthesis, Characterization, and Cytotoxicity of the First Oxaliplatin Pt(IV) Derivative Having a TSPO Ligand in the Axial Position
Abstract
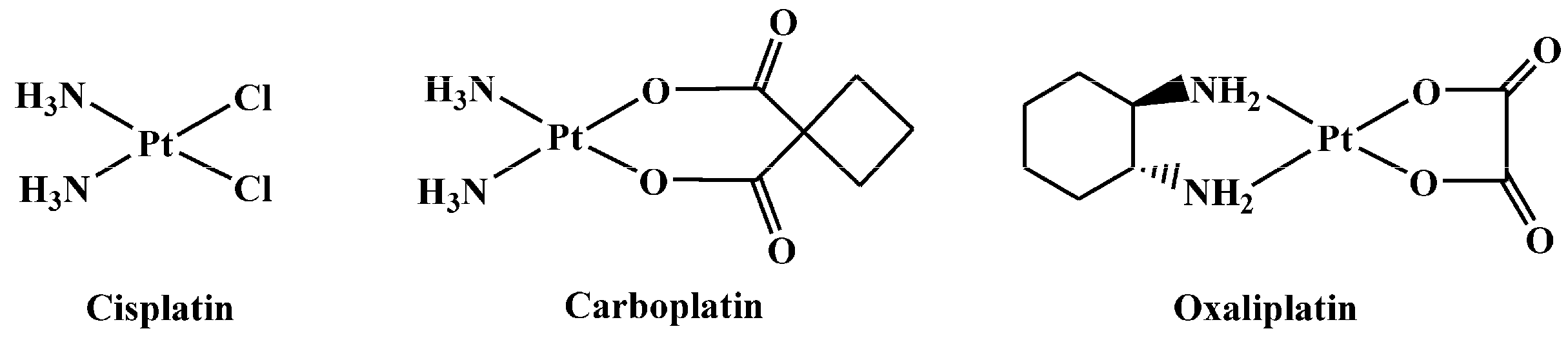
:1. Introduction
2. Results and Discussion
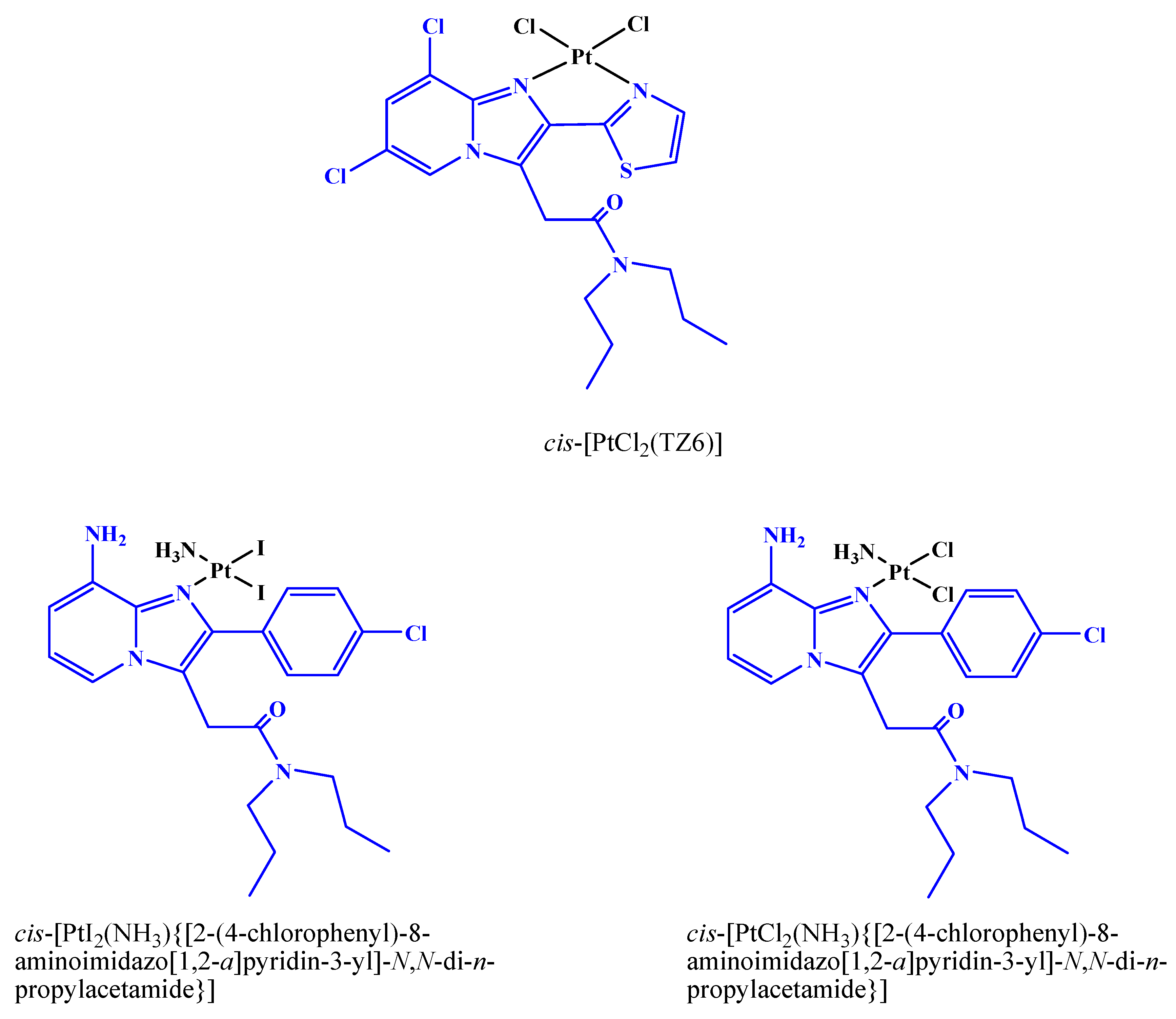
2.1. Synthesis and Characterization of 3
2.2. Stability of 3
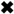 in Figure 8a). After 8 h of incubation we observed a decrease in intensity of the peaks of 3 and the concomitant increase of a new set of peaks, marked with ● in Figure 8b (H5, 8.09 ppm; H7, 7.44 ppm; H16/20 7.35 ppm), that was assigned to compound 2 by comparison with a spectrum of the TSPO ligand recorded in similar conditions. This new set of signals indicates that hydrolysis of the ester bond linking 2 to the ethanolato linker occurs in physiological-like conditions. The spectra recorded at 24, and 56 h showed a further decrease of the signals of 3, which disappeared completely after 108 h. The half-life of the complex, as calculated from the NMR experiment, was determined to be approximately 24 h.
in Figure 8a). After 8 h of incubation we observed a decrease in intensity of the peaks of 3 and the concomitant increase of a new set of peaks, marked with ● in Figure 8b (H5, 8.09 ppm; H7, 7.44 ppm; H16/20 7.35 ppm), that was assigned to compound 2 by comparison with a spectrum of the TSPO ligand recorded in similar conditions. This new set of signals indicates that hydrolysis of the ester bond linking 2 to the ethanolato linker occurs in physiological-like conditions. The spectra recorded at 24, and 56 h showed a further decrease of the signals of 3, which disappeared completely after 108 h. The half-life of the complex, as calculated from the NMR experiment, was determined to be approximately 24 h.2.3. Biological Assays
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of cis,trans,cis-[Pt(ethanedioato)Cl{2-(2-(4-(6,8-dichloro-3-(2-(dipropylamino)-2-oxoethyl)imidazo[1,2-a]pyridin-2-yl)phenoxy)acetate)-ethanolato}(1R,2R-DACH)] (3)
3.3. Stability of Compound 3
3.4. Biological Assays
3.4.1. Cell Lines
3.4.2. Membrane Preparation
3.4.3. Receptor Binding Assays
3.4.4. Cell Proliferation Assay
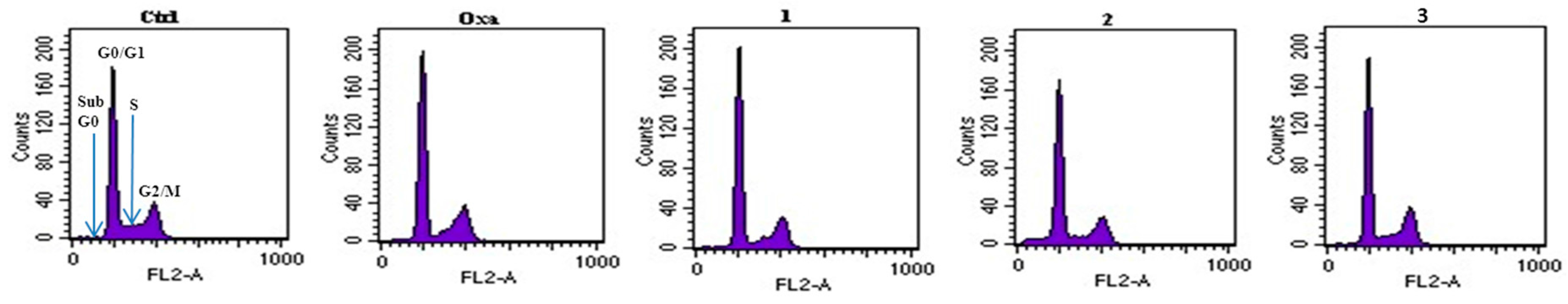
3.4.5. Cell Cycle Analysis
3.4.6. Cellular Uptake
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| COSY | correlation spectroscopy |
| DACH | diaminocyclohexane |
| DMF | dimethylformamide |
| DMSO | dimethylsulfoxide |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| ESI-MS | electrospray mass spectrometry |
| HOBt | 1-hydroxybenzotriazole hydrate |
| HSQC | heteronuclear single quantum coherence spectroscopy |
| ICP-MS | inductively coupled plasma mass spectrometry |
| NOESY | nuclear overhauser enhanced spectroscopy |
| OCT | organic cation transporters |
| PEG | polyethylene glycol |
| PLGA | poly(lactic-co-glycolic acid) |
| PET | positron emission tomography |
| TEA | triethylamine |
| TOCSY | total correlation spectroscopy |
| TSPO | 18-kDa mitochondrial translocator protein |
References
- Rosenberg, B.; van Camp, L.; Trosko, J.E.; Mansour, V.H. Platinum compounds: A new class of potent antitumour agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.; Hollander, S. Review of platinum anticancer compounds. J. Med. 1993, 24, 209–265. [Google Scholar] [PubMed]
- Weiss, R.B.; Christian, M.C. New cisplatin analogues in development a review. Drugs 1993, 46, 360–377. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, D.; Canetta, R. Clinical development of platinum complexes in cancer therapy: An historical perspective and an update. Eur. J. Cancer 1998, 34, 1522–1534. [Google Scholar] [CrossRef]
- Wong, E.; Giandomenico, C.M. Current status of platinum-based antitumor drugs. Chem. Rev. 1999, 99, 2451–2566. [Google Scholar] [CrossRef] [PubMed]
- Goddard, P.; Valenti, M.; Kelland, L.R. The role of glutathione (GSH) in determining sensitivity to platinum drugsin vivo in platinum-sensitive and -resistant murine leukaemia and plasmacytoma and human ovarian carcinoma xenografts. Anticancer Res. 1994, 14, 1065–1070. [Google Scholar] [PubMed]
- Faivre, S.; Chan, D.; Salinas, R.; Woynarowska, B.; Woynarowski, J.M. DNA strand breaks and apoptosis induced by oxaliplatin in cancer cells. Biochem. Pharmacol. 2003, 66, 225–237. [Google Scholar] [CrossRef]
- Di Francesco, A.M.; Ruggiero, A.; Riccardi, R. Cellular and molecular aspects of drugs of the future: Oxaliplatin. CMLS 2002, 59, 1914–1927. [Google Scholar] [CrossRef] [PubMed]
- Extra, J.M.; Espie, M.; Calvo, F.; Ferme, C.; Mignot, L.; Marty, M. Phase I study of oxaliplatin in patients with advanced cancer. Cancer Chemother. Pharmacol. 1990, 25, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-P.; Au-Yeung, S.C.F.; To, K.K.W. Platinum-based anticancer agents: Innovative design strategies and biological perspectives. Med. Res. Rev. 2003, 23, 633–655. [Google Scholar] [CrossRef] [PubMed]
- Drougge, L.I.; Elding, L. Mechanisms for acceleration of halide anation reactions of platinum(IV) complexes. REOA versus ligand assistance and platinum(II) catalysis without central ion exchange. Inorg. Chim. Acta 1986, 121, 175–183. [Google Scholar] [CrossRef]
- Hall, M.D.; Hambley, T.W. Platinum(IV) antitumour compounds: Their bioinorganic chemistry. Coord. Chem. Rev. 2002, 232, 49–67. [Google Scholar] [CrossRef]
- Ellis, L.A.; Er, H.M.; Hambley, T.W. The influence of the axial ligands of a series of platinum(IV) anti-cancer complexes on their reduction to platinum(II) and reaction with DNA. Aust. J. Chem. 1995, 48, 793–806. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z. Targeting and delivery of platinum-based anticancer drugs. Chem. Soc. Rev. 2013, 42, 202–224. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, R.; Papadopoulos, V.; Rammes, G.; Baghai, T.C.; Fan, J.; Akula, N.; Groyer, G.; Adams, D.; Schumacher, M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2010, 9, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Trapani, G.; Denora, N.; Trapani, A.; Laquintana, V. Recent advances in ligand targeted therapy. J. Drug Target. 2012, 20, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Denora, N.; Cassano, T.; Laquintana, V.; Lopalco, A.; Trapani, A.; Cimmino, C.S.; Laconca, L.; Giuffrida, A.; Trapani, G. Novel codrugs with GABAergic activity for dopamine delivery in the brain. Int. J. Pharm. 2012, 437, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Galiegue, S.; Tinel, N.; Casellas, P. The peripheral benzodiazepine receptor: A promising therapeutic drug target. Curr. Med. Chem. 2003, 10, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Maaser, K.; Grabowski, P.; Sutter, A.P.; Höpfner, M.; Foss, H.D.; Stein, H.; Berger, G.; Gavish, M.; Zeitz, M.; Scherübl, H. Overexpression of the peripheral benzodiazepine receptor is a relevant prognostic factor in stage III colorectal cancer. Clin. Cancer Res. 2002, 8, 3205–3209. [Google Scholar] [PubMed]
- Veenman, L.; Levin, E.; Weisinger, G.; Leschiner, S.; Spanier, I.; Snyder, S.H.; Weizman, A.; Gavish, M. Peripheral-type benzodiazepine receptor density and in vitro tumorigenicity of glioma cell lines. Biochem. Pharmacol. 2004, 68, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Scarf, A.M.; Ittner, L.M.; Kassiou, M. The translocator protein (18 kDa): Central nervous system disease and drug design. J. Med. Chem. 2009, 52, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Denora, N.; Laquintana, V.; Trapani, A.; Lopedota, A.; Latrofa, A.; Gallo, J.M.; Trapani, G. Translocator protein (TSPO) ligand–Ara-C (Cytarabine) conjugates as a strategy to deliver antineoplastic drugs and to enhance drug clinical potential. Mol. Pharm. 2010, 7, 2255–2269. [Google Scholar] [CrossRef] [PubMed]
- Laquintana, V.; Denora, N.; Lopedota, A.; Suzuki, H.; Sawada, M.; Serra, M.; Biggio, G.; Latrofa, A.; Trapani, G.; Liso, G. N-benzyl-2-(6,8-dichloro-2-(4-chlorophenyl)imidazo [1,2-a]pyridin-3-yl)-N-(6-(7-nitrobenzo[c][1,2,5]oxadiazol 4 ylamino)hexyl)acetamide as a new fluorescent probe for peripheral benzodiazepine receptor and microglial cell visualization. Bioconj. Chem. 2007, 18, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Laquintana, V.; Denora, N.; Musacchio, T.; Lasorsa, M.; Latrofa, A.; Trapani, G. Peripheral benzodiazepine receptor ligand–PLGA polymer conjugates potentially useful as delivery systems of apoptotic agents. J. Control. Release 2009, 137, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Bornhop, D.J. Recent avances in receptor-targeted fluorescent probes forin vivo cancer imaging. Curr. Med. Chem. 2012, 19, 4742–4758. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.; Moon, B.S.; Park, H.S.; Laquintana, V.; Jung, J.H.; Cutrignelli, A.; Lopedota, A.; Franco, M.; Kim, S.E.; Lee, B.C.; et al. A Novel PET Imaging Probe for the Detection and Monitoring of Translocator Protein 18 kDa Expression in Pathological Disorders. Sci. Rep. 2016, 6, 20422. [Google Scholar] [CrossRef] [PubMed]
- Margiotta, N.; Ostuni, R.; Ranaldo, R.; Denora, N.; Laquintana, V.; Trapani, G.; Liso, G.; Natile, G. Synthesis and Characterization of a Platinum(II) Complex Tethered to a Ligand of the Peripheral Benzodiazepine Receptor. J. Med. Chem. 2007, 50, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Romaniewska, A.; Jasztold-Howorko, R.; Regiec, A.; Lis, T.; Kuduk-Jaworska, J. Synthesis, structure and characterization of new olivacine derivatives and their platinum(II) complexes. Eur. J. Inorg. Chem. 2003, 2003, 4043–4054. [Google Scholar] [CrossRef]
- Bentzion, D.; Lipatov, O.; Polyakov, I.; MacKintosh, R.; Eckardt, J.; Breitz, H. A phase II study of picoplatin (pico) as second-line therapy for patients (pts) with small cell lung cancer (SCLC) who have resistant or refractory disease or have relapsed within 180 days of completing first-line, platinum (plat)-containing chemotherapy. In Proceedings of the ASCO Annual Meeting (Part I), Chicago, IL, USA, 1–5 June 2007; p. 7722.
- Margiotta, N.; Denora, N.; Ostuni, R.; Laquintana, V.; Anderson, A.; Johnson, S.W.; Trapani, G.; Natile, G. Platinum(II) complexes with bioactive carrier ligands having high affinity for the translocator protein. J. Med. Chem. 2010, 53, 5144–5154. [Google Scholar] [CrossRef] [PubMed]
- Mailliet, P.; Bourrie, B.; Normand, A. Derives de Platine(IV)—Couples a un Agent de Ciblage Antitumoral. French Patent FR 2 954 321 A1, 15 July 2010. [Google Scholar]
- Choi, S.; Filotto, C.; Bisanzo, M.; Delaney, S.; Lagasee, D.; Whitworth, J.L.; Jusko, A.; Li, C.R.; Wood, N.A.; Willingham, J.; et al. reduction and anticancer activity of platinum(IV) complexes. Inorg. Chem. 1998, 37, 2500–2504. [Google Scholar] [CrossRef]
- Chen, L.; Lee, P.F.; Ranford, J.D.; Vittal, J.J.; Wong, S.Y. Reduction of the anti-cancer analogue cis, trans, cis-[PtCl2(OCOCH3)NH3)2] by l-methionine and l-cysteine and its crystal structure. J. Chem. Soc. Dalton Trans. 1999, 1209–1212. [Google Scholar] [CrossRef]
- Denora, N.; Laquintana, V.; Lopalco, A.; Iacobazzi, R.M.; Lopedota, A.; Cutrignelli, A.; Iacobellis, G.; Annese, C.; Cascione, M.; Leporatti, S.; et al. In vitro targeting and imaging the translocator protein TSPO 18-kDa through G(4)-PAMAM-FITC labeled dendrimer. J. Control. Release 2013, 172, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Rendina, L.M.; Puddephatt, R. Oxidative Addition Reactions of Organoplatinum(II) Complexes with Nitrogen-Donor Ligands. J. Chem. Rev. 1997, 97, 1735–1754. [Google Scholar] [CrossRef]
- Gossage, R.A.; Ryabov, A.D.; Spek, A.L.; Stufkens, D.J.; van Beek, J.A.M.; van Eldik, R.; van Koten, G. Models for the Initial Stages of Oxidative Addition. Synthesis, Characterization, and Mechanistic Investigation of η1-I2 Organometallic “Pincer” Complexes of Platinum. X-ray Crystal Structures of [PtI(C6H3{CH2NMe2}2–2,6)(η1-I2)] and exo-meso-[Pt(η1-I3)(η1-I2)(C6H3{CH2N(t-Bu)Me}2–2,6)]. J. Am. Chem. Soc. 1999, 121, 2488–2497. [Google Scholar]
- Margiotta, N.; Ranaldo, R.; Intini, F.P.; Natile, G. Cationic intermediates in oxidative addition reactions of Cl2 to [PtCl2(cis-1,4-DACH)]. Dalton Trans. 2011, 40, 12877–12885. [Google Scholar] [CrossRef] [PubMed]
- Giandomenico, C.M.; Abrams, M.J.; Murrer, B.A.; Vollano, J.F.; Rheinheimer, M.I.; Wyer, S.B.; Bossard, G.E.; Higgins, J.D. Carboxylation of Kinetically Inert Platinum(IV) Hydroxy Complexes. An Entr.acte.ee into Orally Active Platinum(IV) Antitumor Agents. Inorg. Chem. 1995, 34, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Pichler, V.; Valiahdi, S.M.; Jakupec, M.A.; Arion, V.B.; Galanski, M.; Keppler, B.K. Mono-carboxylated diaminedichloridoplatinum(IV) complexes—Selective synthesis, characterization, and cytotoxicity. Dalton Trans. 2011, 40, 8187–8192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Bonnitcha, P.; Wexselblatt, E.; Klein, A.V.; Najajreh, Y.; Gibson, D.; Hambley, T.W. Facile preparation of mono-, di- and mixed-carboxylato platinum(IV) complexes for versatile anticancer prodrug design. Chem. Eur. J. 2013, 19, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Ravera, M.; Gabano, E.; Pelosi, G.; Fregonese, F.; Tinello, S.; Osella, D. A new entry to asymmetric platinum(IV) complexes via oxidative chlorination. Inorg. Chem. 2014, 53, 9326–9335. [Google Scholar] [CrossRef] [PubMed]
- Dunham, S.O.; Larsen, R.D.; Abbott, E.H. Nuclear magnetic resonance investigation of the hydrogen peroxide oxidation of platinum(II) complexes. Crystal and molecular structures of sodium trans-dihydroxobis(malonato)platinate(IV) hexahydrate and sodium trans-dihydroxobis(oxalato)platinate(IV) hexahydrate. Inorg. Chem. 1993, 32, 2049–2055. [Google Scholar]
- Petruzzella, E.; Margiotta, N.; Ravera, M.; Natile, G. NMR Investigation of the Spontaneous Thermal- and/or Photoinduced Reduction of trans Dihydroxido Pt(IV) Derivatives. Inorg. Chem. 2013, 52, 2393–2403. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Teply, B.A.; Sherifi, I.; Sung, J.; Luther, G.; Gu, F.X.; Levy-Nissenbaum, E.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Formulation of functionalized PLGA–PEG nanoparticles forin vivo targeted drug delivery. Biomaterials 2007, 28, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Kugler, W.; Veenman, L.; Shandalov, Y.; Leschiner, S.; Spanier, I.; Lakomek, M.; Gavish, M. Ligands of the mitochondrial 18 kDa translocator protein attenuate apoptosis of human glioblastoma cells exposed to erucylphosphohomocholine. Cell. Oncol. 2008, 30, 435–450. [Google Scholar] [PubMed]
- Cubo, L.; Hambley, T.W.; Miguel, P.J.S.; Carnero, A.; Navarro-Ranninger, C.; Quiroga, A.G. The preparation and characterization of trans-platinum(IV) complexes with unusually high cytotoxicity. Dalton Trans. 2011, 40, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lovejoy, K.S.; Shima, J.E.; Lagpacan, L.L.; Shu, Y.; Lapuk, A.; Chen, Y.; Komori, T.; Gray, J.W.; Chen, X.; et al. Organic Cation Transporters Are Determinants of Oxaliplatin Cytotoxicity. Cancer Res. 2006, 66, 8847–8857. [Google Scholar] [CrossRef] [PubMed]
- William-Faltaos, S.; Rouillard, D.; Lechat, P.; Bastian, G. Cell cycle arrest by oxaliplatin on cancer cells. Fundam. Clin. Pharmacol. 2007, 21, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Pregosin, P.S. Platinum-195 nuclear magnetic resonance. Coord. Chem. Rev. 1982, 44, 247–291. [Google Scholar] [CrossRef]
- Kidani, Y.; Inagaki, K. Cis-platinum(ii) complex of trans-l-1,2-diaminocyclohexane. Patent US 4169846 A, 2 October 1979. [Google Scholar]
- Piccinonna, S.; Denora, N.; Margiotta, N.; Laquintana, V.; Trapani, G.; Natile, G. Synthesis, characterization, and binding to the translocator protein (18 kDa, TSPO) of a new rhenium complex as a model of radiopharmaceutical agents. Z. Anorg. Allg. Chem. 2013, 639, 1606–1612. [Google Scholar] [CrossRef]
- Denora, N.; Laquintana, V.; Trapani, A.; Suzuki, H.; Sawada, M.; Trapani, G. New fluorescent probes targeting the mitochondrial-located translocator protein 18 kDa (TSPO) as activated microglia imaging agents. Pharm. Res. 2011, 28, 2820–2832. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, L.; Guida, G.; Tommasi, S.; Guida, M.; Azzariti, A. Metastatic melanoma cells with BRAF G469A mutation: Nab-paclitaxel better than vemurafenib? Cancer Chemother. Pharmacol. 2015, 76, 433–438. [Google Scholar] [CrossRef] [PubMed]
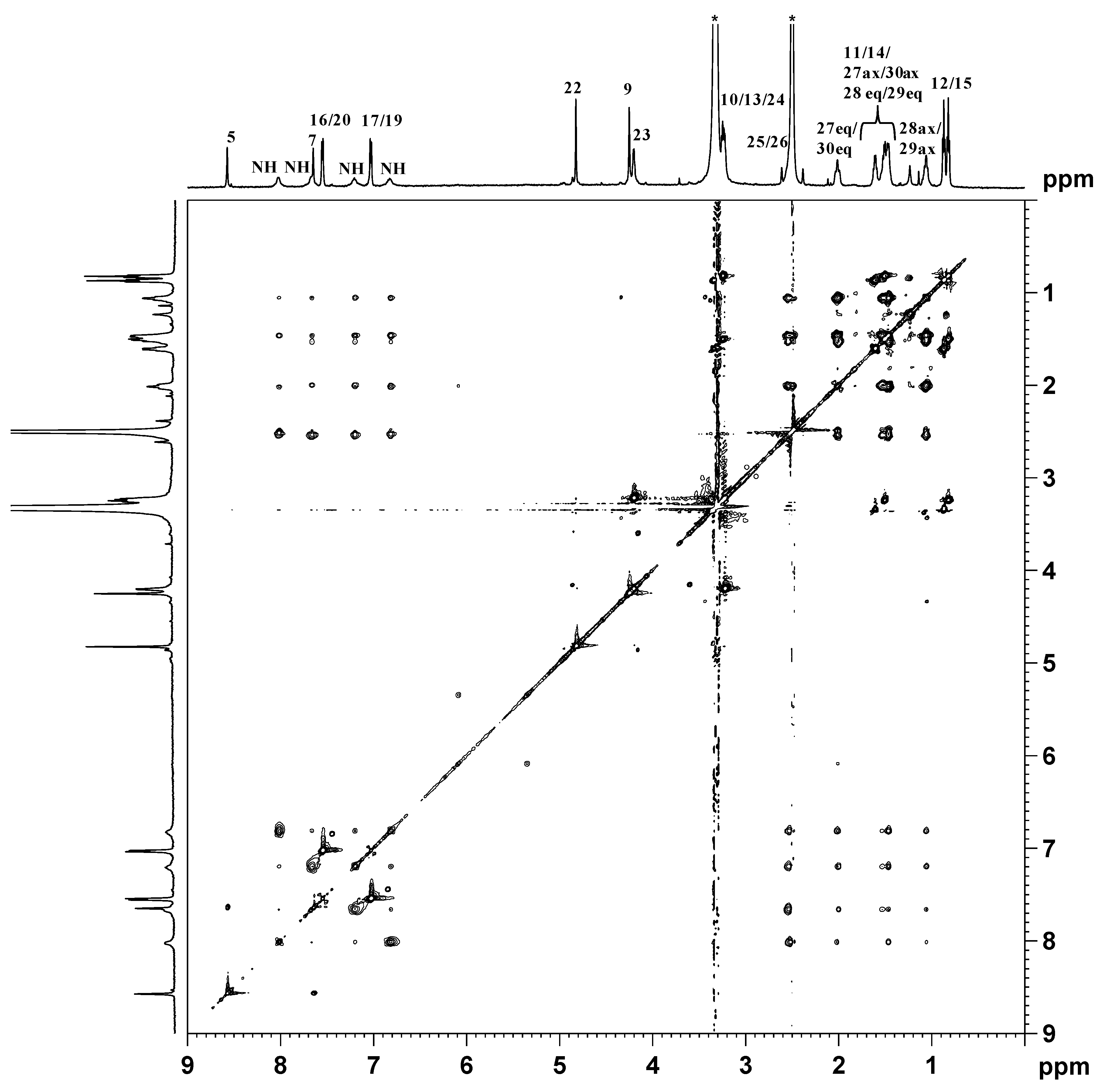
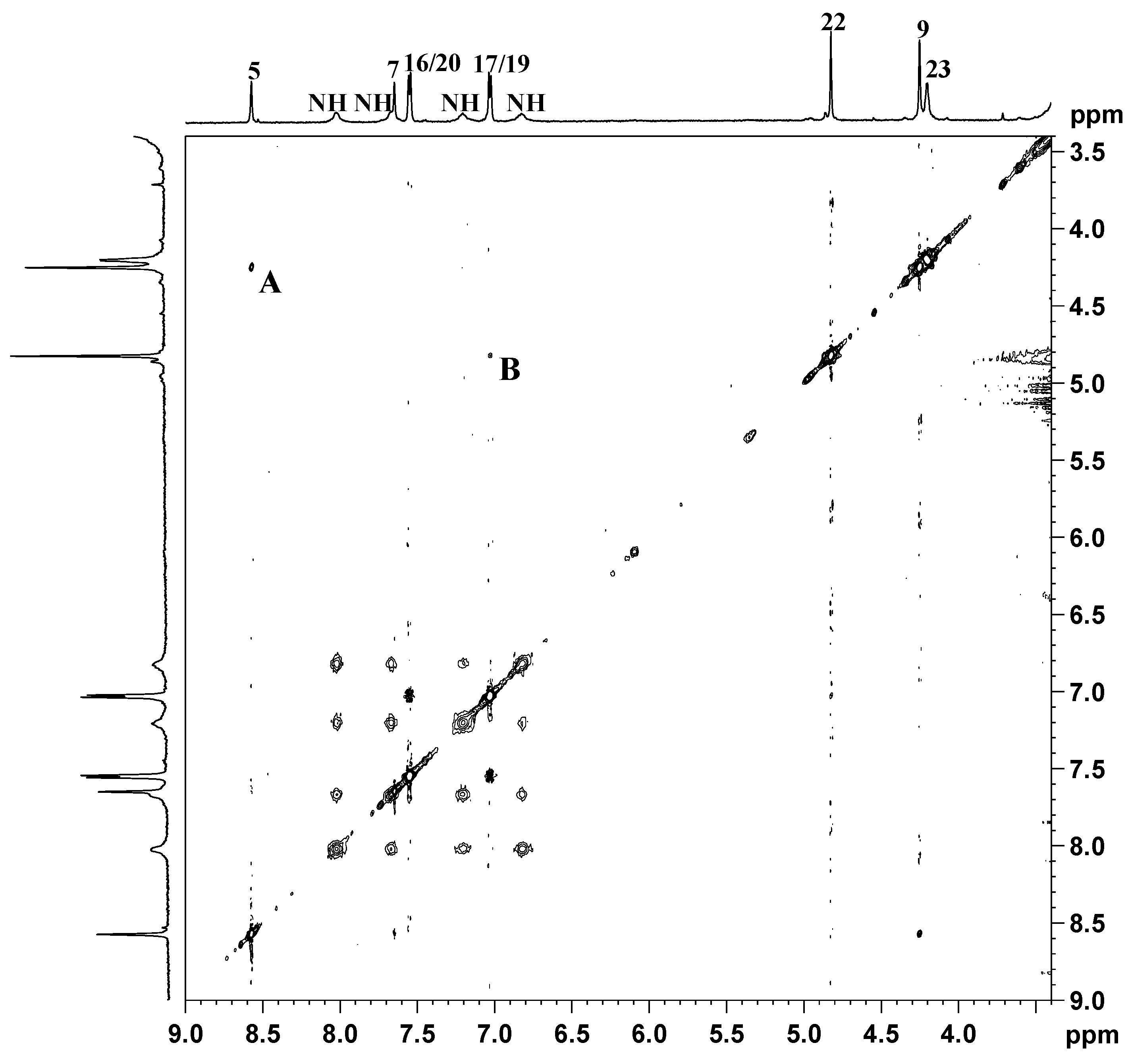
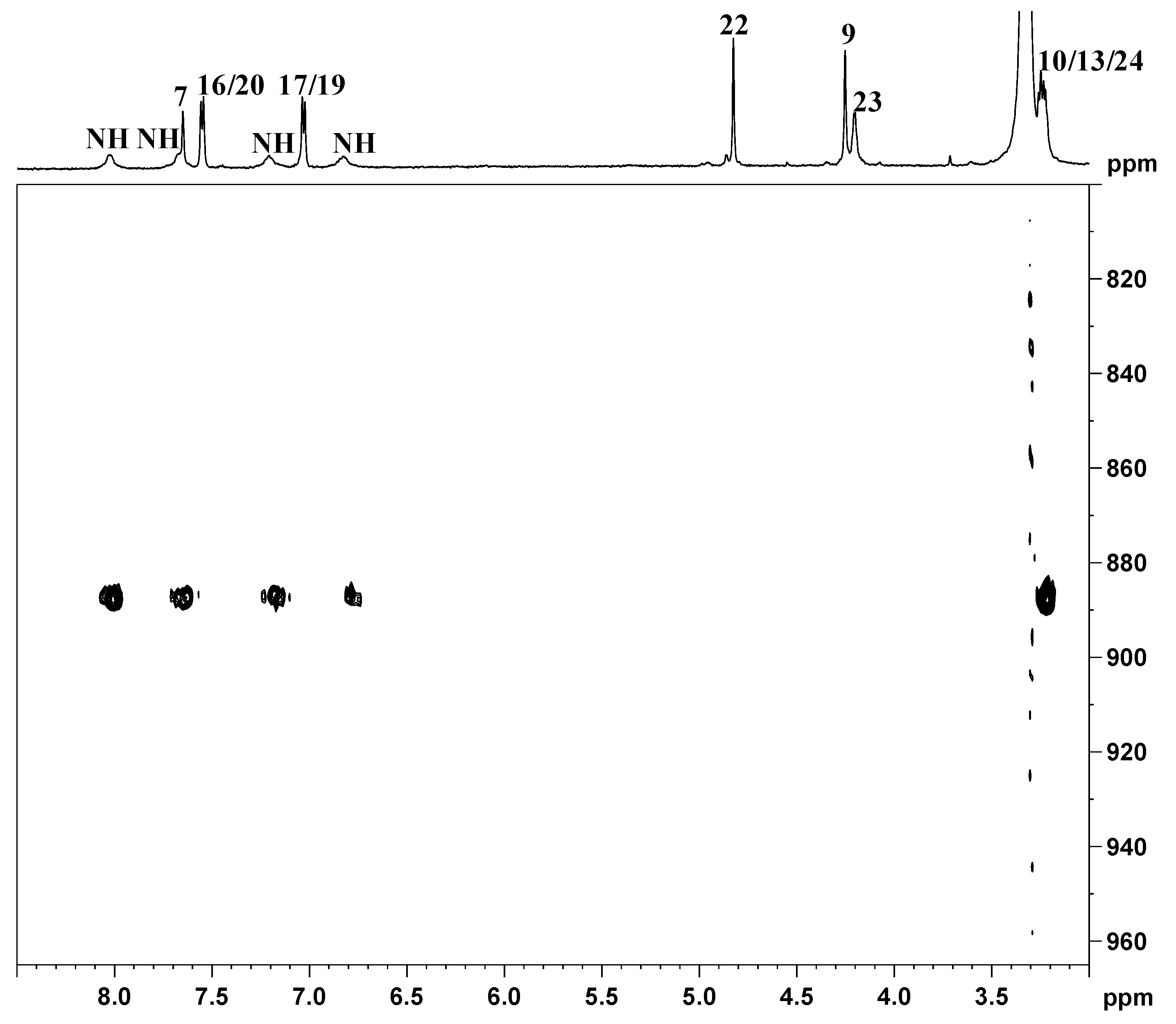
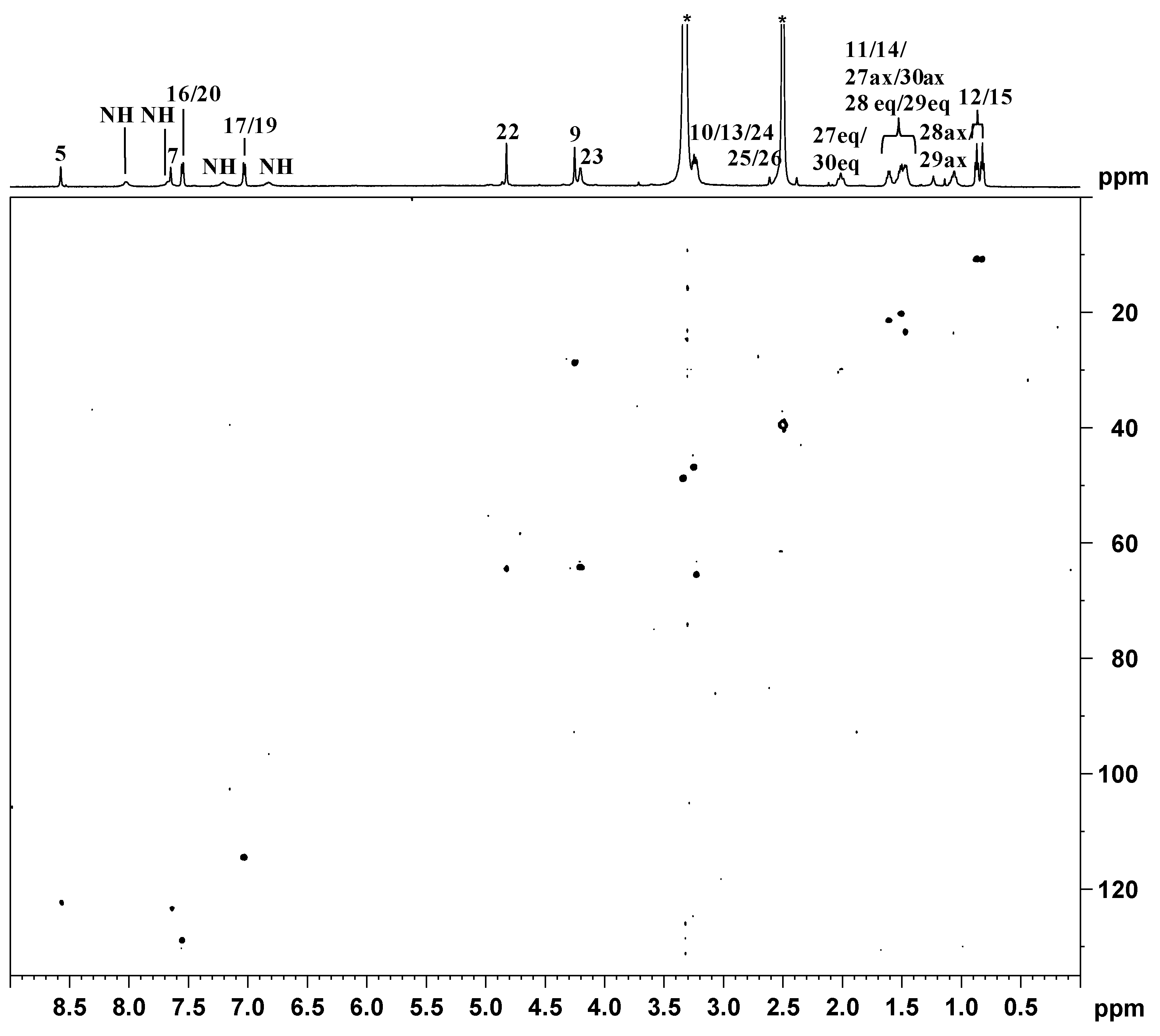
 indicates peaks relevant to the portion of TSPO ligand in complex 3. ● indicates peaks of free TSPO ligand (2). The amine protons were deuterated due to the fast H/D exchange.
indicates peaks relevant to the portion of TSPO ligand in complex 3. ● indicates peaks of free TSPO ligand (2). The amine protons were deuterated due to the fast H/D exchange.
 indicates peaks relevant to the portion of TSPO ligand in complex 3. ● indicates peaks of free TSPO ligand (2). The amine protons were deuterated due to the fast H/D exchange.
indicates peaks relevant to the portion of TSPO ligand in complex 3. ● indicates peaks of free TSPO ligand (2). The amine protons were deuterated due to the fast H/D exchange.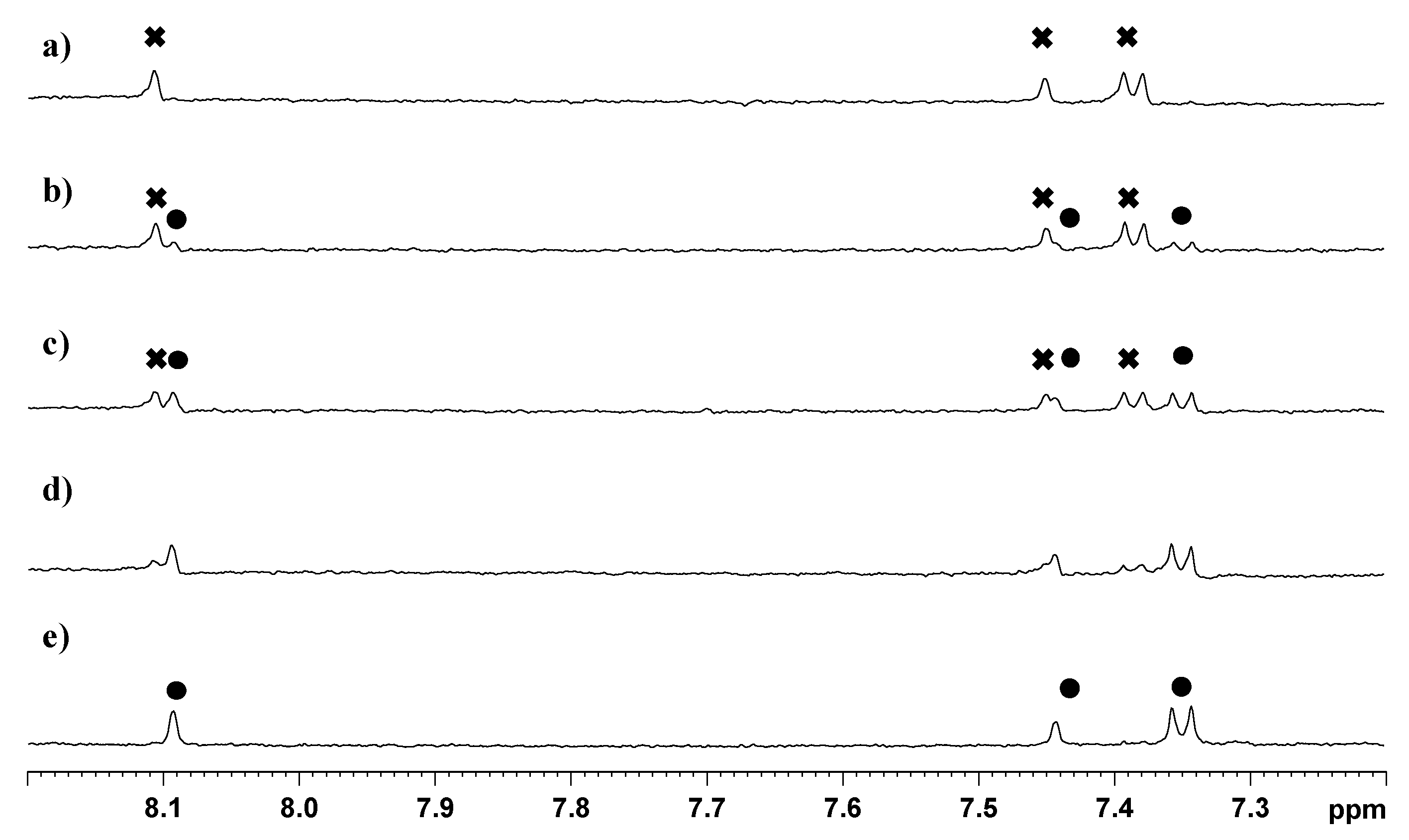
| C | δ 13C (ppm) |
|---|---|
| 5 | 122.3 |
| 7 | 123.5 |
| 9 | 28.7 |
| 10 or 13 | 46.9 |
| 10 or 13 | 48.9 |
| 11 or 14 | 20.3 |
| 11 or 14 | 21.5 |
| 12/15 | 10.9 |
| 16/20 | 129 |
| 17/19 | 114.5 |
| 22 | 64.4 |
| 23 | 64.3 |
| 24 | 65.5 |
| CH2 DACH | 23.5 |
| CH2 DACH | 30.1 |
| CH DACH | 61.5 |
| Compound | IC50 (nM) a |
|---|---|
| TSPO b | |
| 2 | 2.12 ± 0.10 |
| 3 | 18.64 ± 0.84 |
| PK 11195 | 4.27 ± 0.22 |
| Cell Lines | IC50 (µM) or % Cell Viability at 100 µM | |||
|---|---|---|---|---|
| 1 | 2 | 3 | Oxaliplatin | |
| MCF7 | 8.2 ± 0.4 | 53% ± 1% | 14.1 ± 0.1 | 5. 4 ± 0.4 |
| U87 | 9.1 ± 0.4 | 70.2 ± 0.3 (µM) | 16.1 ± 0.3 | 3.1 ± 0.2 |
| LoVo | 2.5 ± 0.5 | 65% ± 4% | 2.3 ± 0.1 | 0.46 ± 0.01 |
| Treatment Time | Uptake by LoVo Cells (ppb of Pt) | ||
|---|---|---|---|
| Oxaliplatin | 1 | 3 | |
| after 4 h treatment | 0.24 ± 0.04 | 0.18 ± 0.01 | 0.33 ± 0.02 |
| after 24 h treatment | 0.38 ± 0.01 | 0.59 ± 0.03 | 0.77 ± 0.02 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savino, S.; Denora, N.; Iacobazzi, R.M.; Porcelli, L.; Azzariti, A.; Natile, G.; Margiotta, N. Synthesis, Characterization, and Cytotoxicity of the First Oxaliplatin Pt(IV) Derivative Having a TSPO Ligand in the Axial Position. Int. J. Mol. Sci. 2016, 17, 1010. https://doi.org/10.3390/ijms17071010
Savino S, Denora N, Iacobazzi RM, Porcelli L, Azzariti A, Natile G, Margiotta N. Synthesis, Characterization, and Cytotoxicity of the First Oxaliplatin Pt(IV) Derivative Having a TSPO Ligand in the Axial Position. International Journal of Molecular Sciences. 2016; 17(7):1010. https://doi.org/10.3390/ijms17071010
Chicago/Turabian StyleSavino, Salvatore, Nunzio Denora, Rosa Maria Iacobazzi, Letizia Porcelli, Amalia Azzariti, Giovanni Natile, and Nicola Margiotta. 2016. "Synthesis, Characterization, and Cytotoxicity of the First Oxaliplatin Pt(IV) Derivative Having a TSPO Ligand in the Axial Position" International Journal of Molecular Sciences 17, no. 7: 1010. https://doi.org/10.3390/ijms17071010
APA StyleSavino, S., Denora, N., Iacobazzi, R. M., Porcelli, L., Azzariti, A., Natile, G., & Margiotta, N. (2016). Synthesis, Characterization, and Cytotoxicity of the First Oxaliplatin Pt(IV) Derivative Having a TSPO Ligand in the Axial Position. International Journal of Molecular Sciences, 17(7), 1010. https://doi.org/10.3390/ijms17071010











