The Potential for Microalgae as Bioreactors to Produce Pharmaceuticals
Abstract
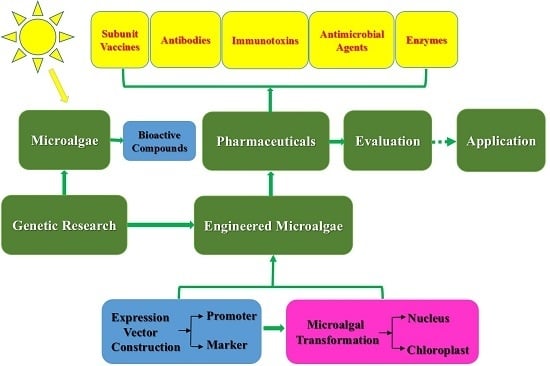
:1. Introduction
2. Brief Introduction to Microalgae
2.1. Growth Characteristics
2.2. Nutrient Value
2.3. Genetic Research on Microalgae
3. Genetic Engineering and Production of Pharmaceuticals and Recombinant Proteins
3.1. Microalgal Transformation
3.2. Genetically Engineered Microalgae
3.3. Production of Pharmaceuticals and Therapeutic Proteins
3.3.1. Subunit Vaccines
3.3.2. Antibodies, Immunotoxins, Antimicrobial Agents and Others
4. Discussions and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Demain, A.L.; Vaishnav, P. Production of recombinant proteins by microbes and higher organisms. Biotechnol. Adv. 2009, 27, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Miralles, N.; Domingo-Espín, J.; Corchero, J.L.; Vázquez, E.; Villaverde, A. Microbial factories for recombinant pharmaceuticals. Microb. Cell Fact. 2009, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Corchero, J.L.; Gasser, B.; Resina, D.; Smith, W.; Parrilli, E.; Vázquez, F.; Abasolo, I.; Giuliani, M.; Jäntti, J.; Ferrer, P. Unconventional microbial systems for the cost-efficient production of high-quality protein therapeutics. Biotechnol. Adv. 2013, 31, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.; Demain, A.L. Enzymes and bioconversions of industrial, pharmaceutical, and biotechnological significance. Org. Process Res. Dev. 2010, 15, 224–230. [Google Scholar] [CrossRef]
- Cereghino, G.P.L.; Cregg, J.M. Applications of yeast in biotechnology: Protein production and genetic analysis. Curr. Opin. Biotechnol. 1999, 10, 422–427. [Google Scholar] [CrossRef]
- Swartz, J.R. Advances in Escherichia coli production of therapeutic proteins. Curr. Opin. Biotechnol. 2001, 12, 195–201. [Google Scholar] [CrossRef]
- Fischer, R.; Liao, Y.-C.; Hoffmann, K.; Schillberg, S.; Emans, N. Molecular farming of recombinant antibodies in plants. Biol. Chem. 1999, 380, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Kelley, B. Biochemical engineering: Bioprocessing of therapeutic proteins. Curr. Opin. Biotechnol. 2001, 12, 173–174. [Google Scholar] [CrossRef]
- Chu, L.; Robinson, D.K. Industrial choices for protein production by large-scale cell culture. Curr. Opin. Biotechnol. 2001, 12, 180–187. [Google Scholar] [CrossRef]
- Jänne, J.; Alhonen, L.; Hyttinen, J.-M.; Peura, T.; Tolvanen, M.; Korhonen, V.-P. Transgenic bioreactors. Biotechnol. Ann. Rev. 1998, 4, 55–74. [Google Scholar] [CrossRef]
- Giddings, G.; Allison, G.; Brooks, D.; Carter, A. Transgenic plants as factories for biopharmaceuticals. Nat. Biotechnol. 2000, 18, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Echelard, Y. Recombinant protein production in transgenic animals. Curr. Opin. Biotechnol. 1996, 7, 536–540. [Google Scholar] [CrossRef]
- Scott, M.R.; Will, R.; Ironside, J.; Nguyen, H.-O.B.; Tremblay, P.; DeArmond, S.J.; Prusiner, S.B. Compelling transgenetic evidence for transmission of bovine spongiform encephalopathy prions to humans. Proc. Natl. Acad. Sci. USA 1999, 96, 15137–15142. [Google Scholar] [CrossRef] [PubMed]
- Mason, H.S.; Arntzen, C.J. Transgenic plants as vaccine production systems. Trends Biotechnol. 1995, 13, 388–392. [Google Scholar] [CrossRef]
- Cabanes-Macheteau, M.; Fitchette-Lainé, A.-C.; Loutelier-Bourhis, C.; Lange, C.; Vine, N.D.; Ma, J.K.; Lerouge, P.; Faye, L. N-Glycosylation of a mouse IgG expressed in transgenic tobacco plants. Glycobiology 1999, 9, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Herbers, K.; Sonnewald, U. Production of new/modified proteins in transgenic plants. Curr. Opin. Biotechnol. 1999, 10, 163–168. [Google Scholar] [CrossRef]
- Mason, H.S.; Lam, D.; Arntzen, C.J. Expression of hepatitis B surface antigen in transgenic plants. Proc. Natl. Acad. Sci. USA 1992, 89, 11745–11749. [Google Scholar] [CrossRef] [PubMed]
- De Cosa, B.; Moar, W.; Lee, S.-B.; Miller, M.; Daniell, H. Overexpression of the Bt CRY2Aa2 operon in chloroplasts leads to formation of insecticidal crystals. Nat. Biotechnol. 2001, 19, 71–74. [Google Scholar] [PubMed]
- Sijmons, P.C.; Dekker, B.M.; Schrammeijer, B.; Verwoerd, T.C.; van den Elzen, P.J.; Hoekema, A. Production of correctly processed human serum albumin in transgenic plants. Nat. Biotechnol. 1990, 8, 217–221. [Google Scholar] [CrossRef]
- Cramer, C.; Boothe, J.; Oishi, K. Transgenic plants for therapeutic proteins: Linking upstream and downstream strategies. Plant Biotechnol. 2000, 95–118. [Google Scholar]
- Mor, T.S.; Sternfeld, M.; Soreq, H.; Arntzen, C.J.; Mason, H.S. Expression of recombinant human acetylcholinesterase in transgenic tomato plants. Biotechnol. Bioeng. 2001, 75, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Sheldrake, A. The production of hormones in higher plants. Biol. Rev. 1973, 48, 509–559. [Google Scholar] [CrossRef]
- Streatfield, S.J.; Lane, J.R.; Brooks, C.A.; Barker, D.K.; Poage, M.L.; Mayor, J.M.; Lamphear, B.J.; Drees, C.F.; Jilka, J.M.; Hood, E.E. Corn as a production system for human and animal vaccines. Vaccine 2003, 21, 812–815. [Google Scholar] [CrossRef]
- Hiatt, A.; Caffferkey, R.; Bowdish, K. Production of antibodies in transgenic plants. Nature 1989, 342, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Ram, N.; Ayala, M.; Lorenzo, D.; Palenzuela, D.; Herrera, L.; Doreste, V.; Pérez, M.; Gavilondo, J.V.; Oramas, P. Expression of a single-chain Fv antibody fragment specific for the hepatitis B surface antigen in transgenic tobacco plants. Transgenic Res. 2002, 11, 61–64. [Google Scholar] [CrossRef]
- Borisjuk, N.V.; Borisjuk, L.G.; Logendra, S.; Petersen, F.; Gleba, Y.; Raskin, I. Production of recombinant proteins in plant root exudates. Nat. Biotechnol. 1999, 17, 466–469. [Google Scholar] [PubMed]
- Fischer, R.; Emans, N. Molecular farming of pharmaceutical proteins. Transgenic Res. 2000, 9, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Arntzen, C. Plant-made pharmaceuticals: From ‘Edible Vaccines’ to Ebola therapeutics. Plant Biotechnol. J. 2015, 13, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Zhu, L.; Sherman, A.; Wang, X.; Lin, S.; Kamesh, A.; Norikane, J.H.; Streatfield, S.J.; Herzog, R.W.; Daniell, H. Low cost industrial production of coagulation factor IX bioencapsulated in lettuce cells for oral tolerance induction in hemophilia B. Biomaterials 2015, 70, 84–93. [Google Scholar]
- Su, J.; Sherman, A.; Doerfler, P.A.; Byrne, B.J.; Herzog, R.W.; Daniell, H. Oral delivery of Acid Alpha Glucosidase epitopes expressed in plant chloroplasts suppresses antibody formation in treatment of Pompe mice. Plant Biotechnol. J. 2015, 13, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.T.; Daniell, H. Plant-made oral vaccines against human infectious diseases—Are we there yet? Plant Biotechnol. J. 2015, 13, 1056–1070. [Google Scholar] [CrossRef] [PubMed]
- Takaiwa, F.; Wakasa, Y.; Takagi, H.; Hiroi, T. Rice seed for delivery of vaccines to gut mucosal immune tissues. Plant Biotechnol. J. 2015, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.L. First plant-made biologic approved. Nat. Biotechnol. 2012, 30, 472. [Google Scholar] [CrossRef]
- Daniell, H.; Streatfield, S.J.; Rybicki, E.P. Advances in molecular farming: Key technologies, scaled up production and lead targets. Plant Biotechnol. J. 2015, 13, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Quist, D.; Chapela, I.H. Transgenic DNA introgressed into traditional maize landraces in Oaxaca, Mexico. Nature 2001, 414, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Rieger, M.A.; Lamond, M.; Preston, C.; Powles, S.B.; Roush, R.T. Pollen-mediated movement of herbicide resistance between commercial canola fields. Science 2002, 296, 2386–2388. [Google Scholar] [CrossRef] [PubMed]
- Griesbeck, C.; Kobl, I.; Heitzer, M. Chlamydomonas reinhardtii: A protein expression system for pharmaceutical and biotechnological proteins. Mol. Biotechnol. 2006, 34, 213–223. [Google Scholar] [CrossRef]
- Potvin, G.; Zhang, Z. Strategies for high-level recombinant protein expression in transgenic microalgae: A review. Biotechnol. Adv. 2010, 28, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-K.; Hu, Y.-R.; Wang, F.; Stiles, A.R.; Liu, C.-Z. Scale-up cultivation of Chlorella ellipsoidea from indoor to outdoor in bubble column bioreactors. Bioresour. Technol. 2014, 156, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.R. Photosynthesis fifth edition: By D O Hall and K K Rao, pp 211. Cambridge University Press, Cambridge. 1994. £9.95/$14.95 ISBN 0-521-43622-2. Biochem. Educ. 1995, 23, 47. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Olaizola, M. Commercial development of microalgal biotechnology: From the test tube to the marketplace. Biomol. Eng. 2003, 20, 459–466. [Google Scholar] [CrossRef]
- Morris, H.J.; Almarales, A.; Carrillo, O.; Bermúdez, R.C. Utilisation of Chlorella vulgaris cell biomass for the production of enzymatic protein hydrolysates. Bioresour. Technol. 2008, 99, 7723–7729. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Li, B. Microalgae as a source of bioactive molecules: Special problems and methodology. In Frontiers in Marine Biotechnology; Proksch, P., Muller, W.E.G., Eds.; Horizon Biosciences: Wymondham, UK, 2006; pp. 145–174. [Google Scholar]
- Borowitzka, M.A. Commercial production of microalgae: Ponds, tanks, tubes and fermenters. J. Biotechnol. 1999, 70, 313–321. [Google Scholar] [CrossRef]
- Kumar, R.R.; Rao, P.H.; Subramanian, V.V.; Sivasubramanian, V. Enzymatic and non-enzymatic antioxidant potentials of Chlorella vulgaris grown in effluent of a confectionery industry. J. Food Sci. Technol. 2011, 51, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Microalgal Biotechnology: Potential and Production; Posten, C., Walter, C., Eds.; De Gruyter: Berlin, Germany, 2012. [Google Scholar]
- Das, P.; Aziz, S.S.; Obbard, J.P. Two phase microalgae growth in the open system for enhanced lipid productivity. Renew. Energ. 2011, 36, 2524–2528. [Google Scholar] [CrossRef]
- Richmond, A. Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Wiley-Blackwell: Oxford, UK, 2008. [Google Scholar]
- Abreu, A.P.; Fernandes, B.; Vicente, A.A.; Teixeira, J.; Dragone, G. Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour. Technol. 2012, 118, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Basaran, P.; Rodríguez-Cerezo, E. Plant molecular farming: Opportunities and challenges. J. Serb. Chem. Soc. 2008, 28, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Weng, Y.; Dickey, A.; Wang, K.Y. Plants as factories for human pharmaceuticals: Applications and challenges. Int. J. Mol. Sci. 2015, 16, 28549–28565. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.-L. Biotechnological applications of microalgae. IeJSME 2012, 6, S24–S37. [Google Scholar]
- Kaplan, D.; Richmond, A.; Dubinsky, Z.; Aaronson, S. Algal nutrition. In Handbook of Microalgal Mass Culture; CRC Press: Boca Raton, FL, USA, 1986; pp. 147–198. [Google Scholar]
- Cunningham, A.; Maas, P. Time lag and nutrient storage effects in the transient growth response of Chlamydomonas reinhardii in nitrogen-limited batch and continuous culture. Microbiology 1978, 104, 227–231. [Google Scholar]
- Garcia-Ferris, C.; Rios, A.; Ascaso, C.; Moreno, J. Correlated biochemical and ultrastructural changes in nitrogen-starved Euglena gracilis. J. Phycol. 1996, 32, 953–963. [Google Scholar] [CrossRef]
- Přibyl, P.; Cepák, V.; Zachleder, V. Production of lipids in 10 strains of Chlorella and Parachlorella, and enhanced lipid productivity in Chlorella vulgaris. Appl. Microbiol. Biotechnol. 2012, 94, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Zachleder, V.; Brányiková, I. Starch overproduction by means of algae. In Algal Biorefineries; Bajpai, R., Prokop, A., Zappi, M., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 1, pp. 217–240. [Google Scholar]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Soletto, D.; Binaghi, L.; Lodi, A.; Carvalho, J.; Converti, A. Batch and fed-batch cultivations of Spirulina platensis using ammonium sulphate and urea as nitrogen sources. Aquaculture 2005, 243, 217–224. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.; Navarro-Juárez, R.; López-Martınez, J.; Campra-Madrid, P.; Rebolloso-Fuentes, M. Functional properties of the biomass of three microalgal species. J. Food Eng. 2004, 65, 511–517. [Google Scholar] [CrossRef]
- Becker, W.; Richmond, A. Microalgae in human and animal nutrition. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Wiley-Blackwell: Oxford, UK, 2004; pp. 312–351. [Google Scholar]
- Metting, F., Jr. Biodiversity and application of microalgae. J. Ind. Microbiol. 1996, 17, 477–489. [Google Scholar] [CrossRef]
- Borowitzka, M. Fats, oils and hydrocarbons. In Micro-Algal Biotechnology; Cambridge University Press: Cambridge, UK, 1988; pp. 257–287. [Google Scholar]
- Brown, M.; Jeffrey, S.; Volkman, J.; Dunstan, G. Nutritional properties of microalgae for mariculture. Aquaculture 1997, 151, 315–331. [Google Scholar] [CrossRef]
- Tonon, T.; Harvey, D.; Larson, T.R.; Graham, I.A. Long chain polyunsaturated fatty acid production and partitioning to triacylglycerols in four microalgae. Phytochemistry 2002, 61, 15–24. [Google Scholar] [CrossRef]
- Tzovenis, I.; de Pauw, N.; Sorgeloos, P. Optimisation of T-ISO biomass production rich in essential fatty acids: I. Effect of different light regimes on growth and biomass production. Aquaculture 2003, 216, 203–222. [Google Scholar] [CrossRef]
- Apt, K.E.; Behrens, P.W. Commercial developments in microalgal biotechnology. J. Phycol. 1999, 35, 215–226. [Google Scholar] [CrossRef]
- Rasoul-Amini, S.; Ghasemi, Y.; Morowvat, M.H.; Mohagheghzadeh, A. PCR amplification of 18S rRNA, single cell protein production and fatty acid evaluation of some naturally isolated microalgae. Food Chem. 2009, 116, 129–136. [Google Scholar] [CrossRef]
- Calvin, M.; Benson, A. The Path of Carbon in Photosynthesis; Ernest Orlando Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 1948. [Google Scholar]
- Hirose, Y.; Fujisawa, T.; Ohtsubo, Y.; Katayama, M.; Misawa, N.; Wakazuki, S.; Shimura, Y.; Nakamura, Y.; Kawachi, M.; Yoshikawa, H. Complete genome sequence of cyanobacterium Nostoc sp. NIES-3756, a potentially useful strain for phytochrome-based bioengineering. J. Biotechnol. 2016, 218, 51–52. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Tabata, S. Complete genome structure of the unicellular cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol. 1997, 38, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, M.; Misumi, O.; Shin-i, T.; Maruyama, S.; Takahara, M.; Miyagishima, S.-Y.; Mori, T.; Nishida, K.; Yagisawa, F.; Nishida, K. Genome sequence of the ultrasmall unicellular red alga Cyanidioschyzon merolae 10D. Nature 2004, 428, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Armbrust, E.V.; Berges, J.A.; Bowler, C.; Green, B.R.; Martinez, D.; Putnam, N.H.; Zhou, S.; Allen, A.E.; Apt, K.E.; Bechner, M. The genome of the diatom Thalassiosira pseudonana: Ecology, evolution, and metabolism. Science 2004, 306, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S.S.; Prochnik, S.E.; Vallon, O.; Harris, E.H.; Karpowicz, S.J.; Witman, G.B.; Terry, A.; Salamov, A.; Fritz-Laylin, L.K.; Maréchal-Drouard, L. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 2007, 318, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Ning, K.; Zeng, X.; Luo, Y.; Wang, D.; Hu, J.; Li, J.; Xu, H.; Huang, J.; Wan, M. Genomic foundation of starch-to-lipid switch in oleaginous Chlorella spp. Plant Physiol. 2015, 169, 2444–2461. [Google Scholar] [PubMed]
- Radakovits, R.; Jinkerson, R.E.; Fuerstenberg, S.I.; Tae, H.; Settlage, R.E.; Boore, J.L.; Posewitz, M.C. Draft genome sequence and genetic transformation of the oleaginous alga nannochloropsis gaditana. Nat. Commun. 2012, 3, 686. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Luo, G.Z.; Chen, K.; Deng, X.; Yu, M.; Han, D.; Hao, Z.; Liu, J.; Lu, X.; Doré, L. N6-Methyldeoxyadenosine marks active transcription start sites in Chlamydomonas. Cell 2015, 161, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, R.; Patena, W.; Gang, S.S.; Blum, S.R.; Ivanova, N.; Yue, R.; Robertson, J.M.; Lefebvre, P.; Fitz-Gibbon, S.T. An indexed, mapped mutant library enables reverse genetics studies of biological processes in Chlamydomonas reinhardtii. Plant Cell 2016, 28, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wittkopp, T.M.; Li, X.; Warakanont, J.; Dubini, A.; Catalanotti, C.; Kim, R.G.; Nowack, E.C.M.; Mackinder, L.C.M.; Aksoy, M. Critical role of Chlamydomonas reinhardtii ferredoxin-5 in maintaining membrane structure and dark metabolism. Proc. Natl. Acad. Sci. USA 2015, 112, 14978–14983. [Google Scholar] [CrossRef] [PubMed]
- Dejtisakdi, W.; Miller, S.M. Overexpression of calvin cycle enzyme fructose 1,6-bisphosphatase in Chlamydomonas reinhardtii has a detrimental effect on growth. Algal Res. 2016, 14, 116–126. [Google Scholar] [CrossRef]
- Sirikhachornkit, A.; Vuttipongchaikij, S.; Suttangkakul, A.; Yokthongwattana, K.; Juntawong, P.; Pokethitiyook, P.; Kangvansaichol, K.; Meetam, M. Increasing the triacylglycerol content in Dunaliella tertiolecta through isolation of starch-deficient mutants. J. Microbiol. Biotechnol. 2016, 28, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Kira, N.; Ohnishi, K.; Miyagawa-Yamaguchi, A.; Kadono, T.; Adachi, M. Nuclear transformation of the diatom Phaeodactylum tricornutum using PCR-amplified DNA fragments by microparticle bombardment. Mar. Genom. 2016, 25, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Camargo, L.S.; Tran, M.; Beld, J.; Burkart, M.D.; Mayfield, S.P. Selenocystamine improves protein accumulation in chloroplasts of eukaryotic green algae. AMB Express 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Hu, H.; Gao, Y.; Xu, X.; Gao, H. Microalgae as platforms for production of recombinant proteins and valuable compounds: Progress and prospects. J. Ind. Microbiol. Biotechnol. 2011, 38, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Kindle, K. Nuclear transformation: Technology and applications. In The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas; Rochaix, J.-D., Goldschmidt-Clermont, M., Merchant, S., Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 7, pp. 41–61. [Google Scholar]
- Dunahay, T. Transformation of Chlamydomonas reinhardtii with silicon carbide whiskers. Biotechniques 1993, 15, 452–455, 457–458, 460. [Google Scholar] [PubMed]
- Te, M.R.; Miller, D.J. Genetic transformation of dinoflagellates (Amphidinium and Symbiodinium): Expression of GUS in microalgae using heterologous promoter constructs. Plant J. 1998, 13, 427–435. [Google Scholar] [CrossRef]
- Shimogawara, K.; Fujiwara, S.; Grossman, A.; Usuda, H. High-efficiency transformation of Chlamydomonas reinhardtii by electroporation. Genetics 1998, 148, 1821–1828. [Google Scholar] [PubMed]
- Hayashi, M.; Hirono, M.; Kamiya, R. Recovery of flagellar dynein function in a Chlamydomonas actin/dynein-deficient mutant upon introduction of muscle actin by electroporation. Cell Motil. Cytoskelet. 2001, 49, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Henry, E.C.; Meints, R.H. Recombinant viruses as transformation vectors of marine macroalgae. J. Appl. Phycol. 1994, 6, 247–253. [Google Scholar] [CrossRef]
- Van Etten, J.L.; Meints, R.H. Giant viruses infecting algae. Annu. Rev. Microbiol. 1999, 53, 447–494. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Kawata, Y. A mini-transposon/transposase complex as a new tool for the genetic transformation of microalgae. In Photosynthetic Microorganisms in Environmental Biotechnology; Kojima, H., Lee, Y.K., Eds.; Springer Verlag: Berlin, Germany, 2001; pp. 41–61. [Google Scholar]
- Apt, K.E.; Grossman, A.; Kroth-Pancic, P. Stable nuclear transformation of the diatom Phaeodactylum tricornutum. Mol. Gen. Genet. 1996, 252, 572–579. [Google Scholar] [PubMed]
- Dunahay, T.G.; Jarvis, E.E.; Roessler, P.G. Genetic transformation of the diatoms Cyclotella cryptica and Navicula saprophila. J. Phycol. 1995, 31, 1004–1012. [Google Scholar] [CrossRef]
- Falciatore, A.; Casotti, R.; Leblanc, C.; Abrescia, C.; Bowler, C. Transformation of nonselectable reporter genes in marine diatoms. Mar. Biotechnol. 1999, 1, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Zaslavskaia, L.A.; Lippmeier, J.C.; Kroth, P.G.; Grossman, A.R.; Apt, K.E. Transformation of the diatom Phaeodactylum tricornutum (bacillariophyceae) with a variety of selectable marker and reporter genes. J. Phycol. 2000, 36, 379–386. [Google Scholar] [CrossRef]
- Lapidot, M.; Raveh, D.; Sivan, A.; Arad, S.M.; Shapira, M. Stable chloroplast transformation of the unicellular red alga Porphyridium species. Plant Physiol. 2002, 129, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Boynton, J.E.; Gillham, N.W.; Harris, E.H.; Hosler, J.P.; Johnson, A.M.; Jones, A.R.; Randolph-Anderson, B.L.; Robertson, D.; Klein, T.M.; Shark, K.B. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science 1988, 240, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Doetsch, N.A.; Favreau, M.R.; Kuscuoglu, N.; Thompson, M.D.; Hallick, R.B. Chloroplast transformation in Euglena gracilis: Splicing of a group III twintron transcribed from a transgenic psbK operon. Curr. Genet. 2001, 39, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Randolph-Anderson, B.L.; Boynton, J.E.; Gillham, N.W.; Harris, E.H.; Johnson, A.M.; Dorthu, M.-P.; Matagne, R.F. Further characterization of the respiratory deficient dum-1 mutation of Chlamydomonas reinhardtii and its use as a recipient for mitochondrial transformation. Mol. Gen. Genet. 1993, 236, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Fernández, E.; Schnell, R.; Ranum, L.; Hussey, S.C.; Silflow, C.D.; Lefebvre, P.A. Isolation and characterization of the nitrate reductase structural gene of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 1989, 86, 6449–6453. [Google Scholar] [CrossRef] [PubMed]
- Debuchy, R.; Purton, S.; Rochaix, J. The argininosuccinate lyase gene of Chlamydomonas reinhardtii: An important tool for nuclear transformation and for correlating the genetic and molecular maps of the ARG7 locus. EMBO J. 1989, 8, 2803–2809. [Google Scholar] [PubMed]
- Shrager, J.; Hauser, C.; Chang, C.-W.; Harris, E.H.; Davies, J.; McDermott, J.; Tamse, R.; Zhang, Z.; Grossman, A.R. Chlamydomonas reinhardtii genome project. A guide to the generation and use of the cDNA information. Plant Physiol. 2003, 131, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Dawson, H.N.; Burlingame, R.; Cannons, A.C. Stable transformation of Chlorella: Rescue of nitrate reductase-deficient mutants with the nitrate reductase gene. Curr. Microbiol. 1997, 35, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Georgianna, D.R.; Hannon, M.J.; Marcuschi, M.; Wu, S.; Botsch, K.; Lewis, A.J.; Hyun, J.; Mendez, M.; Mayfield, S.P. Production of recombinant enzymes in the marine alga Dunaliella tertiolecta. Algal Res. 2013, 2, 2–9. [Google Scholar] [CrossRef]
- Gutierrez, C.L.; Gimpel, J.; Escobar, C.; Marshall, S.H.; Henríquez, V. Chloroplast genetic tool for the green microalgae Haematococcus pluvialis (chlorophyceae, volvocales). J. Phycol. 2012, 48, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Robl, I.; Sumper, M.; Kröger, N. Targeting and covalent modification of cell wall and membrane proteins heterologously expressed in the diatom Cylindrotheca fusiformis (bacillariophyceae). J. Phycol. 1999, 35, 113–120. [Google Scholar] [CrossRef]
- Poulsen, N.; Chesley, P.M.; Kröger, N. Molecular genetic manipulation of the diatom Thalassiosira pseudonana (bacillariophyceae). J. Phycol. 2006, 42, 1059–1065. [Google Scholar] [CrossRef]
- Miyagawa-Yamaguchi, A.; Okami, T.; Kira, N.; Yamaguchi, H.; Ohnishi, K.; Adachi, M. Stable nuclear transformation of the diatom Chaetoceros sp. Phycol. Res. 2011, 59, 113–119. [Google Scholar] [CrossRef]
- Walker, T.L.; Purton, S.; Becker, D.K.; Collet, C. Microalgae as bioreactors. Plant Cell Rep. 2005, 24, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Kindle, K.L.; Schnell, R.A.; Fernández, E.; Lefebvre, P.A. Stable nuclear transformation of Chlamydomonas using the Chlamydomonas gene for nitrate reductase. J. Cell Biol. 1989, 109, 2589–2601. [Google Scholar] [CrossRef] [PubMed]
- Schiedlmeier, B.; Schmitt, R.; Müller, W.; Kirk, M.M.; Gruber, H.; Mages, W.; Kirk, D.L. Nuclear transformation of Volvox carteri. Proc. Natl. Acad. Sci. USA 1994, 91, 5080–5084. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, X.; Li, Q.; Zhang, Q.; Xu, Z. Functional complementation of a nitrate reductase defective mutant of a green alga Dunaliella viridis by introducing the nitrate reductase gene. Gene 2006, 377, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, H.; Johnson, A.M.; Gillham, N.W.; Boynton, J.E. A eubacterial gene conferring spectinomycin resistance on Chlamydomonas reinhardtii: Integration into the nuclear genome and gene expression. Genetics 1997, 145, 97. [Google Scholar] [PubMed]
- Sizova, I.; Fuhrmann, M.; Hegemann, P. A Streptomyces rimosus aphVIII gene coding for a new type phosphotransferase provides stable antibiotic resistance to Chlamydomonas reinhardtii. Gene 2001, 277, 221–229. [Google Scholar] [CrossRef]
- Stevens, D.R.; Purton, S.; Rochaix, J.-D. The bacterial phleomycin resistance gene ble as a dominant selectable marker in Chlamydomonas. Mol. Gen. Genet. 1996, 251, 23–30. [Google Scholar] [PubMed]
- Kovar, J.L.; Zhang, J.; Funke, R.P.; Weeks, D.P. Molecular analysis of the acetolactate synthase gene of Chlamydomonas reinhardtii and development of a genetically engineered gene as a dominant selectable marker for genetic transformation. Plant J. 2002, 29, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, A.; Rappel, A. Genetic engineering of the multicellular green alga Volvox: A modified and multiplied bacterial antibiotic resistance gene as a dominant selectable marker. Plant J. 1999, 17, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.-L.; Yin, W.-B.; Chen, Y.-H.; Niu, L.-L.; Sun, Y.-R.; Zhao, S.-M.; Yang, F.; Wang, R.R.-C.; Wu, Q.; Zhang, X. A new strategy to produce a defensin: Stable production of mutated NP-1 in nitrate reductase-deficient Chlorella ellipsoidea. PLoS ONE 2013, 8, e54966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hao, Q.; Bai, L.; Xu, J.; Yin, W.; Song, L.; Xu, L.; Guo, X.; Fan, C.; Chen, Y. Overexpression of the soybean transcription factor GmDof4 significantly enhances the lipid content of Chlorella ellipsoidea. Biotechnol. Biofuels 2014, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, A.; Okami, T.; Kira, N.; Yamaguchi, H.; Ohnishi, K.; Adachi, M. High efficiency transformation of the diatom Phaeodactylum tricornutum with a promoter from the diatom Cylindrotheca fusiformis. Phycol. Res. 2009, 57, 142–146. [Google Scholar] [CrossRef]
- Hempel, F.; Lau, J.; Klingl, A.; Maier, U.G. Algae as protein factories: Expression of a human antibody and the respective antigen in the diatom Phaeodactylum tricornutum. PLoS ONE 2011, 6, e28424. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, N.; Kröger, N. A new molecular tool for transgenic diatoms. FEBS J. 2005, 272, 3413–3423. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, N.H.; Felsner, G.; Schramm, F.D.; Klingl, A.; Maier, U.-G.; Bolte, K. A single peroxisomal targeting signal mediates matrix protein import in diatoms. PLoS ONE 2011, 6, e25316. [Google Scholar] [CrossRef] [PubMed]
- Hempel, F.; Maier, U.G. An engineered diatom acting like a plasma cell secreting human lgG antibodies with high efficiency. Microb. Cell Fact. 2012, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Stork, S.; Moog, D.; Przyborski, J.M.; Wilhelmi, I.; Zauner, S.; Maier, U.G. Distribution of the selma translocon in secondary plastids of red algal origin and predicted uncoupling of ubiquitin-dependent translocation from degradation. Eukaryot. Cell 2012, 11, 1472–1481. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Kim, Y.T.; Cho, J.J.; Bae, J.-H.; Hur, S.-B.; Hwang, I.; Choi, T.-J. Stable integration and functional expression of flounder growth hormone gene in transformed microalga, Chlorella ellipsoidea. Mar. Biotechnol. 2002, 4, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, E.E.; Brown, L.M. Transient expression of firefly luciferase in protoplasts of the green alga Chlorella ellipsoidea. Curr. Genet. 1991, 19, 317–321. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Sun, Y.; Zhang, L.; Li, W. Highly efficient expression of rabbit neutrophil peptide-1 gene in Chlorella ellipsoidea cells. Curr. Genet. 2001, 39, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.L.; Nakamura, M. Expression of human growth hormone by the eukaryotic alga, Chlorella. Curr. Microbiol. 1999, 38, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, S.; Chandrashekar, A.; Ravishankar, G.; Sarada, R. Agrobacterium-mediated transformation in the green alga Haematococcus pluvialis (Chlorophyceae, Volvocales). J. Phycol. 2009, 45, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Wang, Y.; Wang, P.; Li, W.; Sun, Y. Stable expression of hepatitis B surface antigen gene in Dunaliella salina (chlorophyta). J. Appl. Phycol. 2003, 15, 451–456. [Google Scholar] [CrossRef]
- Feng, S.; Xue, L.; Liu, H.; Lu, P. Improvement of efficiency of genetic transformation for Dunaliella salina by glass beads method. Mol. Biol. Rep. 2009, 36, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.-J.; Chen, H.-X.; Xu, W.-Q.; Xu, Y.-W. Expression of soybean kunitz trypsin inhibitor gene SKTI in Dunaliella salina. J. Appl. Phycol. 2013, 25, 139–144. [Google Scholar] [CrossRef]
- Seo, S.; Jeon, H.; Hwang, S.; Jin, E.S.; Chang, K.S. Development of a new constitutive expression system for the transformation of the diatom Phaeodactylum tricornutum. Algal Res. 2015, 11, 50–54. [Google Scholar] [CrossRef]
- Maruyama, M.; Horáková, I.; Honda, H.; Xing, X.H.; Shiragami, N.; Unno, H. Introduction of foreign DNA into Chlorella saccharophila by electroporation. Biotechnol. Tech. 1994, 8, 821–826. [Google Scholar] [CrossRef]
- Yang, A.; Bai, L.; Chen, Y.; Hu, Z.; Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing. The expression of NP-1 in Chlorella ellipsoidea. unpublished work. 2009. [Google Scholar]
- Wang, Y.; Chen, Y.; Zhang, X.; Wang, P.; Geng, D.; Zhao, S.; Zhang, L.; Sun, Y. Isolation and characterization of a nitrate reductase deficient mutant of Chlorella ellipsoidea (Chlorophyta). J. Appl. Phycol. 2005, 17, 281–286. [Google Scholar] [CrossRef]
- Niu, Y.F.; Zhang, M.H.; Xie, W.H.; Li, J.N.; Gao, Y.F.; Yang, W.D.; Liu, J.S.; Li, H.Y. A new inducible expression system in a transformed green alga, Chlorella vulgaris. Genet. Mol. Res. 2011, 10, 3427–3434. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, H.; Johnson, A.M.; Gillham, N.W.; Boynton, J.E. Epigenetic silencing of a foreign gene in nuclear transformants of Chlamydomonas. Plant Cell 1997, 9, 925–945. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, H.; Ma, X.; Msanne, J.; Repas, T. RNA-mediated silencing in algae: Biological roles and tools for analysis of gene function. Eukaryot. Cell 2011, 10, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Ma, X.; Cerutti, H. Gene silencing in microalgae: Mechanisms and biological roles. Bioresour. Technol. 2015, 184, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Doron, L.; Segal, N.A.; Shapira, M.; Wilhelm, R.A.; Geisler, K. Transgene expression in microalgae—From tools to applications. Front. Plant Sci. 2016, 7, 505. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M. Stable transformation of the intact cells of Chlorella kessleri with high velocity microprojectiles. Biol. Plant. 1999, 42, 209–216. [Google Scholar] [CrossRef]
- Ahmed, E.G.; Sung Kwon, L.; Sang, M.S.; Seung, H.Y.; Gyuhwa, C. Development of stable marker-free nuclear transformation strategy in the green microalga Chlorella vulgaris. Afr. J. Biotechnol. 2015, 14, 2715–2723. [Google Scholar] [CrossRef]
- Pulz, O.; Scheibenbogen, K.; Groß, W. Biotechnology with cyanobacteria and microalgae. In Biotechnology Set, 2nd ed.; Rehm, H.-J., Reed, G., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008; pp. 105–136. [Google Scholar]
- Dunahay, T.G.; Jarvis, E.E.; Dais, S.S.; Roessler, P.G. Manipulation of microalgal lipid production using genetic engineering. Appl. Biochem. Biotechnol. 1996, 57, 223–231. [Google Scholar] [CrossRef]
- Cai, X.-H.; Brown, C.; Adhiya, J.; Traina, S.J.; Sayre, R.T. Growth and heavy metal binding properties of transgenic Chlamydomonas expressing a foreign metallothionein gene. Int. J. Phytoremediat. 1999, 1, 53–65. [Google Scholar] [CrossRef]
- Siripornadulsil, S.; Traina, S.; Verma, D.P.S.; Sayre, R.T. Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell 2002, 14, 2837–2847. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, S.P.; Franklin, S.E.; Lerner, R.A. Expression and assembly of a fully active antibody in algae. Proc. Natl. Acad. Sci. USA 2003, 100, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.; Henry, R.E.; Siefker, D.; Van, C.; Newkirk, G.; Kim, J.; Bui, J.; Mayfield, S.P. Production of anti-cancer immunotoxins in algae: Ribosome inactivating proteins as fusion partners. Biotechnol. Bioeng. 2013, 110, 2826–2835. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.; Van, C.; Barrera, D.J.; Pettersson, P.L.; Peinado, C.D.; Bui, J.; Mayfield, S.P. Production of unique immunotoxin cancer therapeutics in algal chloroplasts. Proc. Natl. Acad. Sci. USA 2013, 110, E15–E22. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Qian, K.; Su, N.; Chang, H.; Liu, J.; Shen, G. Foot-and-mouth disease virus VP1 protein fused with cholera toxin B subunit expressed in Chlamydomonas reinhardtii chloroplast. Biotechnol. Lett. 2003, 25, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Demurtas, O.C.; Massa, S.; Ferrante, P.; Venuti, A.; Franconi, R.; Giuliano, G. A Chlamydomonas-derived human papillomavirus 16 E7 vaccine induces specific tumor protection. PLoS ONE 2013, 8, e61473. [Google Scholar] [CrossRef] [PubMed]
- Rasala, B.A.; Muto, M.; Lee, P.A.; Jager, M.; Cardoso, R.M.; Behnke, C.A.; Kirk, P.; Hokanson, C.A.; Crea, R.; Mendez, M. Production of therapeutic proteins in algae, analysis of expression of seven human proteins in the chloroplast of Chlamydomonas reinhardtii. Plant Biotechnol. J. 2010, 8, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Shen, G.F.; Ru, B.G. Survival of human metallothionein-2 transplastomic Chlamydomonas reinhardtii to ultraviolet B exposure. Acta Biochim. Biophys. Sin. 2006, 38, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, F.; Li, D.; Zhang, Z.; Liu, Y.; Zheng, D.; Wang, Y.; Shen, G. Expression of human soluble TRAIL in Chlamydomonas reinhardtii chloroplast. Chin. Sci. Bull. 2006, 51, 1703–1709. [Google Scholar] [CrossRef]
- Su, Z.L.; Qian, K.X.; Tan, C.P.; Meng, C.X.; Qin, S. Recombination and heterologous expression of allophycocyanin gene in the chloroplast of Chlamydomonas reinhardtii. Acta Biochim. Biophys. Sin. 2005, 37, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.; Zhou, B.; Pettersson, P.L.; Gonzalez, M.J.; Mayfield, S.P. Synthesis and assembly of a full-length human monoclonal antibody in algal chloroplasts. Biotechnol. Bioeng. 2009, 104, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Eichler-Stahlberg, A.; Weisheit, W.; Ruecker, O.; Heitzer, M. Strategies to facilitate transgene expression in Chlamydomonas reinhardtii. Planta 2009, 229, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Manuell, A.L.; Beligni, M.V.; Elder, J.H.; Siefker, D.T.; Tran, M.; Weber, A.; McDonald, T.L.; Mayfield, S.P. Robust expression of a bioactive mammalian protein in Chlamydomonas chloroplast. Plant Biotechnol. J. 2007, 5, 402–412. [Google Scholar] [CrossRef] [PubMed]
- He, D.-M.; Qian, K.-X.; Shen, G.-F.; Zhang, Z.-F.; Yi-Nü, L.; Su, Z.-L.; Shao, H.-B. Recombination and expression of classical swine fever virus (CSFV) structural protein E2 gene in Chlamydomonas reinhardtii chroloplasts. Colloids Surf. B Biointerfaces 2007, 55, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Surzycki, R.; Greenham, K.; Kitayama, K.; Dibal, F.; Wagner, R.; Rochaix, J.-D.; Ajam, T.; Surzycki, S. Factors effecting expression of vaccines in microalgae. Biologicals 2009, 37, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Brandsma, M.; Tremblay, R.; Maxwell, D.; Jevnikar, A.M.; Huner, N.; Ma, S. A novel expression platform for the production of diabetes-associated autoantigen human glutamic acid decarboxylase (hGAD65). BMC Biotechnol. 2008, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Dreesen, I.A.; Charpin-El Hamri, G.; Fussenegger, M. Heat-stable oral alga-based vaccine protects mice from Staphylococcus aureus infection. J. Biotechnol. 2010, 145, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Dauvillée, D.; Delhaye, S.; Gruyer, S.; Slomianny, C.; Moretz, S.E.; d’Hulst, C.; Long, C.A.; Ball, S.G.; Tomavo, S. Engineering the chloroplast targeted malarial vaccine antigens in Chlamydomonas starch granules. PLoS ONE 2010, 5, e15424. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.A.; Li, F.; Tomosada, L.M.; Cox, C.J.; Topol, A.B.; Vinetz, J.M.; Mayfield, S. Algae-produced Pfs25 elicits antibodies that inhibit malaria transmission. PLoS ONE 2012, 7, e37179. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.S.; Luong, T.; Hannon, M.; Tran, M.; Gregory, J.A.; Shen, Z.; Briggs, S.P.; Mayfield, S.P. Heterologous expression of the C-terminal antigenic domain of the malaria vaccine candidate Pfs48/45 in the green algae Chlamydomonas reinhardtii. Appl. Microbiol. Biotechnol. 2013, 97, 1987–1995. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.A.; Topol, A.B.; Doerner, D.Z.; Mayfield, S. Alga-produced cholera toxin-Pfs25 fusion proteins as oral vaccines. Appl. Environ. Microbiol. 2013, 79, 3917–3925. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-M.; Kim, S.Y.; Li, K.F.; Yoon, B.H.; Choe, S.; Kuo, M.M.-C. Transgenic microalgae expressing Escherichia coli AppA phytase as feed additive to reduce phytate excretion in the manure of young broiler chicks. Appl. Microbiol. Biotechnol. 2011, 91, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Rasala, B.A.; Lee, P.A.; Shen, Z.; Briggs, S.P.; Mendez, M.; Mayfield, S.P. Robust expression and secretion of xylanase1 in Chlamydomonas reinhardtii by fusion to a selection gene and processing with the FMDV 2A peptide. PLoS ONE 2012, 7, e43349. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Qiu, S.; Liu, Q.; Tian, J.; Hu, Z.; Ni, J. Selenoprotein-transgenic Chlamydomonas reinhardtii. Nutrients 2013, 5, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Feng, W.; Zhao, L.; Gu, H.; Li, Q.; Shi, K.; Guo, S.; Zhang, N. Preparation of transgenic Dunaliella salina for immunization against white spot syndrome virus in crayfish. Arch. Virol. 2014, 159, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, Y.; Ishida, K.I. Internal plastid-targeting signal found in a RubisCO small subunit protein of a chlorarachniophyte alga. Plant J. 2010, 64, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Soria-Guerra, R.E.; Ramírez-Alonso, J.I.; Ibáñez-Salazar, A.; Govea-Alonso, D.O.; Paz-Maldonado, L.M.T.; Bañuelos-Hernández, B.; Korban, S.S.; Rosales-Mendoza, S. Expression of an HBcAg-based antigen carrying angiotensin II in Chlamydomonas reinhardtii as a candidate hypertension vaccine. Plant Cell Tissue Organ Cult. 2014, 116, 133–139. [Google Scholar] [CrossRef]
- Barahimipour, R.; Neupert, J.; Bock, R. Efficient expression of nuclear transgenes in the green alga Chlamydomonas: Synthesis of an HIV antigen and development of a new selectable marker. Plant Mol. Biol. 2016, 90, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-López, J.I.; Romero-Maldonado, A.; Monreal-Escalante, E.; Bañuelos-Hernández, B.; Paz-Maldonado, L.M.; Rosales-Mendoza, S. Chlamydomonas reinhardtii chloroplasts express an orally immunogenic protein targeting the p210 epitope implicated in atherosclerosis immunotherapies. Plant Cell Rep. 2016, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rasala, B.A.; Mayfield, S.P. Photosynthetic biomanufacturing in green algae; production of recombinant proteins for industrial, nutritional, and medical uses. Photosynth. Res. 2014, 123, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, M.; Eckert, A.; Fuhrmann, M.; Griesbeck, C. Influence of codon bias on the expression of foreign genes in microalgae. Adv. Exp. Med. Biol. 2007, 616, 46–53. [Google Scholar] [PubMed]
- Specht, E.A.; Mayfield, S.P. Algae-based oral recombinant vaccines. Front. Microbiol. 2014, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Van Hulten, M.C.; Witteveldt, J.; Peters, S.; Kloosterboer, N.; Tarchini, R.; Fiers, M.; Sandbrink, H.; Lankhorst, R.K.; Vlak, J.M. The white spot syndrome virus DNA genome sequence. Virology 2001, 286, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Tisch, R.; Yang, X.-D.; Singer, S.M.; Liblau, R.S.; Fugger, L.; McDevitt, H.O. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature 1993, 366, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.A.; Mayfield, S.P. Developing inexpensive malaria vaccines from plants and algae. Appl. Microbiol. Biotechnol. 2014, 98, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Z.; Gerken, H.; Huang, J.; Jiang, Y.; Chen, F. Genetic engineering of the green alga Chlorella zofingiensis: A modified norflurazon-resistant phytoene desaturase gene as a dominant selectable marker. Appl. Microbiol. Biotechnol. 2014, 98, 5069–5079. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, M.; Aoi, M.; Inoue-Kashino, N.; Kashino, Y.; Ifuku, K. Highly efficient transformation of the diatom Phaeodactylum tricornutum by multi-pulse electroporation. Biosci. Biotechnol. Biochem. 2013, 77, 874–876. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, F. Biology and industrial applications of Chlorella: Advances and prospects. Adv. Biochem. Eng. Biotechnol. 2016, 135, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Shaaltiel, Y.; Bartfeld, D.; Hashmueli, S.; Baum, G.; Brill-Almon, E.; Galili, G.; Dym, O.; Boldin-Adamsky, S.A.; Silman, I.; Sussman, J.L. Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of gaucher’s disease using a plant cell system. Plant Biotechnol. J. 2007, 5, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Hollak, C. An evidence-based review of the potential benefits of taliglucerase alfa in the treatment of patients with Gaucher disease. Core Evid. 2012, 7, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Shaaltiel, Y.; Gingis-Velitski, S.; Tzaban, S.; Fiks, N.; Tekoah, Y.; Aviezer, D. Plant-based oral delivery of β-glucocerebrosidase as an enzyme replacement therapy for Gaucher’s disease. Plant Biotechnol. J. 2015, 13, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Rosalesmendoza, S.; Angulo, C.; Meza, B. Food-grade organisms as vaccine biofactories and oral delivery vehicles. Trends Biotechnol. 2015, 34, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.B.; Abranches, R.; Fischer, R.; Sack, M.; Holland, T. Putting the spotlight back on plant suspension cultures. Front. Plant Sci. 2016, 7, 297. [Google Scholar] [CrossRef] [PubMed]
- Tacket, C.O.; Mason, H.S.; Losonsky, G.; Estes, M.K.; Levine, M.M.; Arntzen, C.J. Human Immune Responses to a Novel Norwalk Virus Vaccine Delivered in Transgenic Potatoes. J. Infect. Dis. 2000, 182, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Yusibov, V.; Hooper, D.; Spitsin, S.; Fleysh, N.; Kean, R.; Mikheeva, T.; Deka, D.; Karasev, A.; Cox, S.; Randall, J. Expression in plants and immunogenicity of plant virus-based experimental rabies vaccine. Vaccine 2002, 20, 3155–3164. [Google Scholar] [CrossRef]
- Thanavala, Y.; Mahoney, M.; Pal, S.; Scott, A.; Richter, L.; Natarajan, N.; Goodwin, P.; Arntzen, C.J.; Mason, H.S. Immunogenicity in humans of an edible vaccine for hepatitis B. Proc. Natl. Acad. Sci. USA 2005, 102, 3378–3382. [Google Scholar] [CrossRef] [PubMed]
- Hefferon, K. Clinical trials fuel the promise of plant-derived vaccines. Am. J. Clin. Med. 2010, 7, 30–37. [Google Scholar]
- Heinzelmann, E. The management centre innsbruck keeping one step ahead with algae innovation. Chim. Int. J. Chem. 2015, 69, 362–364. [Google Scholar] [CrossRef] [PubMed]
| Expression Systems | Bacteria | Yeasts | Cultured Mammalian Cells | Animals | Plants | Microalgae |
|---|---|---|---|---|---|---|
| Protein folding accuracy | Low | Medium | High | High | High | High |
| Glycosylation | None | Incorrect | Correct | Correct | Minor Differences | Minor Differences |
| Product quality | Low | Medium | High | High | High | High |
| Protein yield | Medium | High | High | High | High | High |
| Production scale | Limited | Limited | Limited | Limited | Worldwide | High |
| Production time | Short | Medium | Long | Long | Long | Short |
| Scale-up cost | High | High | High | High | Medium | Low |
| Overall cost | Medium | Medium | High | High | Low | Low |
| Contamination risk | Endotoxins | Low | High | High | Low | Low |
| Safety | Low | Unknown | High | High | High | High |
| Storage cost | Moderate | Moderate | Expensive | Expensive | Inexpensive | Low |
| Distribution | Medium | Medium | Difficult | Difficult | Easy | Very easy |
| Reproduction | Easy | Easy | Difficult | Medium | Easy | Very easy |
| Genome Engineering | Nucleus | Chloroplast |
|---|---|---|
| Gene expression machinery | Eukaryotic | Prokaryotic |
| Protein localization | Cytoplasm, nucleus, chloroplast, ER, mitochondria, secretion | Chloroplast |
| Modifications | Phosphorylation, glycosylation, disulfide bond | Phosphorylation, disulfide bond |
| Accumulation levels | Low | High |
| Transformation methods | Electroporation, biolistic, glass beads, silicon whiskers | Biolistic |
| Integration mode | Non-homologous end joining | Homologous recombination |
| Microalgae | Transformation Method | Expressed Genes | Promoters | Selective Marker Genes | Products | Expression Location (Chloroplast/Nucleus) | Product Content or Activity | References |
|---|---|---|---|---|---|---|---|---|
| Amphidinium sp. | SiC whiskers | Gus | CaMV 35S | nptII gene (neomycin resistance), hpt gene (hygromycin resistance) | β-glucoronidase | Nucleus | – | [88] |
| C. reinhardtii | Particle bombardment | lsc | atpA or rbcL | a 16S ribosomal gene (spectinomycin resistance) | Anti-HSV glycoprotein D Isc | Chloroplast | >1% of TSP | [151] |
| C. reinhardtii | Particle bombardment | gelonin | psbA | aphA6 (kanamycin resistance) | Anti-CD22-gelonin sc | Chloroplast | 0.1%–0.2% of TSP | [152] |
| C. reinhardtii | Particle bombardment | Exotoxin A | psbA | aphA6 | Anti-CD22-ETA sc | Chloroplast | 0.2%–0.3% of TSP | [153] |
| C. reinhardtii | Particle bombardment | VP1, CTB | atpA | aadA (spectinomycin resistance) | VP1-CTB | Chloroplast | 3% of TSP | [154] |
| C. reinhardtii | Glass beads method | E7GGG | psbD | aadA | E7GGG | Chloroplast | 0.12% of TSP | [155] |
| C. reinhardtii | Particle bombardment | VEGF | psbA | aphA6 | VEGF | Chloroplast | 2% of TSP | [156] |
| C. reinhardtii | Particle bombardment | HMGB1 | psbA | aphA6 | HMGB1 | Chloroplast | 2.5% of TSP | [156] |
| C. reinhardtii | Particle bombardment | 14FN3 | psbA | aphA6 | 14FN3 | Chloroplast | 3% of TSP | [156] |
| C. reinhardtii | Particle bombardment | metallothionein-2 | psbA | aadA | Metallothionein-2 | Chloroplast | – | [157] |
| C. reinhardtii | Particle bombardment | Strail | atpA | aadA | TRAIL | Chloroplast | 0.43%–0.67% of TSP | [158] |
| C. reinhardtii | Particle bombardment | apcA and apcB | psbA | aadA | Allophycocyanin | Chloroplast | 2%–3% of TSP | [159] |
| C. reinhardtii | – | IgG1 lc, hc | psbA | aadA | Anti-PA 83 anthrax IgG1 | Chloroplast | 100 μg/g of dry algal biomass | [160] |
| C. reinhardtii | Glass beads method | crEpo | HSP70A/RBCS2 | ARG7 | Erythropoietin | Nucleus | 100 μg /L | [161] |
| C. ellipsoidea | Electroporation | mNP-1 | Ubi1 | NptII and NR genes | mNP-1 | Nucleus | 11.42 mg/L | [120] |
| C. reinhardtii | Particle Bombardment | m-saa | psbA | – | M-SAA (bovine mammary-associated serum amyloid) | Chloroplast | 5% of TSP | [162] |
| C. reinhardtii | Particle bombardment | E2 | atpA | aadA | Swine fever virus (CSFV) structural protein | Chloroplast | 1.5%–2% of TSP | [163] |
| C. reinhardtii | Particle bombardment | VP28 | psbA | aadA | VP28 | Chloroplast | 21% of TCP (about 42% of TSP) | [164] |
| C. reinhardtii | Particle bombardment | hGAD65 | rbcL | a 16S ribosomal gene | hGAD65 | Chloroplast | 0.3% of TSP | [165] |
| C. reinhardtii | Particle bombardment | CTB, D2 | rbcL | aadA | CTB-D2 | Chloroplast | 0.7% of TSP | [166] |
| C. reinhardtii | Glass beads method | PfMSP1-19 | HSP70A/RbcS2 | AphVIII gene (paromomycin resistance) | GBSS-PfMSP1–19 | Nucleus | – | [167] |
| C. reinhardtii | Glass beads method | PbAMA1-C | HSP70A/RbcS2 | AphVIII gene | GBSS-PbAMA1-C | Nucleus | – | [167] |
| C. reinhardtii | Particle bombardment | Pfs25 | psbA | kanR (a kanamycin resistance cassette) | Pfs25 | Chloroplast | – | [168] |
| C. reinhardtii | Particle bombardment | Pfs28 | psbA | kanR | Pfs28 | Chloroplast | – | [168] |
| C. reinhardtii | Particle bombardment | c.r.pfs48/45 | psbA/psbD | – | c.r.Pfs48/45 | Chloroplast | – | [169] |
| C. reinhardtii | Particle bombardment | Cr.ctxB-pfs25 | psbA | kanR | Cr.CtxB-Pfs25 | Chloroplast | – | [170] |
| C. reinhardtii | Particle bombardment | appA | atpA | – | AppA phytase | Chloroplast | – | [171] |
| C. reinhardtii | Electroporation | xyn1 | PAR4 (hsp70A and the rbcs2) | aph7 gene (hygromycin B resistance), sh-ble gene (zeocin resistance) | β-1,4-endoxylanase | Nucleus | – | [172] |
| C. reinhardtii | LiAc/PEG | fGH | CaMV 35S | Sh ble gene (phleomycin resistance) | Flounder growth hormone | Nucleus | 420 μg of fGH protein per liter of culture | [128] |
| C. reinhardtii | Glass beads method | human Sep15 | Hsp70-RBCS2 | ble gene (zeocin resistance) | Human Sep15 protein | Nucleus | – | [173] |
| D. salina | Electroporation | HBsAg | ubiquitin-Ω | CAT gene (chloramphenicol resistance) | HBsAg | Nucleus | 1.6–3.1 ng/mg | [133] |
| D. salina | Glass beads method | VP28 | Ubi1-Ω | – | VP28 | Nucleus | 3 ng/mg | [174] |
| D.tertiolecta | Particle bombardment | xylanase/α-galactosidase/phytase | psbD | Erythromycin esterase (erythromycin resistance) | Xylanase/α-galactosidase/phytase | Chloroplast | – | [106] |
| L. amoebiformis | Particle bombardment | RbcS | LarbcS1 promoter | – | Rubisco small subunit (RbcS) protein | Nucleus | – | [175] |
| Porphyridium sp. | Particle bombardment | AHAS (W492S) | Endogenous AHAS promoter | AHAS | acetohydroxyacid synthase | Chloroplast | – | [98] |
| P.tricornutum | Particle bombardment | IgG LC, HC | Nitrate reductase promoter | – | Monoclonal human IgG antibody against HBsAg | Chloroplast | 8.7% of TSP, 21 mg/g of dry algal biomass | [123] |
| S.microadriaticum | SiC whiskers | Gus | CaMV 35S | nptII gene, hpt gene | β-glucoronidase | Nucleus | – | [88] |
| C. reinhardtii | Agrobacterium | HBcAgII | CaMV 35S | NptII (kanamycin resistance) | HBcAg-GS-AgII-GS-HBcAg | Chloroplast | 0.02%–0.05% of TSP | [176] |
| C. reinhardtii | Glass beads method | P24 | PSAD promoter | aphVIII gene (paromomycin resistance) | subunit of HIV-1 viral particles | Nucleus | 0.25% of the total cellular protein | [177] |
| C. reinhardtii | Particle bombardment | CTB:p210 | atpA | aadA gene (spectinomycin resistance) | the p210 epitope from ApoB100 | Chloroplast | up to 60 μg/g of fresh weight biomass | [178] |
| Recombinant Proteins | Preclinical Trials | Activity Analysis | References |
|---|---|---|---|
| Anti-CD22-ETA sc | Compared with mice treated with the antibody deficient immune toxin, mice treated with immunotoxin produced by algae can significantly inhibit the propagation of tumor, suggesting immunotoxins expressed in algae significantly affect tumor progression in animal experiments. | – | [153] |
| VP1-CTB | The CTB-VP1, fusion protein expressed in C. reinhardtii chloroplast, exhibited significant binding affinity to GM1-ganglioside. | Southern blot, Western blot, ELISA | [154] |
| E7GGG | E7GGG expressed in Chlamydomonas elicited tumor protection in 60% of mice. Mice vaccinated with the purified E7GGG-FLAG protein were 100% tumor-free after 9 weeks. | Western blot, ELISA | [155] |
| E2 | Responsing against CSFV, E2 can induce significant serum antibody by subcutaneous immunization, but oral immunization resulted in no detectable serum response. | Southern blot, Western blot | [163] |
| hGAD65 | Algal-derived hGAD65 had higher binding capacity to diabetic serum samples and can stimulate proliferation of splenic T cells from NOD mice in vitro, demonstrating its antigenic and immunogenic characteristics. | Western blot | [165] |
| CTB-D2 | Oral vaccination of CTB-D2 to mice induced responses of specific systemic and mucosal immune against S. aureus. | – | [166] |
| GBSS-pfMSP1-19 GBSS-pfAMA1-C | The starch antigens accumulated in the chloroplast can protect against lethal P. berghei infection in mice. | Western blot | [167] |
| Cr.CtxB-Pfs25 | Oral vaccination of Cr.CtxB-Pfs25-producing algae to mice elicited IgG and IgA antibodies to CTB, and IgG antibodies to Pfs25. | ELISA | [170] |
| AppA phytase | AppA-producing algae can significantly reduce the phytate content and increase the content of inorganic phosphate content in chick manure. | – | [171] |
| fGH | Flounder fry fed with transgenic Chlorella increased by 25% within 30 days in length and width. | Southern blot, immunoblot | [128] |
| VP28 | Survival rates of crayfish vaccinated by Ds-VP28 were significantly higher (59% mortality) than the positive control groups and Ds empty control (100% mortality). | Western blot, ELISA | [174] |
| p210 epitope from ApoB100 | The immunogenic activity was tested by orally administration in BALB/c mice, and elicited anti-p210 serum antibodies, which can last for at least 80 days. | Western blot, ELISA | [178] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, N.; Fan, C.; Chen, Y.; Hu, Z. The Potential for Microalgae as Bioreactors to Produce Pharmaceuticals. Int. J. Mol. Sci. 2016, 17, 962. https://doi.org/10.3390/ijms17060962
Yan N, Fan C, Chen Y, Hu Z. The Potential for Microalgae as Bioreactors to Produce Pharmaceuticals. International Journal of Molecular Sciences. 2016; 17(6):962. https://doi.org/10.3390/ijms17060962
Chicago/Turabian StyleYan, Na, Chengming Fan, Yuhong Chen, and Zanmin Hu. 2016. "The Potential for Microalgae as Bioreactors to Produce Pharmaceuticals" International Journal of Molecular Sciences 17, no. 6: 962. https://doi.org/10.3390/ijms17060962
APA StyleYan, N., Fan, C., Chen, Y., & Hu, Z. (2016). The Potential for Microalgae as Bioreactors to Produce Pharmaceuticals. International Journal of Molecular Sciences, 17(6), 962. https://doi.org/10.3390/ijms17060962