Enriched Astaxanthin Extract from Haematococcus pluvialis Augments Growth Factor Secretions to Increase Cell Proliferation and Induces MMP1 Degradation to Enhance Collagen Production in Human Dermal Fibroblasts
Abstract
:1. Introduction
2. Results
2.1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Free Radical Scavenging Activity Assay
2.2. Ferrous Ions Chelating Capacity
2.3. Reducing Power
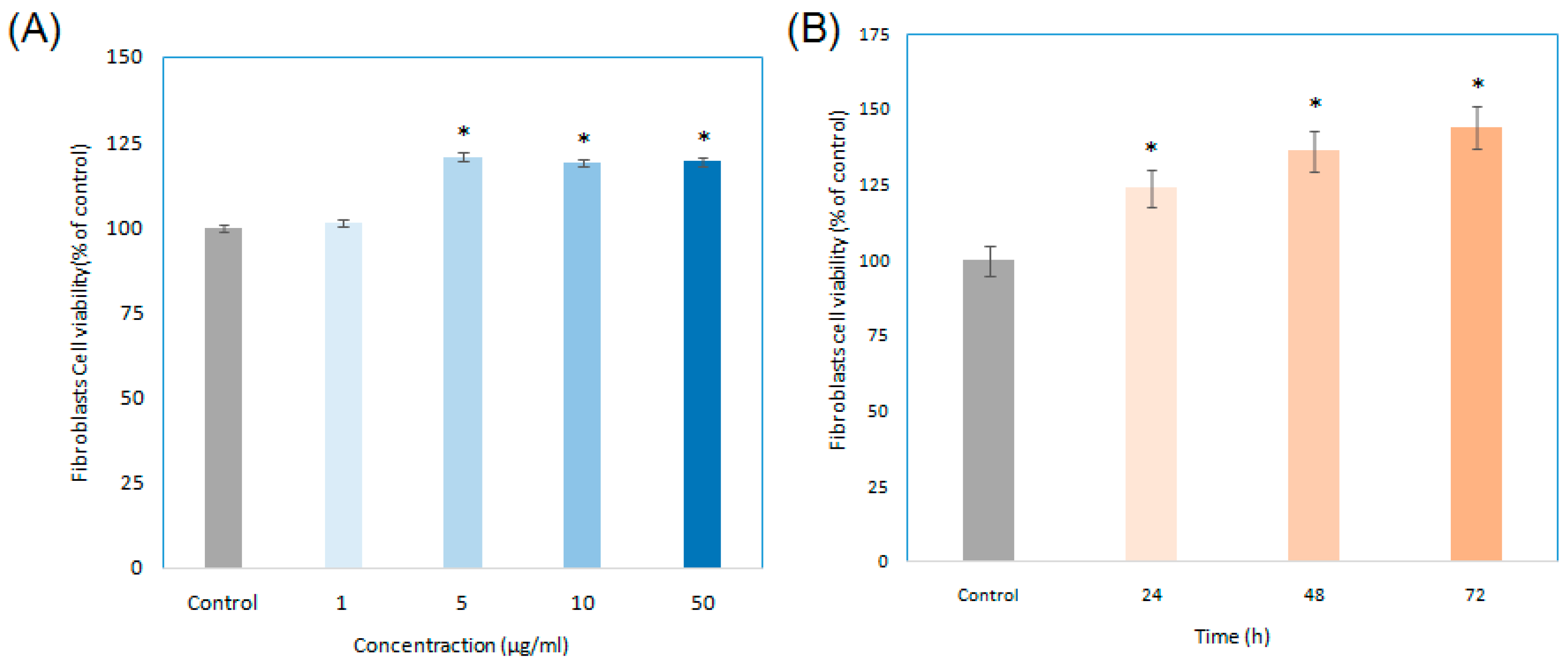
2.4. Cell Growth of Enriched Astaxanthin Extract (EAE) Treated in Human Fibroblasts
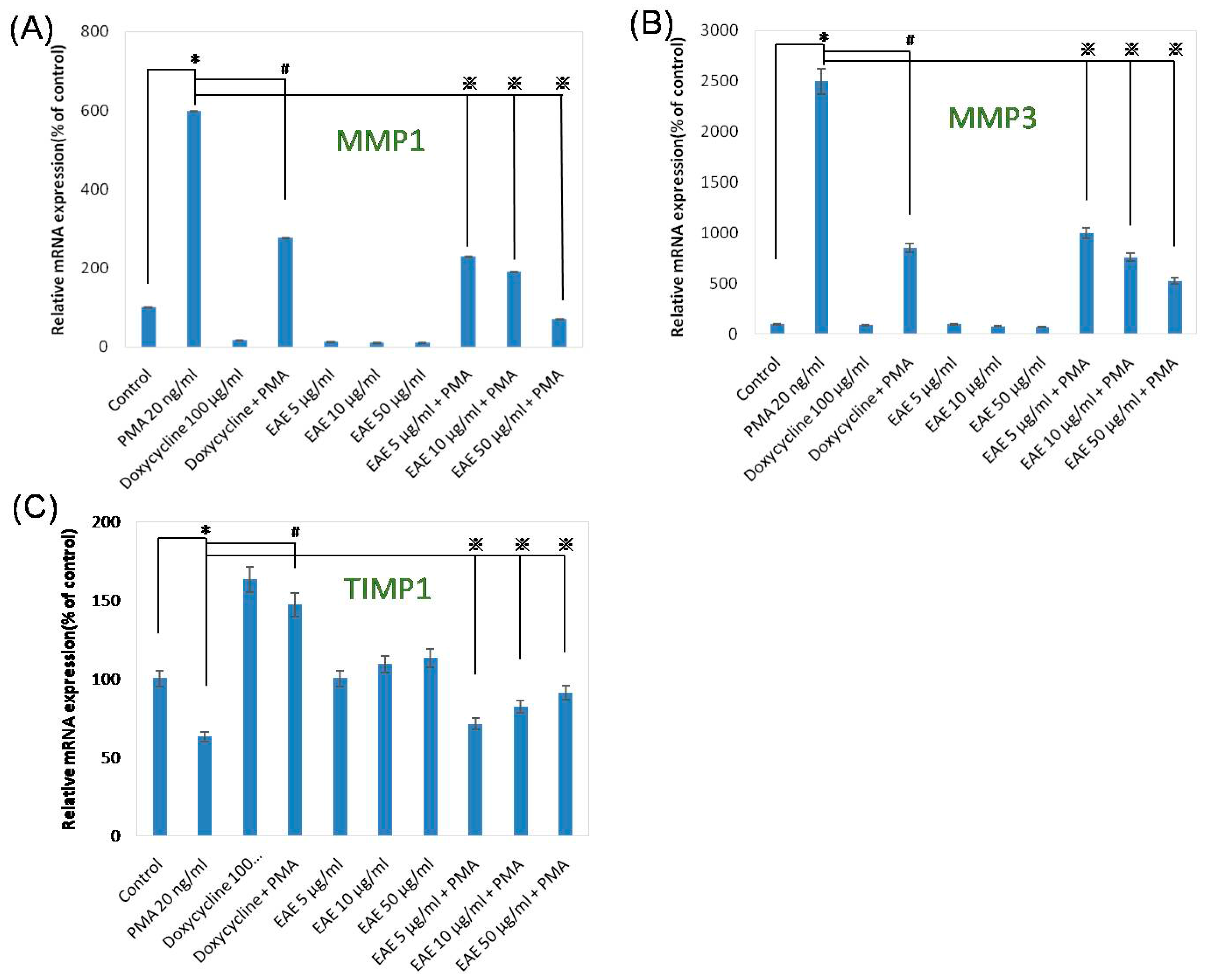
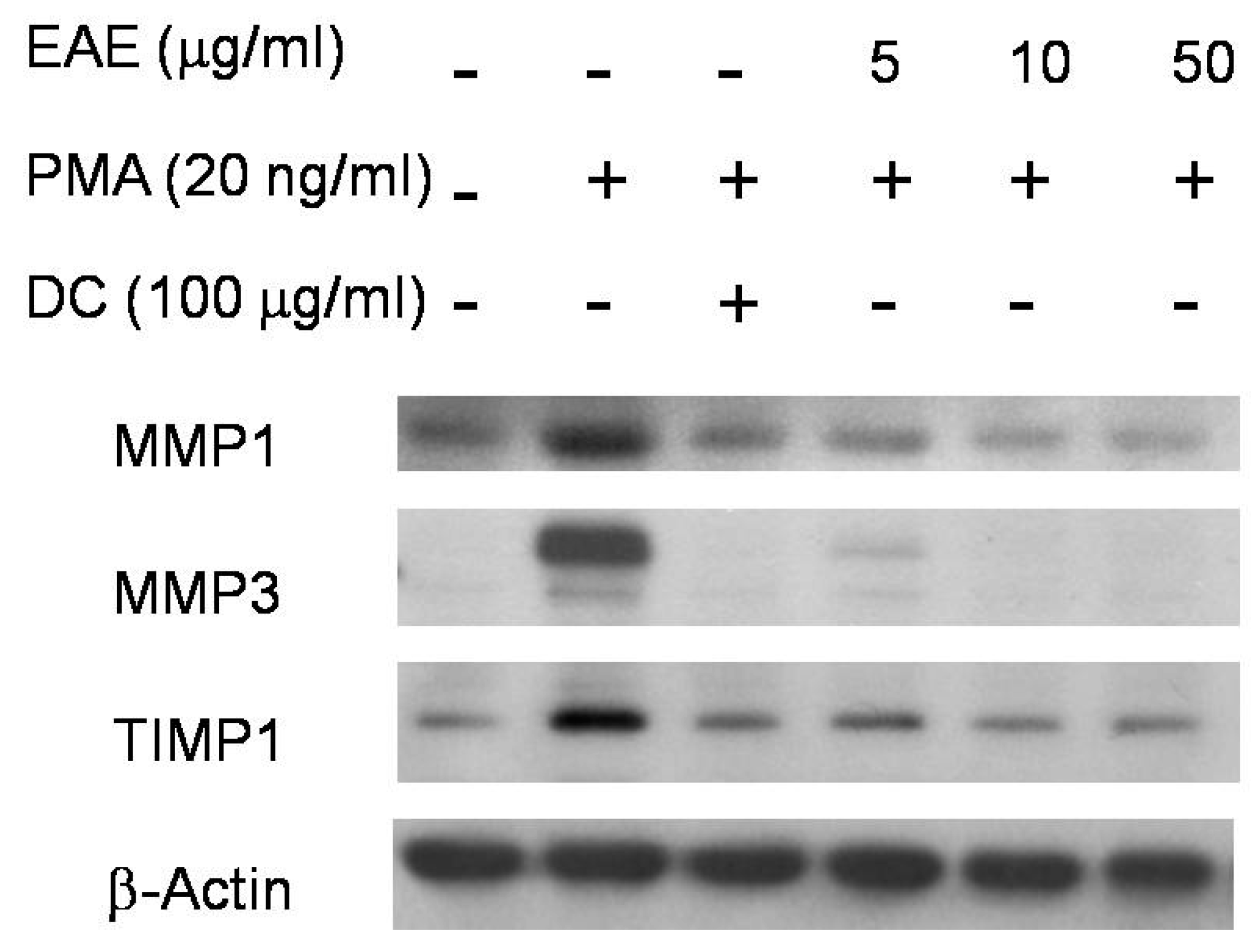
2.5. Effects of EAE on Phorbol 12-Myristate 13-Acetate (PMA)-Stimulated MMP1 and TIMP1 Production in Human Fibroblasts
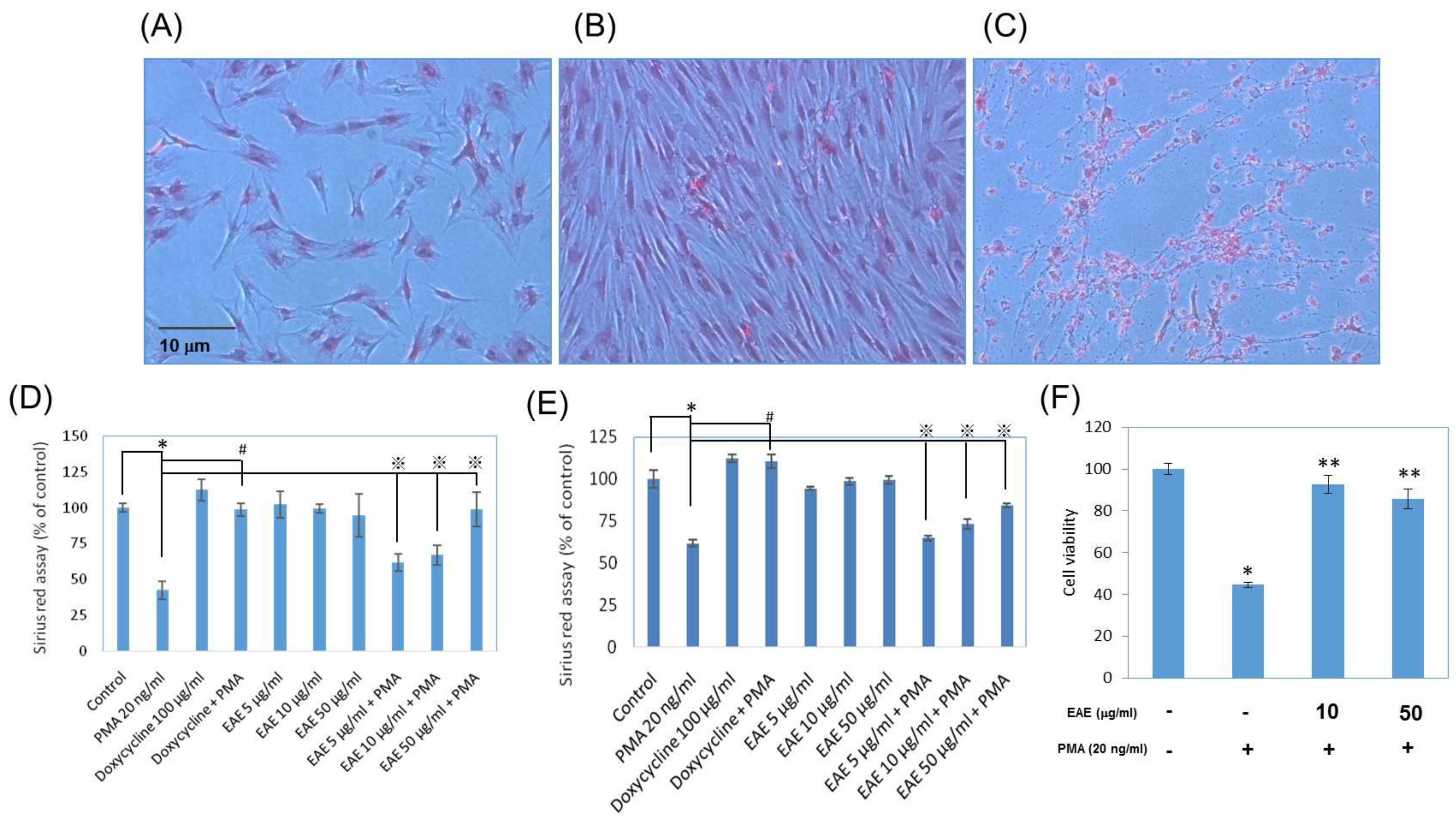
2.6. Collagen Productions in Sirius Red Assays
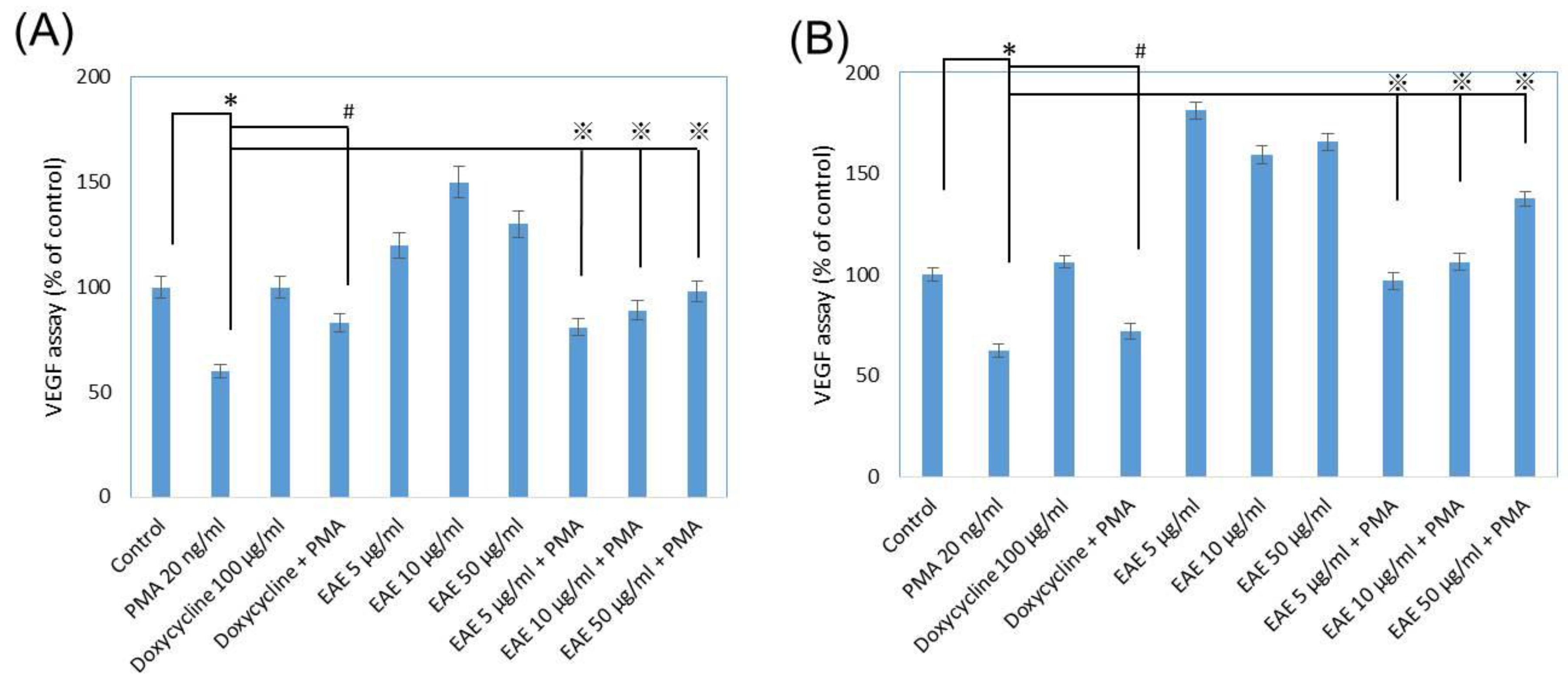
2.7. Vascular Endothelial Growth Factor (VEGF) Secretions from Human Fibroblasts
3. Discussion
4. Experimental Section
4.1. Supercritical Fluid Carbon Dioxide Extraction (SFE-CO2)
4.2. Saponification of Astaxanthin Esters
4.3. Determination of 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Capacity
4.4. Metal Chelating Activity
4.5. Reducing Power
4.6. Cell Cultures
4.7. Cell Proliferation Examinations
4.8. Quantitative Real Time Polymerase Chain Reactions
4.9. Western Blotting
4.10. Collagen Measurements
4.11. VEGF Secretion Assays
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Implications
References
- Wang, H.M.; Wu, P.F. 7-Hydroxydehydronuciferine induces human melanoma A375.S2 cell death via autophagy and apoptosis in vitro and in vivo investigations. Toxicol. Lett. 2013, 221. [Google Scholar] [CrossRef]
- Wu, S.-Y.S.; Wang, H.-M.D.; Wen, Y.-S.; Liu, W.; Li, P.-H.; Chiu, C.-C.; Chen, P.-C.; Huang, C.-Y.; Sheu, J.-H.; Wen, Z.-H. 4-(Phenylsulfanyl) butan-2-one suppresses melanin synthesis and melanosome maturation in vitro and in vivo. Int. J. Mol. Sci. 2015, 16, 20240–20257. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Lin, Y.C.; Wu, P.F.; Wen, Z.H.; Liu, P.L.; Chen, C.Y.; Wang, H.M. Biofunctional constituents from Liriodendron tulipifera with antioxidants and anti-melanogenic properties. Int. J. Mol. Sci. 2013, 14, 1698–1712. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Chou, Y.T.; Wen, Z.H.; Wang, Z.R.; Chen, C.H.; Ho, M.L. Novel biodegradable porous scaffold applied to skin regeneration. PLoS ONE 2015, 8, e56330. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Wolber, R.; Gerwat, W.; Mann, T.; Batzer, J.; Smuda, C.; Liu, H.; Kolbe, L.; Hearing, V.J. The fibroblast-derived paracrine factor neuregulin-1 has a novel role in regulating the constitutive color and melanocyte function in human skin. J. Cell Sci. 2010, 123, 3102–3111. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, M.; Papp, S.; Schaffer, L.; Pouyani, T. Hydrocortisone and triiodothyronine regulate hyaluronate synthesis in a tissue-engineered human dermal equivalent through independent pathways. J. Biosci. Bioeng. 2015, 119, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-induced skin damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef]
- Elwood, J.M.; Janet, J. Melanoma and sun exposure: An overview of published studies. Int. J. Cancer 1997, 73, 198–203. [Google Scholar] [CrossRef]
- Bae, C.H.; Chen, S.M.; Lee, H.M.; Song, S.Y.; Kim, Y.D. The effect of doxycycline on PMA-induced MUC5B expression via MMP9 and p38 in NCI-H292 cells. Clin. Exp. Otorhinolaryngol. 1997, 4, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.Y.; Chou, T.H.; Lin, R.J.; Chan, L.P.; Wang, G.H.; Liang, C.H. Antioxidant and antimelanogenic behaviors of Paeonia suffruticosa. Plant Foods Hum. Nutr. 2011, 66, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.K.; Damaghi, N.; Picart, S.D.; Markova, N.G.; Obayashi, K.; Okano, Y.; Masaki, H.; Grether-Beck, S.; Krutmann, J.; Smiles, K.A.; et al. UV-induced DNA damage initiates release of MMP1 in human skin. Exp. Dermatol. 2008, 17, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Cheng, K.C.; Chang, A.Y.; Lin, Y.T.; Hseu, Y.C.; Wang, H.M. 10-Shogaol, an antioxidant from Zingiber officinale for skin cell proliferation and migration enhancer. Int. J. Mol. Sci. 2012, 13, 1762–1777. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, D.N.; Premkumar, E.R. JNK signaling in apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, N.A.; Nikonorova, Y.V.; Surovtseva, M.A.; Lykov, A.P.; Poveshchenko, O.V.; Poveshchenko, A.F.; Pokushalov, E.A.; Romanov, A.B.; Konenkov, V.I. Effect of vascular endothelial growth factor and erythropoietin on functional activity of fibroblasts and multipotent mesenchymal stromal cells. Bull. Exp. Biol. Med. 2016, 160. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Chiu, C.C.; Liao, W.T.; Wu, P.F.; Chen, Y.T.; Huang, K.C.; Chou, Y.T.; Wen, Z.H.; Wang, H.M. Alpinia oxyphylla Miq. bioactive extracts from supercritical fluid carbon dioxide extraction. Biochem. Eng. J. 2013, 78, 101–107. [Google Scholar] [CrossRef]
- Maoka, T.; Yasui, H.; Ohmori, A.; Tokuda, H.; Suzuki, N.; Osawa, A.; Shindo, k.; Ishibashi, T. Anti-oxidative, anti-tumor-promoting, and anti-carcinogenic activities of adonirubin and adonixanthin. J. Oleo Sci. 2013, 62, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Régnier, P.; Bastias, J.; Rodriguez-Ruiz, V.; Caballero-Casero, N.; Caballo, C.; Sicilia, D.; Fuentes, A.; Maire, M.; Crepin, M.; Letourneur, D.; et al. Astaxanthin from Haematococcus pluvialis prevents oxidative stress on human endothelial cells without toxicity. Mar. Drugs 2015, 13, 2857–2874. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.R.; Sindhuja, H.N.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.Q.; Xie, M.; Gao, Y.; Zhang, X. Influence of Erigeron breviscapus on the expression of collagen type I, MMP1 and TIMP1 of MRC-5 cells under hypoxia. Sichuan Da Xue Xue Bao Yi Xue Ban = J. Sichuan Unive. Med. Sci. Ed. 2012, 43, 325–330. [Google Scholar]
- Wang, H.M.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Lin, L.C.; Yang, W.F.; Bordon, J.; Wang, H.M.D. An Updated Organic Classification of Tyrosinase Inhibitors on Melanin Biosynthesis. Curr. Org. Chem. 2015, 19, 4–18. [Google Scholar] [CrossRef]
- Ma, D.L.; Liu, L.J.; Leung, K.H.; Chen, Y.T.; Zhong, H.J.; Chan, D.S.H.; Wang, H.M.D.; Leung, C.H. Antagonizing STAT3 dimerization with a rhodium (III) complex. Angew. Chem. Int. Ed. 2014, 53, 9178–9182. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.L.; Wang, H.M.; Chen, C.Y.; Chang, J.S. Extraction of astaxanthin from Haematococcus pluvialis by supercritical carbon dioxide fluid with ethanol modifier. Eng. Life Sci. 2012, 12, 638–647. [Google Scholar] [CrossRef]
- Burnette, W.N. “Western blotting”: Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 1981, 112, 195–203. [Google Scholar] [CrossRef]
- Huang, Y.; Boer, W.B.; Adams, L.A.; MacQuillan, G.; Rossi, E.; Rigby, P.; Raftopoulos, S.C.; Bulsara, M.; Jeffrey, G.P. Image analysis of liver collagen using sirius red is more accurate and correlates better with serum fibrosis markers than trichrome. Liver Int. 2013, 33, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, S.; Zhang, M.; Zhang, Z.; Zhang, B.; Han, D.; Ma, J.; Wang, X.; Hong, J.; Guo, Y.; et al. In vitro Sirius Red collagen assay measures the pattern shift from soluble to deposited collagen. In Oxygen Transport to Tissue XXXIV; Springer: New York, NY, USA, 2013; pp. 47–53. [Google Scholar]
| Samples | Anti-Oxidative Properties | ||
|---|---|---|---|
| DPPH (%) | Chelating (%) | Reducing Power (OD700) | |
| Vitamin C a | 86.5 | - | - |
| EDTA b | - | 87.3 | - |
| BHA c | - | - | 1.88 ± 0.03 |
| EAE | 45.3 | 54.7 | 1.17± 0.11 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, H.-Y.; Lee, C.; Pan, J.-L.; Wen, Z.-H.; Huang, S.-H.; Lan, C.-W.J.; Liu, W.-T.; Hour, T.-C.; Hseu, Y.-C.; Hwang, B.H.; et al. Enriched Astaxanthin Extract from Haematococcus pluvialis Augments Growth Factor Secretions to Increase Cell Proliferation and Induces MMP1 Degradation to Enhance Collagen Production in Human Dermal Fibroblasts. Int. J. Mol. Sci. 2016, 17, 955. https://doi.org/10.3390/ijms17060955
Chou H-Y, Lee C, Pan J-L, Wen Z-H, Huang S-H, Lan C-WJ, Liu W-T, Hour T-C, Hseu Y-C, Hwang BH, et al. Enriched Astaxanthin Extract from Haematococcus pluvialis Augments Growth Factor Secretions to Increase Cell Proliferation and Induces MMP1 Degradation to Enhance Collagen Production in Human Dermal Fibroblasts. International Journal of Molecular Sciences. 2016; 17(6):955. https://doi.org/10.3390/ijms17060955
Chicago/Turabian StyleChou, Hsin-Yu, Chelsea Lee, Jian-Liang Pan, Zhi-Hong Wen, Shu-Hung Huang, Chi-Wei John Lan, Wang-Ta Liu, Tzyh-Chyuan Hour, You-Cheng Hseu, Byeong Hee Hwang, and et al. 2016. "Enriched Astaxanthin Extract from Haematococcus pluvialis Augments Growth Factor Secretions to Increase Cell Proliferation and Induces MMP1 Degradation to Enhance Collagen Production in Human Dermal Fibroblasts" International Journal of Molecular Sciences 17, no. 6: 955. https://doi.org/10.3390/ijms17060955
APA StyleChou, H.-Y., Lee, C., Pan, J.-L., Wen, Z.-H., Huang, S.-H., Lan, C.-W. J., Liu, W.-T., Hour, T.-C., Hseu, Y.-C., Hwang, B. H., Cheng, K.-C., & Wang, H.-M. D. (2016). Enriched Astaxanthin Extract from Haematococcus pluvialis Augments Growth Factor Secretions to Increase Cell Proliferation and Induces MMP1 Degradation to Enhance Collagen Production in Human Dermal Fibroblasts. International Journal of Molecular Sciences, 17(6), 955. https://doi.org/10.3390/ijms17060955