Bioavailability of an R-α-Lipoic Acid/γ-Cyclodextrin Complex in Healthy Volunteers
Abstract
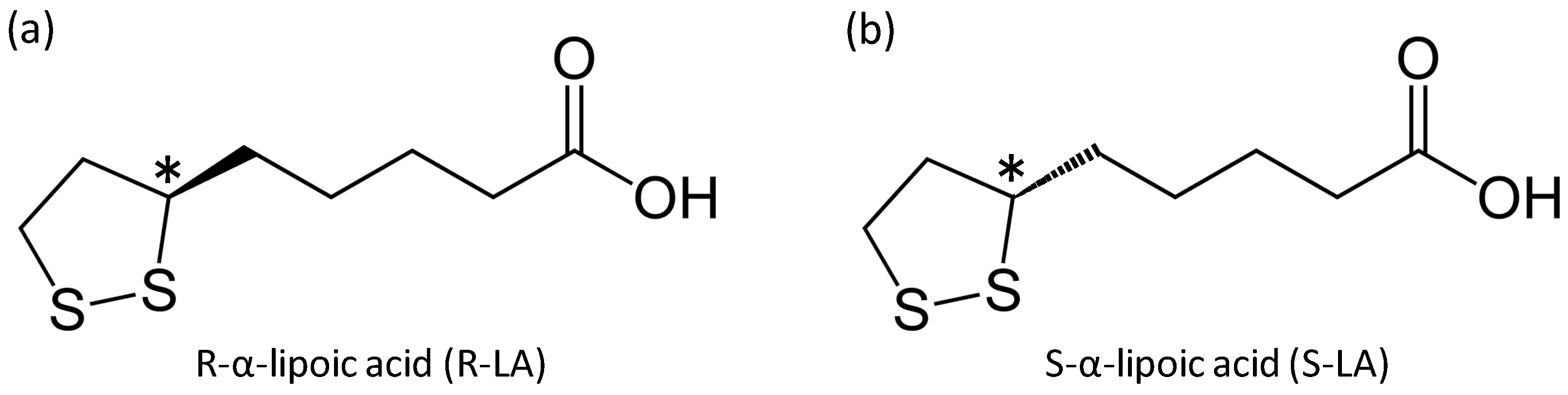
:1. Introduction
2. Results
2.1. Subjects’ Characteristics, Safety, and Tolerability
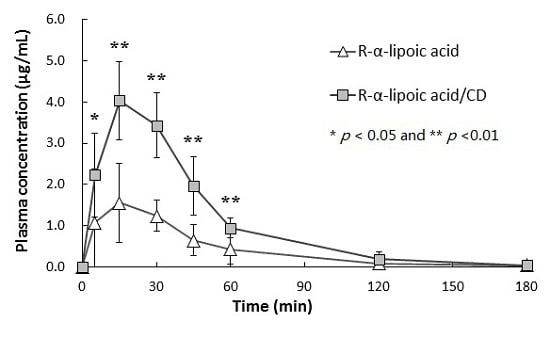
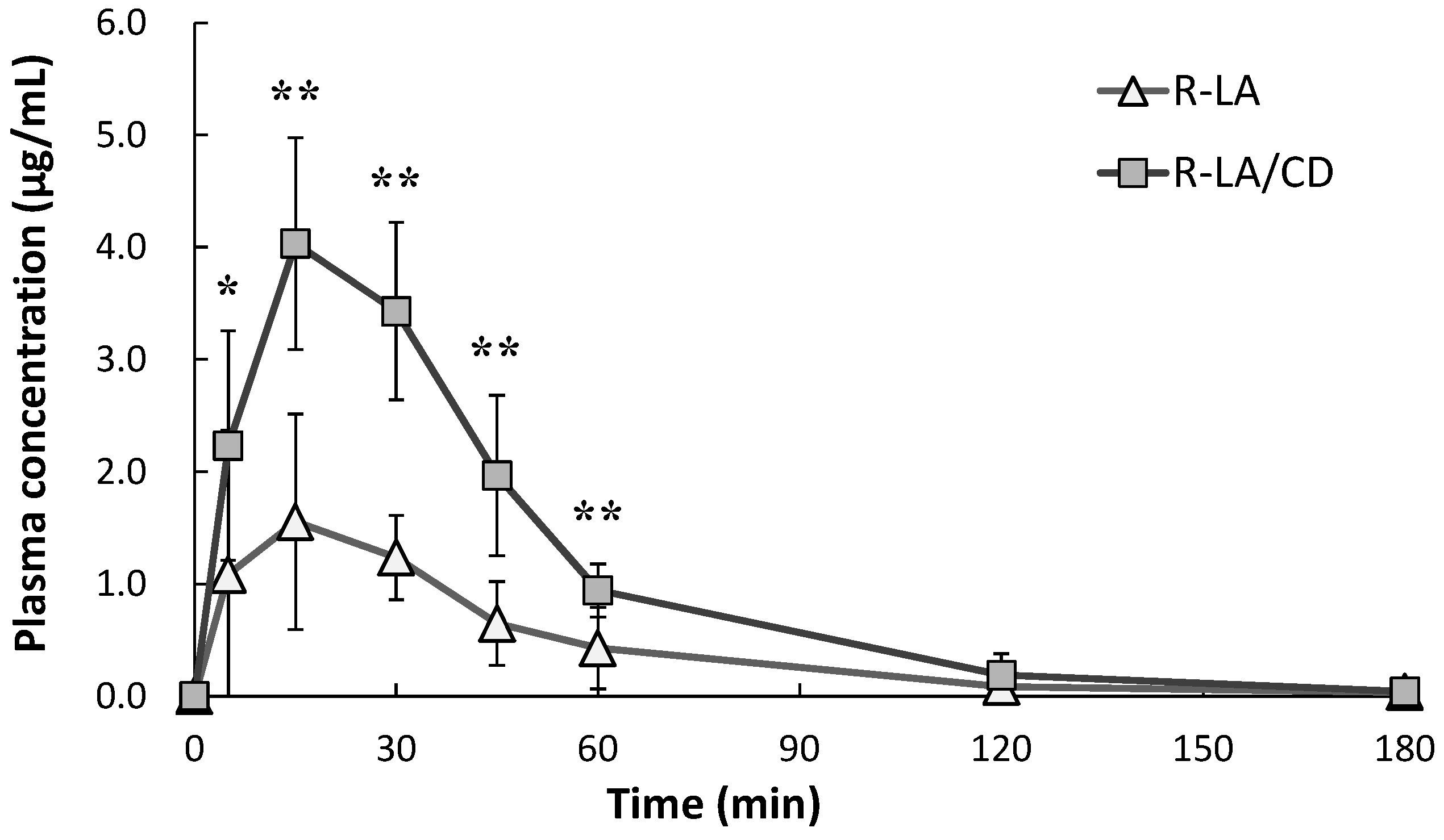
2.2. Plasma Pharmacokinetics of R-α-Lipoic Acid (R-LA)
3. Discussion
4. Materials and Methods
4.1. Subjects and Study Design
4.2. Drug Analysis and Pharmacokinetics
4.3. Plasma Glucose Analysis
4.4. Tolerability
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Packer, L.; Kraemer, K.; Rimbach, G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 2001, 17, 888–895. [Google Scholar] [CrossRef]
- Khanna, S.; Roy, S.; Packer, L.; Sen, C.K. Cytokine-induced glucose uptake in skeletal muscle: Redox regulation and the role of α-lipoic acid. Am. J. Physiol. 1999, 276, R1327–R1333. [Google Scholar] [PubMed]
- Bramanti, V.; Tomassoni, D.; Bronzi, D.; Grasso, S.; Currò, M.; Avitabile, M.; Li Volsi, G.; Renis, M.; Ientile, R.; Amenta, F.; et al. α-Lipoic acid modulates GFAP, vimentin, nestin, cyclin D1 and MAP-kinase espression in astroglial cell cultures. Neurochem. Res. 2010, 35, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.; Packer, L.; Hoppe, P. R-α-lipoic acid. In Nutraceuticals in Health and Disease Prevention; Marcel Dekker, Inc.: New York, NY, USA, 2001; pp. 129–164. [Google Scholar]
- Grasso, S.; Bramanti, V.; Tomassoni, D.; Bronzi, D.; Malfa, G.; Traini, E.; Napoli, M.; Renis, M.; Amenta, F.; Avola, R. Effect of lipoic acid and α-glyceryl-phosphoryl-choline on astroglial cell proliferation and differentiation in primary culture. J. Neurosci. Res. 2014, 92, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Hanefeld, M.; Ruhnau, K.J.; Hasche, H.; Lobisch, M.; Schütte, K.; Kerum, G.; Malessa, R. Treatment of symptomatic diabetic polyneuropathy with the antioxidant α-lipoic acid: A 7-month multicenter randomized controlled trial (ALADIN III Study). ALADIN III study group. α-lipoic acid in diabetic neuropathy. Diabetes Care 1999, 22, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Haak, E.S.; Usadel, K.H.; Kohleisen, M.; Yilmaz, A.; Kusterer, K.; Haak, T. The effect of α-lipoic acid on the neurovascular reflex arc in patients with diabetic neuropathy assessed by capillary microscopy. Microvasc. Res. 1999, 58, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Ametov, A.; Barinov, A.; Dyck, P.J.; Gurieva, I.; Low, P.A.; Munzel, U.; Yakhno, N.; Raz, I.; Novosadova, M.; et al. Oral treatment with α-lipoic acid improves symptomatic diabetic polyneuropathy the SYDNEY 2 trial. Diabetes Care 2006, 29, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Kamenova, P. Improvement of insulin sensitivity in patients with type 2 diabetes mellitus after oral administration of α-lipoic acid. Hormones 2006, 5, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Lee, E.K.; Kim, T.H.; Min, W.K.; Chun, S.; Lee, K.-U.; Kim, S.B.; Park, J.S. Effects of α-lipoic acid on the plasma levels of asymmetric dimethylarginine in diabetic end-stage renal disease patients on hemodialysis: A pilot study. Am. J. Nephrol. 2007, 27, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Gębka, A.; Serkies-Minuth, E.; Raczyńska, D. Effect of the administration of α-lipoic acid on contrast sensitivity in patients with type 1 and type 2 diabetes. Med. Inflamm. 2014, 2014, 131538. [Google Scholar] [CrossRef] [PubMed]
- Morcos, M.; Borcea, V.; Isermann, B.; Gehrke, S.; Ehret, T.; Henkels, M.; Schiekofer, S.; Hofmann, M.; Amiral, J.; Tritschler, H.; et al. Effect of α-lipoic acid on the progression of endothelial cell damage and albuminuria in patients with diabetes mellitus: An exploratory study. Diabetes Res. Clin. Pract. 2001, 52, 175–183. [Google Scholar] [CrossRef]
- El-Nabarawy, S.K.; Mohamed, M.A.; Ahmed, M.M.; El-Arabi, G.H. α-Lipoic acid ameliorates the oxidative status and serum iron in diabetic patients. J. Pharm. Biomed. Sci. 2011, 1, 97–103. [Google Scholar]
- Huang, E.A.; Gitelman, S.E. The effect of oral α-lipoic acid on oxidative stress in adolescents with type 1 diabetes mellitus. Pediatr. Diabetes 2008, 9 Pt 2, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Hager, K.; Marahrens, A.; Kenklies, M.; Riederer, P.; Münch, G. α-Lipoic acid as a new treatment option for Alzheimer type dementia. Arch. Gerontol. Geriatr. 2001, 32, 275–282. [Google Scholar] [CrossRef]
- Maczurek, A.; Hager, K.; Kenklies, M.; Sharman, M.; Martins, R.; Engel, J.; Carlson, D.A.; Münch, G. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer’s disease. Adv. Drug Deliv. Rev. 2008, 60, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Hager, K.; Kenklies, M.; McAfoose, J.; Engel, J.; Münch, G. α-Lipoic acid as a new treatment option for Alzheimer’s disease—A 48 months follow-up analysis. J. Neural Transm. 2007, 72, 189–193. [Google Scholar]
- Bustamante, J.; Lodge, J.K.; Marcocci, L.; Tritschler, H.J.; Packer, L.; Rihn, B.H. α-Lipoic acid in liver metabolism and disease. Free Radic. Biol. Med. 1998, 24, 1023–1039. [Google Scholar] [CrossRef]
- Vincent, H.K.; Bourguignon, C.M.; Vincent, K.R.; Taylor, A.G. Effects of α-lipoic acid supplementation in peripheral arterial disease: A pilot study. J. Altern. Complement. Med. 2007, 13, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Huerta, A.E.; Navas-Carretero, S.; Prieto-Hontoria, P.L.; Martínez, J.A.; Moreno-Aliaga, M.J. Effects of α-lipoic acid and eicosapentaenoic acid in overweight and obese women during weight loss. Obesity 2015, 23, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.H.; Lee, W.J.; Lee, S.A.; Kim, E.H.; Cho, E.H.; Jeong, E.; Kim, D.W.; Kim, M.-S.; Park, J.-Y.; Park, K.-G.; et al. Effects of α-lipoic acid on body weight in obese subjects. Am. J. Med. 2011, 124, 85.e1–85.e8. [Google Scholar] [CrossRef] [PubMed]
- Carbonelli, M.G.; Renzo, L.D.; Bigioni, M.; Daniele, N.D.; Lorenzo, A.D.; Fusco, M.A. α-Lipoic acid supplementation: A tool for obesity therapy? Curr. Pharm. Des. 2010, 16, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, N.; Sugiyama, H.; Shimosegawa, H.; Nakane, R.; Ishida, Y.; Uekaji, Y.; Nakata, D.; Pallauf, K.; Rimbach, G.; Terao, K.; et al. Analysis of the enhanced stability of R(+)-α lipoic acid by the complex formation with cyclodextrins. Int. J. Mol. Sci. 2013, 14, 3639–3655. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, N.; Tanaka, A.; Otsubo, A.; Ogawa, N.; Yamamoto, H.; Mizukami, T.; Arai, S.; Okuno, M.; Terao, K.; Matsugo, S. Spectroscopic studies of R(+)-α-lipoic acid—Cyclodextrin complexes. Int. J. Mol. Sci. 2014, 15, 20469–20485. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, N.; Endo, T.; Hosomi, S.; Setou, K.; Tanaka, S.; Ogawa, N.; Yamamoto, H.; Mizukami, T.; Arai, S.; Okuno, M.; et al. Structural analysis of crystalline R(+)-α-lipoic acid-α-cyclodextrin complex based on microscopic and spectroscopic studies. Int. J. Mol. Sci. 2015, 16, 24614–24628. [Google Scholar] [CrossRef] [PubMed]
- Uchida, R.; Iwamoto, K.; Nagayama, S.; Miyajima, A.; Okamoto, H.; Ikuta, N.; Fukumi, H.; Terao, K.; Hirota, T. Effect of γ-cyclodextrin inclusion complex on the absorption of R-α-lipoic acid in rats. Int. J. Mol. Sci. 2015, 16, 10105–10120. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kishino, E.; Mikuni, K.; Kiuchi, Y.; Beppu, H.; Okazaki, H.; Shimpo, K.; Sonoda, S. Effect of different types of cyclodextrins on gastrointestinal absorption of α-lipoic acid in rats and humans. J. Appl. Glycosci. 2012, 59, 97–103. [Google Scholar] [CrossRef]
- Gleiter, C.H.; Schung, B.S.; Hermann, R.; Elze, M.; Blume, H.H.; Gundert-Remy, U. Influence of food intake on the bioavailability of thioctic acid enantiomers. Eur. J. Pharmacol. 1996, 50, 513–514. [Google Scholar] [CrossRef]
- Breithaupt-Grögler, K.; Niebch, G.; Schneider, E.; Erb, K.; Hermann, R.; Blume, H.H.; Schug, B.S.; Belz, G.G. Dose-proportionality of oral thioctic acid—Coincidence of assessments via pooled plasma and individual data. Eur. J. Pharm. Sci. 1999, 8, 57–65. [Google Scholar] [CrossRef]
- Teichert, J.; Tuemmers, T.; Achenbach, H.; Preiss, C.; Hermann, R.; Ruus, P.; Preiss, R. Pharmacokinetics of α-lipoic acid in subjects with severe kidney damage and end-stage renal disease. J. Clin. Pharmacol. 2005, 45, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Schatz, H.; Conrad, F.; Gries, F.A.; Ulrich, H.; Reichel, G. Effects of treatment with the antioxidant α-lipoic acid on cardiac autonomic neuropathy in NIDDM patients. A 4-month randomized controlled multicenter trial (DEKAN study). Diabetes Care 1997, 20, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Reljanovic, M.; Reichel, G.; Rett, K.; Lobisch, M.; Schuette, K.; Moller, W.; Tritschler, H.J.; Mehnert, H. Treatment of diabetic polyneuropathy with the antioxidant thioctic acid (α-lipoic acid): A two year multicenter randomized double-blind placebo-controlled trial (ALADIN II). Free Radic. Res. 1999, 31, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Uchida, R.; Okamoto, H.; Ikuta, N.; Terao, K.; Hirota, T. Enantioselective pharmacokinetics of α-lipoic acid in rats. Int. J. Mol. Sci. 2015, 16, 22781–22794. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.A.; Smith, A.R.; Fischer, S.J.; Young, K.L.; Packer, L. The plasma pharmacokinetics of R-(+)-lipoic acid administrated as sodium R-(+)-lipoate to healthy human subjects. Altern. Med. Rev. 2007, 12, 343–351. [Google Scholar] [PubMed]
- Hermann, R.; Niebch, G.; Borbe, H.O.; Fieger-Büschges, H.; Ruus, P.; Nowak, H.; Riethmüller-Winzen, H.; Peukert, M.; Blume, H. Enantioselective pharmacokinetics and bioavailability of different racemic α-lipoic acid formulations in healthy volunteers. Eur. J. Pharm. Sci. 1996, 4, 167–174. [Google Scholar] [CrossRef]
- Konrad, D.; Somwar, R.; Sweeney, G.; Yaworsky, K.; Hayashi, M.; Ramlal, T.; Klip, A. The antihyperglycemic drug α-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: Potential role of p38 mitogen-activated protein kinase in GLUT4 activation. Diabetes 2001, 50, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Veiga, F.; Fernandes, C.; Teixeira, F. Oral bioavailability and hypoglycaemic activity of tolbutamide/cyclodextrin inclusion complexes. Int. J. Pharm. 2000, 202, 165–171. [Google Scholar] [CrossRef]
- Terao, K.; Nakata, D.; Fukumi, H.; Schmid, G.; Arima, H.; Hirayama, F.; Uekama, K. Enhancement of oral bioavailability of coenzyme Q10 by complexation with γ-cyclodextrin in healthy adults. Nutr. Res. 2006, 26, 503–508. [Google Scholar] [CrossRef]
- Carrier, R.L.; Miller, L.A.; Ahmed, I. The utility of cyclodextrins for enhancing oral bioavailability. J. Control Release 2007, 123, 78–99. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Moya-Ortega, M.D.; Alvarez-Lorenzo, C.; Concheiro, A. Pharmacokinetics of cyclodextrins and drugs after oral and parenteral administration of drug/cyclodextrin complexes. J. Pharm. Pharmacol. 2015, 16, 312–332. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Teichert, J.; Preiss, R. HPLC-methods for determination of lipoic acid and its reduced form in human plasma. Int. J. Clin. Pharmacol. Ther. Toxicol. 1992, 30, 511–512. [Google Scholar] [PubMed]
- Teichert, J.; Preiss, R. High-performance liquid chromatographic assay for α-lipoic acid and five of its metabolites in human plasma and urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 769, 269–281. [Google Scholar] [CrossRef]
- Tabata, K.; Yamaoka, K.; Kaibara, A.; Suzuki, S.; Terakawa, M.; Hata, T. Moment analysis program available on Microsoft Excel®. Drug Metab. Pharmacokinet. 1999, 14, 286–293. [Google Scholar] [CrossRef]
| Subject | Gender | Age | Body Weight (kg) | Body Mass Index (kg/m2) |
|---|---|---|---|---|
| 1 | Male | 31 | 68 | 22.2 |
| 2 | Male | 34 | 55 | 21.5 |
| 3 | Male | 33 | 54 | 19.1 |
| 4 | Male | 32 | 67 | 23.7 |
| 5 | Male | 34 | 67 | 26.5 |
| 6 | Male | 34 | 60 | 20.8 |
| Mean ± S.D. | 33.0 ± 1.3 | 61.8 ± 6.4 | 22.3 ± 2.6 |
| Time (min) | Plasma Glucose (mg/dL) | |
|---|---|---|
| R-LA | R-LA/CD | |
| 0 | 78 ± 7 | 82 ± 12 |
| 5 | 80 ± 10 | 79 ± 11 |
| 15 | 77 ± 7 | 80 ± 11 |
| 30 | 80 ± 9 | 78 ± 10 |
| 45 | 79 ± 8 | 77 ± 8 |
| 60 | 78 ± 10 | 76 ± 10 |
| 120 | 79 ± 11 | 82 ± 12 |
| 180 | 77 ± 12 | 80 ± 12 |
| Subject | R-LA | R-LA/CD | ||
|---|---|---|---|---|
| Cmax (µg/mL) | AUC0–180min (µg·min/mL) | Cmax (µg/mL) | AUC0–180min (µg·min/mL) | |
| 1 | 1.02 | 43.6 | 2.62 | 186.7 |
| 2 | 1.03 | 46.7 | 4.45 | 214.7 |
| 3 | 1.83 | 74.9 | 5.08 | 216.7 |
| 4 | 1.02 | 53.8 | 4.93 | 197.3 |
| 5 | 3.62 | 158.8 | 4.23 | 189.4 |
| 6 | 1.55 | 90.5 | 3.30 | 170.5 |
| Property | R-LA | R-LA/CD |
|---|---|---|
| Cmax (µg/mL) | 1.68 ± 1.01 | 4.10 ± 0.96 ** |
| AUC0–18 min (µg·min/mL) | 78.0 ± 43.5 | 195.9 ± 17.7 ** |
| Tmax (min) | 20.8 ± 10.7 | 17.5 ± 6.1 |
| T1/2 (min) | 38.9 ± 12.2 | 23.3 ± 10.3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikuta, N.; Okamoto, H.; Furune, T.; Uekaji, Y.; Terao, K.; Uchida, R.; Iwamoto, K.; Miyajima, A.; Hirota, T.; Sakamoto, N. Bioavailability of an R-α-Lipoic Acid/γ-Cyclodextrin Complex in Healthy Volunteers. Int. J. Mol. Sci. 2016, 17, 949. https://doi.org/10.3390/ijms17060949
Ikuta N, Okamoto H, Furune T, Uekaji Y, Terao K, Uchida R, Iwamoto K, Miyajima A, Hirota T, Sakamoto N. Bioavailability of an R-α-Lipoic Acid/γ-Cyclodextrin Complex in Healthy Volunteers. International Journal of Molecular Sciences. 2016; 17(6):949. https://doi.org/10.3390/ijms17060949
Chicago/Turabian StyleIkuta, Naoko, Hinako Okamoto, Takahiro Furune, Yukiko Uekaji, Keiji Terao, Ryota Uchida, Kosuke Iwamoto, Atsushi Miyajima, Takashi Hirota, and Norihiro Sakamoto. 2016. "Bioavailability of an R-α-Lipoic Acid/γ-Cyclodextrin Complex in Healthy Volunteers" International Journal of Molecular Sciences 17, no. 6: 949. https://doi.org/10.3390/ijms17060949
APA StyleIkuta, N., Okamoto, H., Furune, T., Uekaji, Y., Terao, K., Uchida, R., Iwamoto, K., Miyajima, A., Hirota, T., & Sakamoto, N. (2016). Bioavailability of an R-α-Lipoic Acid/γ-Cyclodextrin Complex in Healthy Volunteers. International Journal of Molecular Sciences, 17(6), 949. https://doi.org/10.3390/ijms17060949