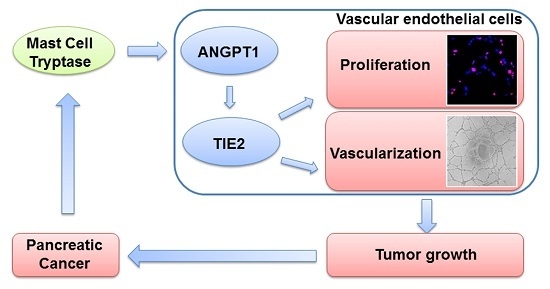
Mast Cell Tryptase Contributes to Pancreatic Cancer Growth through Promoting Angiogenesis via Activation of Angiopoietin-1
Abstract
:1. Introduction
2. Results
2.1. Increased MCT Level in Pancreatic Cancer Patients Correlates with Tumor Angiogenesis
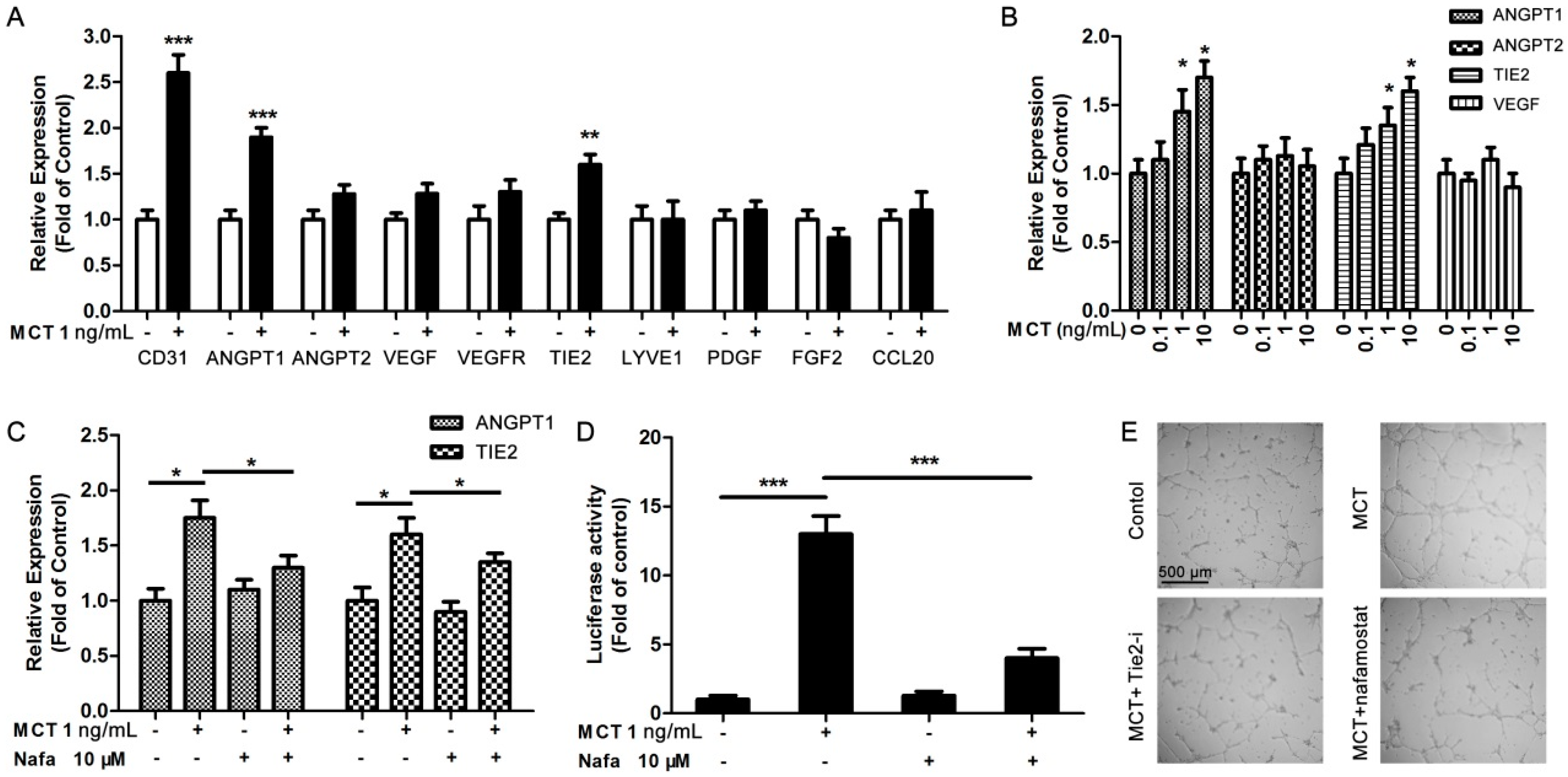
2.2. MCT Promoted Proliferation and Vascularization in HUVEC
2.3. ANGPT1 was Involved in MCT-Induced Promotion of Angiogenesis
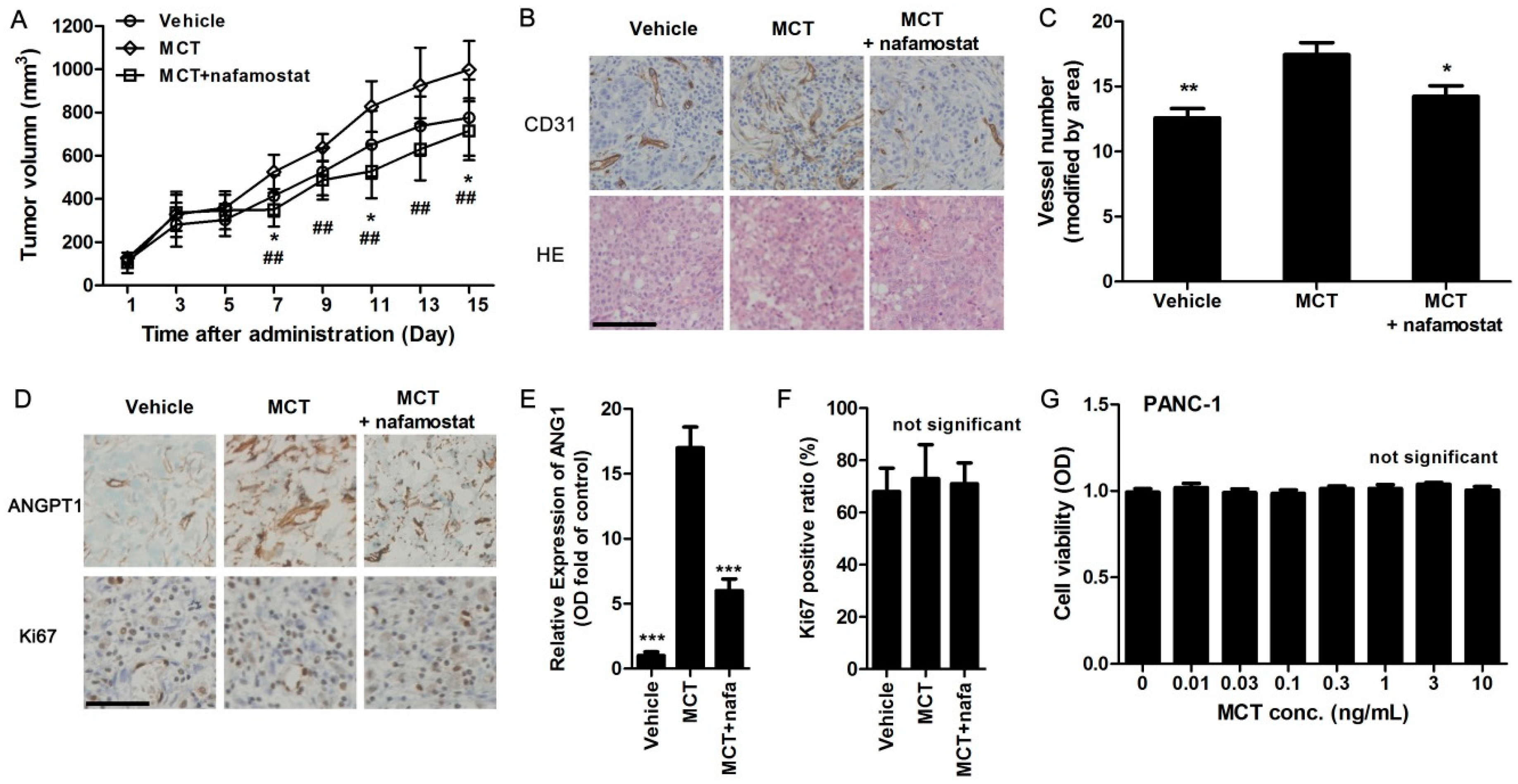
2.4. MCT Promotes Pancreatic Cancer Cells Tumorigenesis and Angiogenesis in Vivo
3. Discussion
4. Materials and Methods
4.1. Serum Analysis and IHC Assay
4.2. qPCR Assay
4.3. Cell Culture
4.4. Cell Proliferation Assays
4.5. Tube Formation Assay
4.6. Western Blotting Analysis
4.7. Tumor Formation Assay in a Nude Mouse Model
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- He, X.Y.; Yuan, Y.Z. Advances in pancreatic cancer research: Moving towards early detection. World J. Gastroenterol. 2014, 20, 11241–11248. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Lan, H.; Liu, F.; Wang, S.; Chen, X.; Jin, K.; Mou, X. Anti-angiogenesis or pro-angiogenesis for cancer treatment: Focus on drug distribution. Int. J. Clin. Exp. Med. 2015, 8, 8369–8376. [Google Scholar] [PubMed]
- Yadav, L.; Puri, N.; Rastogi, V.; Satpute, P.; Sharma, V. Tumour angiogenesis and angiogenic inhibitors: A review. J. Clin. Diagn. Res. 2015, 9, XE01–XE05. [Google Scholar] [CrossRef] [PubMed]
- Barzi, A.; Thara, E. Angiogenesis in esophageal and gastric cancer: A paradigm shift in treatment. Expert Opin. Biol. Ther. 2014, 14, 1319–1332. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Leporini, C.; Marech, I.; Gadaleta, C.D.; Scognamillo, G.; Sacco, R.; Sammarco, G.; De Sarro, G.; Russo, E.; Ranieri, G. Targeting mast cells tryptase in tumor microenvironment: A potential antiangiogenetic strategy. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Marech, I.; Ammendola, M.; Gadaleta, C.; Zizzo, N.; Oakley, C.; Gadaleta, C.D.; Ranieri, G. Possible biological and translational significance of mast cells density in colorectal cancer. World J. Gastroenterol. 2014, 20, 8910–8920. [Google Scholar] [PubMed]
- Kankkunen, J.P.; Harvima, I.T.; Naukkarinen, A. Quantitative analysis of tryptase and chymase containing mast cells in benign and malignant breast lesions. Int. J. Cancer 1997, 72, 385–388. [Google Scholar] [CrossRef]
- Kashiwase, Y.; Morioka, J.; Inamura, H.; Yoshizawa, Y.; Usui, R.; Kurosawa, M. Quantitative analysis of mast cells in benign and malignant breast lesions. Immunohistochemical study on formalin-fixed, paraffin-embedded tissues. Int. Arch. Allergy Immunol. 2004, 134, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Ranieri, G.; Nico, B.; Benagiano, V.; Crivellato, E. Tryptase and chymase are angiogenic in vivo in the chorioallantoic membrane assay. Int. J. Dev. Biol. 2011, 55, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Strouch, M.J.; Cheon, E.C.; Salabat, M.R.; Krantz, S.B.; Gounaris, E.; Melstrom, L.G.; Dangi-Garimella, S.; Wang, E.; Munshi, H.G.; Khazaie, K.; et al. Crosstalk between mast cells and pancreatic cancer cells contributes to pancreatic tumor progression. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, D.D.; Baram, D.; Mekori, Y.A. Mast cells. Physiol. Rev. 1997, 77, 1033–1079. [Google Scholar] [PubMed]
- Ammendola, M.; Sacco, R.; Sammarco, G.; Donato, G.; Zuccala, V.; Luposella, M.; Patruno, R.; Marech, I.; Montemurro, S.; Zizzo, N.; et al. Mast cells density positive to tryptase correlates with angiogenesis in pancreatic ductal adenocarcinoma patients having undergone surgery. Gastroenterol. Res. Pract. 2014, 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Blair, R.J.; Meng, H.; Marchese, M.J.; Ren, S.; Schwartz, L.B.; Tonnesen, M.G.; Gruber, B.L. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J. Clin. Invest. 1997, 99, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Taipale, J.; Lohi, J.; Saarinen, J.; Kovanen, P.T.; Keski-Oja, J. Human mast cell chymase and leukocyte elastase release latent transforming growth factor-β 1 from the extracellular matrix of cultured human epithelial and endothelial cells. J. Biol. Chem. 1995, 270, 4689–4696. [Google Scholar] [CrossRef] [PubMed]
- De Souza Junior, D.A.; Santana, A.C.; da Silva, E.Z.; Oliver, C.; Jamur, M.C. The role of mast cell specific chymases and tryptases in tumor angiogenesis. BioMed Res. Int. 2015, 2015, 142359. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, N.N.; Caughey, G.H. Mast cell peptidases: Chameleons of innate immunity and host defense. Am. J. Respir. Cell Mol. Biol. 2010, 42, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Raymond, W.W.; Bergers, G.; Laig-Webster, M.; Behrendtsen, O.; Werb, Z.; Caughey, G.H.; Hanahan, D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999, 13, 1382–1397. [Google Scholar] [CrossRef] [PubMed]
- Nico, B.; Mangieri, D.; Crivellato, E.; Vacca, A.; Ribatti, D. Mast cells contribute to vasculogenic mimicry in multiple myeloma. Stem Cells Dev. 2008, 17, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Ranieri, G. Tryptase, a novel angiogenic factor stored in mast cell granules. Exp. Cell Res. 2015, 332, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Vacca, A.; Nico, B.; Quondamatteo, F.; Ria, R.; Minischetti, M.; Marzullo, A.; Herken, R.; Roncali, L.; Dammacco, F. Bone marrow angiogenesis and mast cell density increase simultaneously with progression of human multiple myeloma. Br. J. Cancer 1999, 79, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Ennas, M.G.; Vacca, A.; Ferreli, F.; Nico, B.; Orru, S.; Sirigu, P. Tumor vascularity and tryptase-positive mast cells correlate with a poor prognosis in melanoma. Eur. J. Clin. Invest. 2003, 33, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Finato, N.; Crivellato, E.; Marzullo, A.; Mangieri, D.; Nico, B.; Vacca, A.; Beltrami, C.A. Neovascularization and mast cells with tryptase activity increase simultaneously with pathologic progression in human endometrial cancer. Am. J. Obstet. Gynecol. 2005, 193, 1961–1965. [Google Scholar] [CrossRef] [PubMed]
- Marech, I.; Ammendola, M.; Sacco, R.; Capriuolo, G.S.; Patruno, R.; Rubini, R.; Luposella, M.; Zuccala, V.; Savino, E.; Gadaleta, C.D.; et al. Serum tryptase, mast cells positive to tryptase and microvascular density evaluation in early breast cancer patients: Possible translational significance. BMC Cancer 2014, 14, 534. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Sacco, R.; Sammarco, G.; Donato, G.; Montemurro, S.; Ruggieri, E.; Patruno, R.; Marech, I.; Cariello, M.; Vacca, A.; et al. Correlation between serum tryptase, mast cells positive to tryptase and microvascular density in colo-rectal cancer patients: Possible biological-clinical significance. PLoS ONE 2014, 9, e99512. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Guidolin, D.; Marzullo, A.; Nico, B.; Annese, T.; Benagiano, V.; Crivellato, E. Mast cells and angiogenesis in gastric carcinoma. Int. J. Exp. Pathol. 2010, 91, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Carlini, M.J.; Dalurzo, M.C.; Lastiri, J.M.; Smith, D.E.; Vasallo, B.C.; Puricelli, L.I.; Lauria de Cidre, L.S. Mast cell phenotypes and microvessels in non-small cell lung cancer and its prognostic significance. Hum. Pathol. 2010, 41, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Ammendola, M.; Patruno, R.; Celano, G.; Zito, F.A.; Montemurro, S.; Rella, A.; Di Lecce, V.; Gadaleta, C.D.; Battista De Sarro, G.; et al. Tryptase-positive mast cells correlate with angiogenesis in early breast cancer patients. Int. J. Oncol. 2009, 35, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.P.; Zhu, S.; Ko, J.E.; Ashritha, N.; Kandimalla, R.; Snyder, J.P.; Shoji, M.; El-Rayes, B.F. Antiangiogenic effects of a novel synthetic curcumin analogue in pancreatic cancer. Cancer Lett. 2015, 357, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Echavarria, R.; Hussain, S.N. Regulation of angiopoietin-1/Tie-2 receptor signaling in endothelial cells by dual-specificity phosphatases 1, 4, and 5. J. Am. Heart Assoc. 2013, 2, e000571. [Google Scholar] [CrossRef] [PubMed]
- Varinska, L.; Gal, P.; Mojzisova, G.; Mirossay, L.; Mojzis, J. Soy and breast cancer: Focus on angiogenesis. Int. J. Mol. Sci. 2015, 16, 11728–11749. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, M.; Jikuhara, A.; Mori, S.; Iwagaki, H.; Takahashi, H.K.; Nishibori, M.; Tanaka, N. Mast cell tryptase stimulates DLD-1 carcinoma through prostaglandin- and map kinase-dependent manners. J. Pharmacol. Sci. 2005, 98, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. Mast cells as therapeutic target in cancer. Eur. J. Pharmacol. 2015, 778, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Gacche, R.N.; Meshram, R.J. Targeting tumor micro-environment for design and development of novel anti-angiogenic agents arresting tumor growth. Prog. Biophys. Mol. Biol. 2013, 113, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Durkin, A.J.; Bloomston, M.; Yeatman, T.J.; Gilbert-Barness, E.; Cojita, D.; Rosemurgy, A.S.; Zervos, E.E. Differential expression of the Tie-2 receptor and its ligands in human pancreatic tumors. J. Am. Coll. Surg. 2004, 199, 724–731. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Zhai, L.; Xue, R.; Shi, J.; Zeng, Q.; Gao, C. Mast Cell Tryptase Contributes to Pancreatic Cancer Growth through Promoting Angiogenesis via Activation of Angiopoietin-1. Int. J. Mol. Sci. 2016, 17, 834. https://doi.org/10.3390/ijms17060834
Guo X, Zhai L, Xue R, Shi J, Zeng Q, Gao C. Mast Cell Tryptase Contributes to Pancreatic Cancer Growth through Promoting Angiogenesis via Activation of Angiopoietin-1. International Journal of Molecular Sciences. 2016; 17(6):834. https://doi.org/10.3390/ijms17060834
Chicago/Turabian StyleGuo, Xiangjie, Liqin Zhai, Ruobing Xue, Jieru Shi, Qiang Zeng, and Cairong Gao. 2016. "Mast Cell Tryptase Contributes to Pancreatic Cancer Growth through Promoting Angiogenesis via Activation of Angiopoietin-1" International Journal of Molecular Sciences 17, no. 6: 834. https://doi.org/10.3390/ijms17060834
APA StyleGuo, X., Zhai, L., Xue, R., Shi, J., Zeng, Q., & Gao, C. (2016). Mast Cell Tryptase Contributes to Pancreatic Cancer Growth through Promoting Angiogenesis via Activation of Angiopoietin-1. International Journal of Molecular Sciences, 17(6), 834. https://doi.org/10.3390/ijms17060834