Exchange Transfusion in the Treatment of Neonatal Septic Shock: A Ten-Year Experience in a Neonatal Intensive Care Unit
Abstract
:1. Introduction
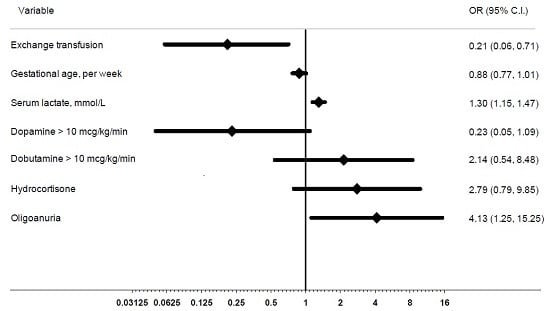
2. Results
3. Discussion
4. Material and Methods
4.1. Study Population
4.2. Definitions
4.3. Data Collection
4.4. Exchange Transfusion (ET) Procedure
4.5. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Palazzi, D.B.; Klein, J.O.; Baker, C.J. Bacterial sepsis and meningitis. In Infectious Diseases of the Fetus and Newborn Infant, 6th ed.; Remington, J.S., Klein, J.O., Wilson, C.B., Baker, C.J., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2006; pp. 247–295. [Google Scholar]
- Shane, A.L.; Stoll, B.J. Neonatal sepsis: Progress towards improved outcomes. J. Infect. 2014, 68, S24–S32. [Google Scholar] [CrossRef] [PubMed]
- Klinger, G.; Levy, I.; Sirota, L.; Boyko, V.; Lerner-Geva, L.; Reichman, B. Israel Neonatal Network. Outcome of early-onset sepsis in a national cohort of very low birth weight infants. Pediatrics 2010, 125, e736–e740. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Hansen, N.I.; Adams-Chapman, I.; Fanaroff, A.A.; Hintz, S.R.; Vohr, B.; Higgins, R.D. National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and growth impairment among extremely low-birth weight infants with neonatal infection. JAMA 2004, 292, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Kermorvant-Duchemin, E.; Laborie, S.; Rabilloud, M.; Lapillonne, A.; Claris, O. Outcome and prognostic factors in neonates with septic shock. Pediatr. Crit. Care Med. 2008, 9, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Tarnow-Mordi, W.; Isaacs, D.; Dutta, S. Adjunctive immunologic interventions in neonatal sepsis. Clin. Perinatol. 2010, 37, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Lacy, J.B. Intravenous immunoglobulin for suspected or proven infection in neonates. Cochrane Database Syst. Rev. 2013, 7, CD001239. [Google Scholar] [PubMed]
- Carr, R.; Modi, N.; Doré, C. G-CSF and GM-CSF for treating or preventing neonatal infections. Cochrane Database Syst. Rev. 2003, 3, CD003066. [Google Scholar] [PubMed]
- Prod’hom, L.S.; Choffat, J.M.; Frenck, N.; Mazoumi, M.; Relier, J.P.; Torrado, A. Care of seriously ill neonate with hyaline membrane disease and with sepsis (Sclerema neonatorum). Pediatrics 1974, 53, 170–181. [Google Scholar] [PubMed]
- Xanthou, M.; Xypolyta, A.; Anagnostakis, D.; Economou-Mavrou, C.; Matsaniotis, N. Exchange transfusion in severe neonatal infection with sclerema. Arch. Dis. Child. 1975, 50, 901–902. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Töllner, U.; Pohlandt, F.; Heinze, F.; Henrichs, I. Treatment of septicaemia in the newborn infant: Choice of initial antimicrobial drugs and the role of exchange transfusion. Acta Paediatr. Scand. 1977, 66, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Vain, N.E.; Mazlumian, J.R.; Swarner, O.W.; Cha, C.C. Role of exchange transfusion in the treatment of severe septicemia. Pediatrics 1980, 66, 693–697. [Google Scholar] [PubMed]
- Lemos, L. Exchange transfusion in treatment of sepsis. Pediatrics 1981, 68, 471–472. [Google Scholar] [PubMed]
- Mathur, N.B. Neonatal sepsis. Indian Pediatr. 1996, 33, 663–674. [Google Scholar] [PubMed]
- Togari, H.; Mikawa, M.; Iwanaga, T.; Matsumoto, N.; Kawase, A.; Hagisawa, M.; Ogino, T.; Goto, R.; Watanabe, I.; Kito, H.; et al. Endotoxin clearance by exchange blood transfusion in septic shock neonates. Acta Paediatr. Scand. 1983, 72, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Sadana, S.; Mathur, N.B.; Thakur, A. Exchange transfusion in septic neonates with sclerema: Effect on immunoglobulin and complement levels. Indian Pediatr. 1997, 34, 20–25. [Google Scholar] [PubMed]
- Xanthou, M.; Nicolopoulos, D.; Gizas, A.; Matsaniotis, N. The response of leukocytes in the peripheral blood during and following exchange transfusion in the newborn. Pediatrics 1973, 51, 570–574. [Google Scholar] [PubMed]
- Christensen, R.D.; Anstall, H.B.; Rothstein, G. Use of whole blood exchange transfusion to supply neutrophils to septic, neutropenic neonates. Transfusion 1982, 22, 504–506. [Google Scholar] [CrossRef] [PubMed]
- De Curtis, M.; Vetrano, G.; Romano, G.; D’Antonio, F.; Paludetto, R.; Ciccimarra, F. Improvement of phagocytosis and nitroblue tetrazolium reduction after exchange transfusions in two preterm infants with severe septicemia. Pediatrics 1982, 70, 829–830. [Google Scholar] [PubMed]
- Mathur, N.B.; Subramanian, B.K.; Sharma, V.K.; Puri, R.K. Exchange transfusion in neutropenic septicemic neonates: Effect on granulocyte functions. Acta Paediatr. 1993, 82, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Bossi, E.; Meister, B.; Pfenninger, J. Exchange transfusion for severe neonatal septicemia. Pediatrics 1981, 67, 941. [Google Scholar] [PubMed]
- Narayanan, I.; Mitter, A.; Gujral, V.V. A comparative study on the value of exchange and blood transfusion in the management of severe neonatal septicemia with sclerema. Indian J. Pediatr. 1982, 49, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, R.; Rao, S.; Rangnekar, J.; Fernandez, A. Exchange transfusions in neonatal sepsis. Indian Pediatr. 1991, 28, 39–43. [Google Scholar] [PubMed]
- Gunes, T.; Koklu, E.; Buyukkayhan, D.; Kurtoglu, S.; Karakukcu, M.; Patiroglu, T. Exchange transfusion or intravenous immunoglobulin therapy as an adjunct to antibiotics for neonatal sepsis in developing countries: A pilot study. Ann. Trop. Paediatr. 2006, 26, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Aradhya, A.S.; Sundaram, V.; Kumar, P.; Ganapathy, S.M.; Jain, A.; Rawat, A. Double Volume Exchange Transfusion in Severe Neonatal Sepsis. Indian J. Pediatr. 2016, 83, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Gross, S.J.; Filston, H.C.; Anderson, J.C. Controlled study of treatment for disseminated intravascular coagulation in the neonate. J. Pediatr. 1982, 100, 445–448. [Google Scholar] [CrossRef]
- Phibbs, R.H. Response of newborn infants to leukocyte depletion during exchange transfusions. Biol. Neonate 1970, 15, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Levinson, S.A.; Hume, D.M. Effect of exchange transfusion with fresh whole blood on refractory septic shock. Am. Surg. 1972, 38, 49–55. [Google Scholar] [PubMed]
- Stoll, B.J.; Hansen, N.; Fanaroff, A.A.; Wright, L.L.; Carlo, W.A.; Ehrenkranz, R.A.; Lemons, J.A.; Donovan, E.F.; Stark, A.R.; Tyson, J.E.; et al. Late-onset sepsis in very low birth weight neonates: The experience of the NICHD Neonatal Research Network. Pediatrics 2002, 110, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Bhagat, I.; Hieber, S.; Donn, S.M. Can neutrophil responses in very low birth weight infants predict the organisms responsible for late-onset bacterial or fungal sepsis? J. Perinatol. 2006, 26, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Steiner, L.A.; Bizzarro, M.J.; Ehrenkranz, R.A.; Gallagher, P.G. A decline in the frequency of neonatal exchange transfusions and its effect on exchange-related morbidity and mortality. Pediatrics 2007, 120, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.; Giroir, B.; Randolph, A. International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 2005, 6, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Wynn, J.L.; Wong, H.R. Pathophysiology and treatment of septic shock in neonates. Clin. Perinatol. 2010, 37, 439–479. [Google Scholar] [CrossRef] [PubMed]
- Bertino, E.; Spada, E.; Occhi, L.; Coscia, A.; Giuliani, F.; Gagliardi, L.; Gilli, G.; Bona, G.; Fabris, C.; de Curtis, M.; et al. Neonatal anthropometric charts: The Italian neonatal study compared with other European studies. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.A.; Wilson, M.E. Feeding intolerance: A concept analysis. Adv. Neonatal. Care 2011, 11, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Papile, L.A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 gm. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef]
- Richardson, D.K.; Corcoran, J.D.; Escobar, G.J.; Lee, S.K. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J. Pediatr. 2001, 138, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Levit, O.; Bhandari, V.; Li, F.Y.; Shabanova, V.; Gallagher, P.G.; Bizzarro, M.J. Clinical and laboratory factors that predict death in very low birth weight infants presenting with late-onset sepsis. Pediatr. Infect. Dis. J. 2014, 33, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Pugni, L.; Riva, E.; Pietrasanta, C.; Rabacchi, C.; Bertolini, S.; Pederiva, C.; Mosca, F.; Calandra, S. Severe hypertriglyceridemia in a newborn with monogenic lipoprotein lipase deficiency: An unconventional therapeutic approach with exchange transfusion. JIMD Rep. 2014, 13, 59–64. [Google Scholar] [PubMed]
- Murki, S.; Kumar, P. Blood exchange transfusion for infants with severe neonatal hyperbilirubinemia. Semin. Perinatol. 2011, 35, 175–184. [Google Scholar] [CrossRef] [PubMed]

| Clinical Variables | ET (n = 50) | ScT (n = 51) | p Value * |
|---|---|---|---|
| Gestational age, wks, median (IQR) | 28 (26–31) | 26 (24–29) | 0.05 |
| Birth weight, g, median (IQR) | 1060 (770–2000) | 750 (580–1100) | 0.003 |
| ≤1000 g, n (%) | 23 (46) | 37 (72.5) | 0.008 |
| 1001–1500 g, n (%) | 11 (22) | 5 (5.8) | 0.11 |
| >1500 g, n (%) | 16 (32) | 9 (17.6) | 0.11 |
| SGA, n (%) | 5 (10) | 13 (25.5) | 0.07 |
| Inborn, n (%) | 44 (88) | 44 (86.3) | 1 |
| Twin, n (%) | 14 (28) | 11 (21.6) | 0.49 |
| Male, n (%) | 31 (62) | 33 (64.7) | 0.83 |
| Apgar 1 min, median (IQR) | 5 (2–7) | 5 (2–6) | 0.43 |
| Apgar 5 min, median (IQR) | 8 (7–8) | 7 (7–8) | 0.66 |
| Resuscitation in delivery room, n (%) 1 | 42 (84) | 45 (88.2) | 0.57 |
| Early-onset sepsis, n (%) | 27 | 14 | 0.008 |
| Late-onset sepsis, n (%) | 23 | 37 | 0.008 |
| Age at sepsis evaluation, d, median (IQR) | 2 (0–11) | 9 (3–22) | 0.003 |
| Weight at sepsis evaluation, g, median (IQR) | 1212.5 (820–1960) | 880 (600–1610) | 0.05 |
| Time between sepsis and septic shock, h, median (IQR) | 4.5 (3–10) | 13 (5–29) | 0.001 |
| Time between septic shock and ET, h, median (IQR) | 7 (4–18) | NA | NA |
| Cultures | ET | ScT | p Value * |
|---|---|---|---|
| Blood culture, n | 47 | 48 | |
| Any bacteria, n (%) | 26 (55.3) | 27 (56.2) | 1 |
| Gram-positive organisms, n (%) | 5 (10.6) | 15 (31.2) | 0.02 |
| S. agalactiae, n (%) | 1 (2) | 2 (3.9) | |
| S. aureus, n (%) | 0 | 1 (1.9) | |
| S. aureus MRSA, n (%) | 0 | 2 (3.9) | |
| S. aureus MSSA, n (%) | 1 (2) | 2 (3.9) | |
| S. epidermidis, n (%) | 3 (6) | 5 (9.8) | |
| E. faecalis, n (%) | 0 | 1 (1.9) | |
| E. faecium, n (%) | 0 | 1 (1.9) | |
| Others, n (%) | 0 | 1 (1.9) | |
| Gram-negative organisms, n (%) | 21 (44.6) | 12 (25) | 0.05 |
| E. coli, n (%) | 3 (6) | 0 | |
| E. coli ESBL neg, n (%) | 2 (4) | 3 (5.8) | |
| E. coli ESBL pos, n (%) | 1 (2) | 0 | |
| E. cloacae, n (%) | 1 (2) | 1 (1.9) | |
| K. pneumoniae, n (%) | 3 (6) | 0 | |
| K. pneumoniae ESBL pos, n (%) | 3 (6) | 2 (3.9) | |
| K. pneumoniae ESBL neg, n (%) | 0 | 1 (1.9) | |
| P. aeruginosa, n (%) | 7 (14) | 3 (5.8) | |
| S. marcescens, n (%) | 1 (2) | 0 | |
| S. maltophilia, n (%) | 0 | 1 (1.9) | |
| M. morganii, n (%) | 0 | 1 (1.9) | |
| Fungi, n (%) | 2 (4) | 0 | 0.24 |
| C. albicans, n (%) | 2 (4) | 0 | |
| Spinal fluid culture, n | 4 | 10 | – |
| Any bacteria, n (%) | 2 (50) | 2 (20) | 0.8 |
| S. marcescens, n (%) | 1 (25) | 0 | |
| S. agalactiae, n (%) | 1 (25) | 1 (10) | |
| E. coli, n (%) | 0 | 1 (10) | |
| Urine culture, n | 4 | 1 | 0.24 |
| P. aeruginosa, n (%) | 1 (25) | 0 |
| Clinical Signs/Symptoms | ET (n = 50) | ScT (n = 51) | p Value * |
|---|---|---|---|
| Hyperthermia, n (%) | 6 (12) | 5 (9.8) | 0.76 |
| Hypothermia, n (%) | 6 (12) | 11 (21.6) | 0.28 |
| Respiratory signs, n (%) | – | – | – |
| Any, n (%) | 48 (96) | 50 (98) | 0.6 |
| Apnea, n (%) | 32 (62.7) | 32 (64) | 1 |
| Tachypnea, n (%) | 48 (96) | 49 (96.1) | 1 |
| Feeding intolerance, n (%) | 16 (32) | 29 (56.9) | 0.01 |
| Tachycardia, n (%) | 45 (90) | 35 (68.6) | 0.01 |
| Hypotension, n (%) | 42 (84) | 45 (88.2) | 0.57 |
| Poor capillary refill, n (%) | 45 (90) | 48 (94.1) | 0.48 |
| Neurologic signs, n (%) | – | – | – |
| Any, n (%) | 36 (72) | 40 (78.4) | 0.49 |
| Hypotonia, n (%) | 28 (56) | 36 (70.6) | 0.15 |
| Lethargy, n (%) | 28 (56) | 40 (78.4) | 0.02 |
| Seizures, n (%) | 2 (3.9) | 2 (4) | 1 |
| Diuresis | – | – | – |
| Oliguria/anuria, n (%) | 24 (48) | 15 (29) | 0.14 |
| Oliguria, n (%) | 7 (14) | 4 (8) | 0.35 |
| Anuria, n (%) | 17 (34) | 11 (21) | 0.19 |
| SNAP-II, score, median (IQR) | 32 (19–50) | 41 (25–50) | 0.32 |
| <20 | 13 | 8 | – |
| 20–40 | 15 | 15 | – |
| >40 | 22 | 28 | – |
| Laboratory Data | ET (n = 50) | ScT (n = 51) | p Value * |
|---|---|---|---|
| Complete Blood Cell Count | |||
| White cell blood count, cells/mm3, median (IQR) | 4750 (2890–11,530) | 9540 (5620–20,100) | 0.0006 |
| Neutrophils, cells/mm3, median (IQR) | 1690 (980–5400) | 4150 (2050–8320) | 0.01 |
| Platelets, n/mm3, median (IQR) | 87,500 (37,500–160,000) | 130,000 (45,000–250,000) | 0.18 |
| C-reactive protein, mg/dL, median (IQR) | 4.71 (0.90–10.13) | 2.65 (1–6.5) | 0.56 |
| Coagulation Tests | |||
| PT ratio, median (IQR) | 1.88 (1.62–2.38) | 1.64 (1.25–1.91) | 0.05 |
| APTT ratio, median (IQR) | 1.88 (1.64–2.73) | 1.95 (1.4–5) | 0.86 |
| Fibrinogen, mg/dL, median (IQR) | 202.5 (161–278) | 233 (199–334) | 0.34 |
| Blood Gas Analysis | |||
| Base excess, mmol/L, median (IQR) | −8.8 (−13.1, −5.75) | -8.1 (−13.1, −3.1) | 0.68 |
| Serum lactate, mmol/L, median (IQR) | 6.95 (3–12.6) | 5.3 (2.5–8.9) | 0.2 |
| Hepatic Assessment | |||
| Serum alanine aminotransferase, U/L, median (IQR) | 11 (6–20) | 8 (5–15) | 0.4 |
| Serum aspartate aminotransferase, U/L, median (IQR) | 46 (32–80) | 42.5 (24–72) | 0.36 |
| Kidney Assessment | |||
| Serum creatinine, U/L, median (IQR) | 0.7 (0.6–1) | 0.9 (0.7–1.12) | 0.09 |
| Serum urea, U/L, median (IQR) | 49 (35–65) | 56 (35–83) | 0.19 |
| Supportive Therapy | ET (n = 50) | ScT (n = 51) | p Value * |
|---|---|---|---|
| Respiratory Support | |||
| Any ventilation, n (%) | 50 (100) | 51 (100) | 1 |
| Non-invasive ventilation, n (%) | 3 (6) | 2 (4) | 0.67 |
| Mechanical ventilation, n (%) | 21 (42) | 31 (61) | 0.07 |
| High frequency oscillatory ventilation, n (%) | 26 (52) | 18 (35) | 0.11 |
| Oxygen requirement, n (%) | 46 (92) | 49 (96) | 0.4 |
| Inhaled nitric oxide, n (%) | 26 (52) | 21 (37) | 0.3 |
| Intravenous Fluid, n (%) | |||
| Any fluid, n (%) | 49 (98) | 49 (96) | – |
| Saline bolus, n (%) | 45 (90) | 44 (86) | 0.75 |
| Plasma bolus, n (%) | 27 (54) | 31 (61) | 0.55 |
| Inotropic Agents | |||
| Any inotropic agents, n (%) | 42 (84) | 45 (88) | 0.6 |
| Dopamine, n (%) | 40 (80) | 41 (80) | 1 |
| Dobutamine, n (%) | 35 (70) | 29 (57) | 0.2 |
| Epinephrine, n (%) | 9 (18) | 2 (4) | 0.03 |
| Hydrocortisone, n (%) | 25 (50) | 16 (31) | 0.07 |
| Laboratory Data | Pre-ET (n = 50) | Post-ET (n = 51) | p Value * |
|---|---|---|---|
| Complete Blood Cell Count | |||
| White cell blood count, cells/mm3, median (IQR) | 4750 (2890–11,530) | 10,630 (6152–16,290) | 0.02 |
| Neutrophils, cells/mm3, median (IQR) | 1690 (980–5095) | 5130 (3245–8665) | 0.08 |
| Platelets, n/mm3, median (IQR) | 87,500 (38,700–155,500) | 37,500 (14,500–61,200) | <0.001 |
| C-reactive Protein, mg/dL, Median (IQR) | 4.71 (0.9–10.13) | 5.52 (1.04–12.03) | 0.18 |
| Blood Gas Analysis | |||
| Base excess, mmol/L, median (IQR) | −8.8 (−13.1, −5.7) | −5.2 (−8.5, −3.2) | <0.001 |
| Serum lactate, mmol/L, median (IQR) | 6.95 (3–12.4) | 5.6 (2.6–12.6) | 0.88 |
| Kidney Assessment | |||
| Serum creatinine, U/L, median (IQR) | 0.75 (0.6–1.02) | 0.95 (0.6–1.22) | 0.22 |
| Serum urea, U/L, median (IQR) | 49 (35.5–64.5) | 78 (43–101) | 0.0002 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugni, L.; Ronchi, A.; Bizzarri, B.; Consonni, D.; Pietrasanta, C.; Ghirardi, B.; Fumagalli, M.; Ghirardello, S.; Mosca, F. Exchange Transfusion in the Treatment of Neonatal Septic Shock: A Ten-Year Experience in a Neonatal Intensive Care Unit. Int. J. Mol. Sci. 2016, 17, 695. https://doi.org/10.3390/ijms17050695
Pugni L, Ronchi A, Bizzarri B, Consonni D, Pietrasanta C, Ghirardi B, Fumagalli M, Ghirardello S, Mosca F. Exchange Transfusion in the Treatment of Neonatal Septic Shock: A Ten-Year Experience in a Neonatal Intensive Care Unit. International Journal of Molecular Sciences. 2016; 17(5):695. https://doi.org/10.3390/ijms17050695
Chicago/Turabian StylePugni, Lorenza, Andrea Ronchi, Bianca Bizzarri, Dario Consonni, Carlo Pietrasanta, Beatrice Ghirardi, Monica Fumagalli, Stefano Ghirardello, and Fabio Mosca. 2016. "Exchange Transfusion in the Treatment of Neonatal Septic Shock: A Ten-Year Experience in a Neonatal Intensive Care Unit" International Journal of Molecular Sciences 17, no. 5: 695. https://doi.org/10.3390/ijms17050695
APA StylePugni, L., Ronchi, A., Bizzarri, B., Consonni, D., Pietrasanta, C., Ghirardi, B., Fumagalli, M., Ghirardello, S., & Mosca, F. (2016). Exchange Transfusion in the Treatment of Neonatal Septic Shock: A Ten-Year Experience in a Neonatal Intensive Care Unit. International Journal of Molecular Sciences, 17(5), 695. https://doi.org/10.3390/ijms17050695