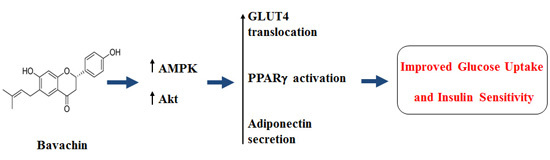
Bavachin from Psoralea corylifolia Improves Insulin-Dependent Glucose Uptake through Insulin Signaling and AMPK Activation in 3T3-L1 Adipocytes
Abstract
:1. Introduction
2. Results
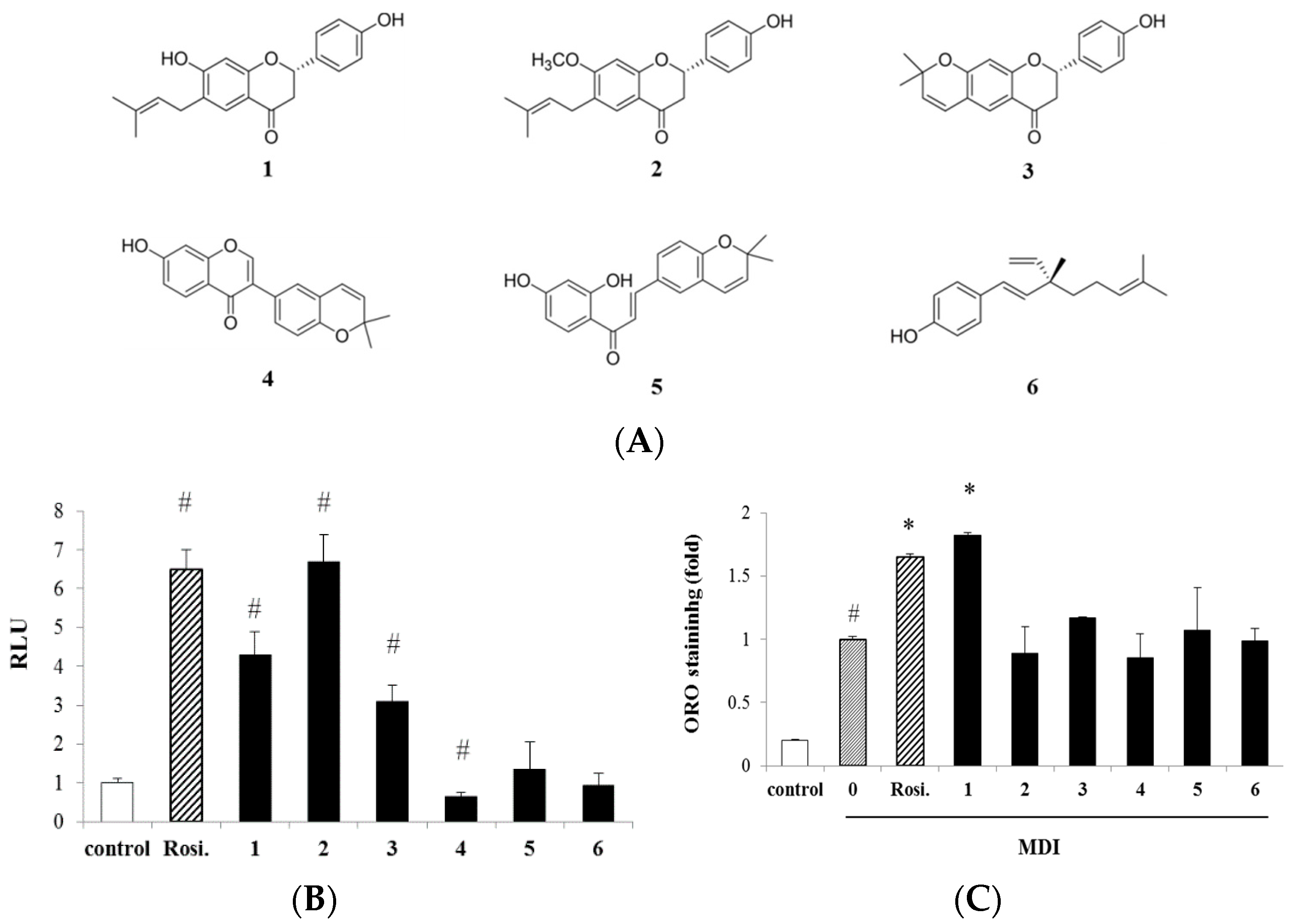
2.1. Purification of Peroxisome Proliferator-Activated Receptorγ (PPARγ) Ligands from Psoralea corylifolia
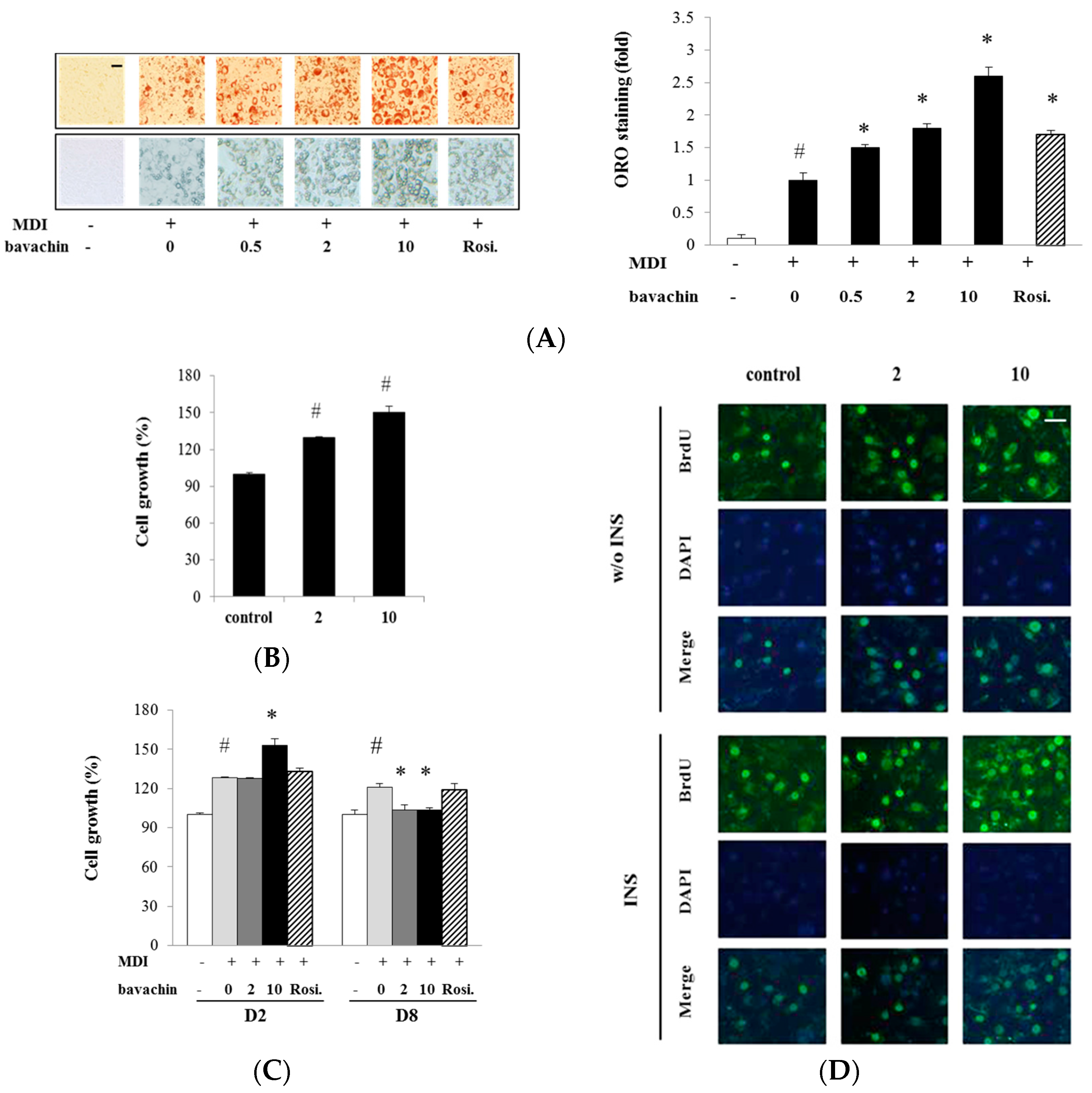
2.2. Bavachin Regulates Proliferation and Differentiation of Adipocyte
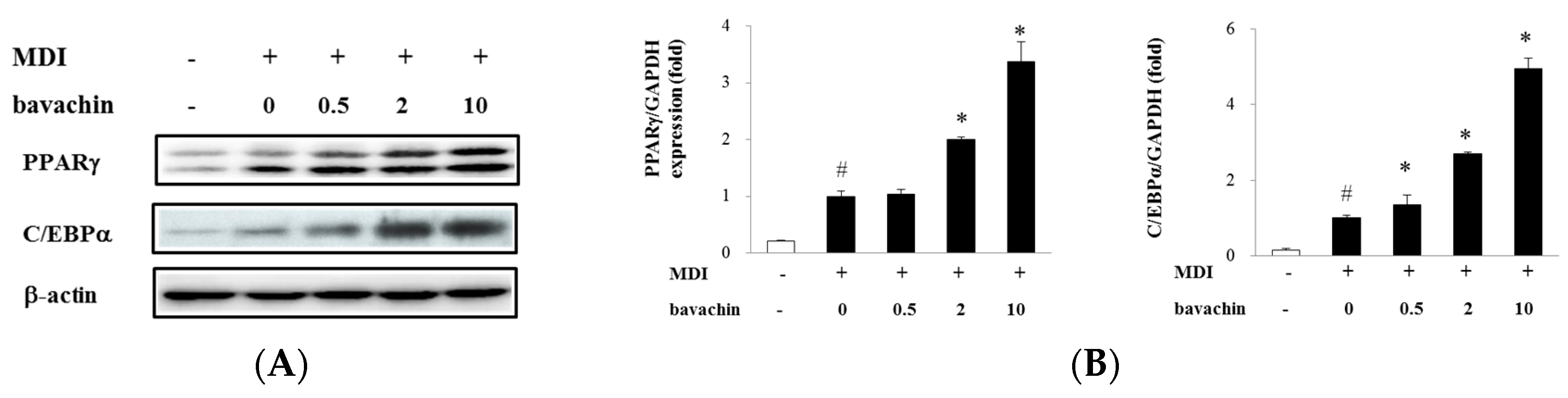
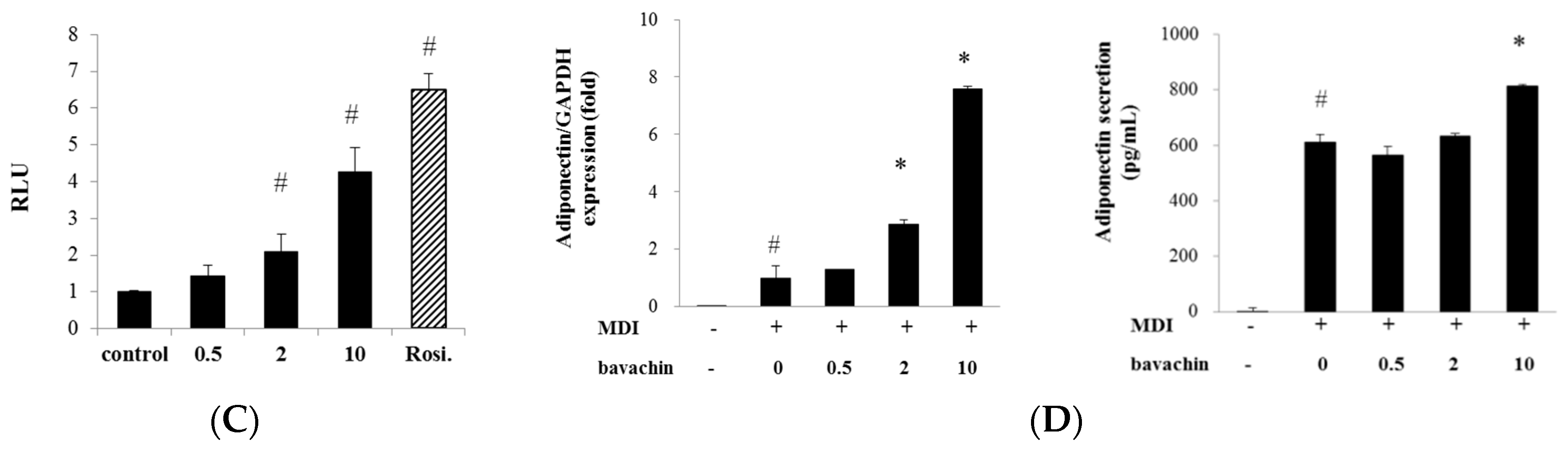
2.3. Bavachin Activates Adipogenic Factors and Increases PPARγ Transcriptional Activity in Differentiated Adipocytes
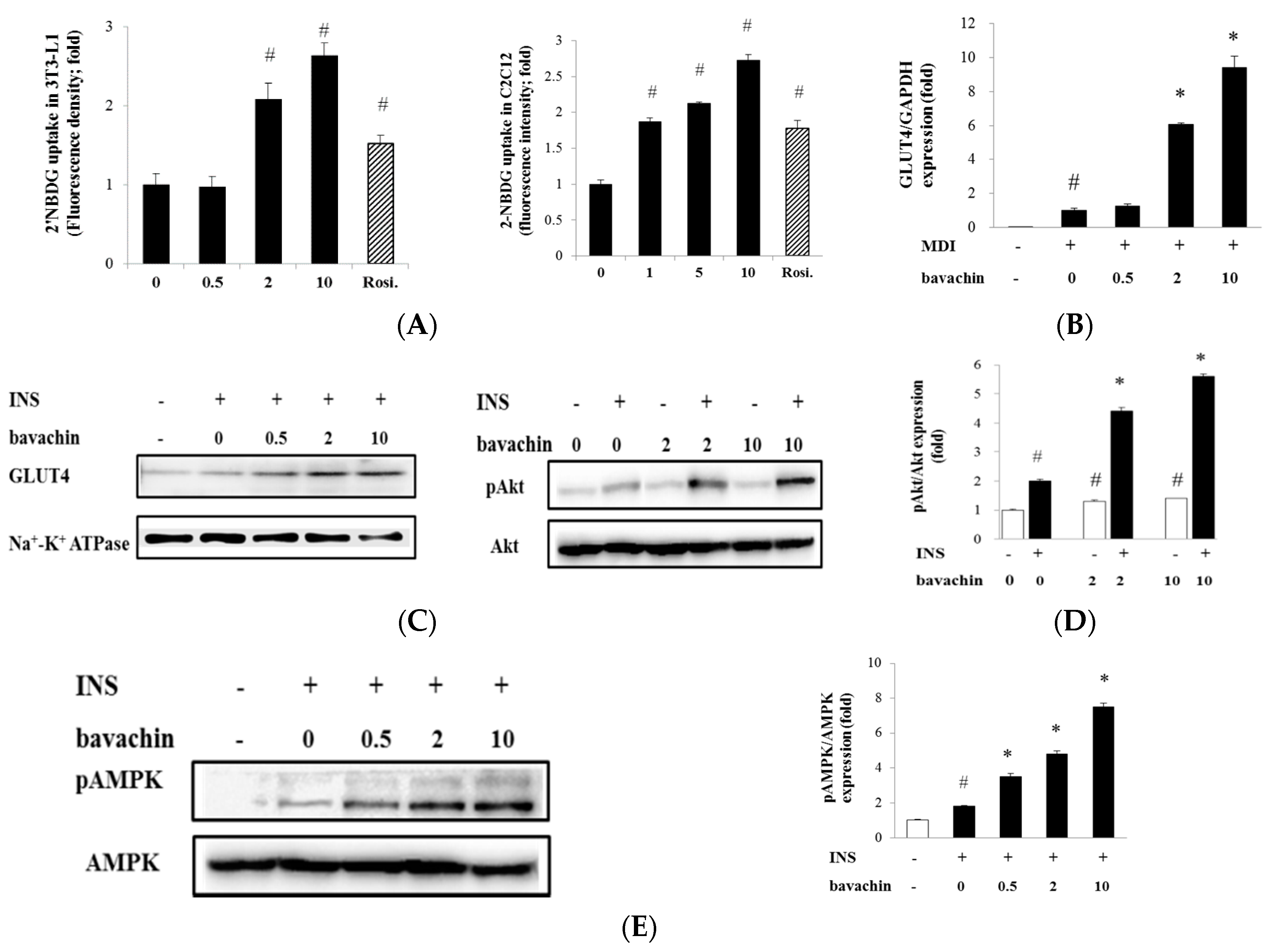
2.4. Bavachin Enhances Insulin-Stimulated Glucose Uptake through GLUT4 Translocation via Akt and AMPK Pathway
3. Discussion
4. Materials and Methods
4.1. Isolation of Compound from Psoralea corylifolia L. (PC)
4.2. Cell Culture and Pre-Adipocyte Differentiation
4.3. Oil Red O (ORO) Staining and Microscopy of Lipid Drop Formation in 3T3-L1 Cells
4.4. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) and Cell Proliferation Assay
4.5. Glucose Uptake Assay
4.6. Adiponectin Secretion Assay
4.7. Peroxisome Proliferator-Activated Receptor (PPAR)γ Reporter Gene Assay
4.8. RNA Extraction and Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qPCR)
4.9. Preparation of the Plasma Membrane Fraction and Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kwon, D.Y.; Kim da, S.; Yang, H.J.; Park, S. The lignan-rich fractions of Fructus Schisandrae improve insulin sensitivity via the PPAR-γ pathways in in vitro and in vivo studies. J. Ethnopharmacol. 2011, 135, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.I.; Kim, D.H.; Yun, J.W. Extract of Chaga mushroom (Inonotus obliquus) stimulates 3T3-L1 adipocyte differentiation. Phytother. Res. 2010, 24, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Khushboo, P.S.; Jadhav, V.M.; Kadam, V.J.; Sathe, N.S. Psoralea corylifolia Linn.—“Kushtanashini”. Pharmacogn. Rev. 2010, 4, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, J.; Sharma, S.; Kumar, S. In vivo Anti-diabetic and anti-oxidant potential of Psoralea corylifolia seeds in Streptozotocin induced type-2 diabetic rats. J. Health Sci. 2011, 57, 225–235. [Google Scholar] [CrossRef]
- Seo, E.; Lee, E.K.; Lee, C.S.; Chun, K.H.; Lee, M.Y.; Jun, H.S. Psoralea corylifolia L. seed extract ameliorates streptozotocin-induced diabetes in mice by inhibition of oxidative stress. Oxid. Med. Cell. Longev. 2014, 2014, 897296. [Google Scholar] [CrossRef] [PubMed]
- Bera, T.K.; Ali, K.M.; Jana, K.; Ghosh, A.; Ghosh, D. Protective effect of aqueous extract of seed of Psoralea corylifolia (Somraji) and seed of Trigonella foenum-graecum L. (Methi) in streptozotocin-induced diabetic rat: A comparative evaluation. Pharmacogn. Res. 2013, 5, 277–285. [Google Scholar]
- Suhashini, R.; Sindhu, S.; Sagadevan, E. In vitro evaluation of anti diabetic potential and phytochemical profile of Psoralea corylifolia seeds. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 414–419. [Google Scholar]
- Krenisky, J.M.; Luo, J.; Reed, M.J.; Carney, J.R. Isolation and antihyperglycemic activity of bakuchiol from Otholobium pubescens (Fabaceae), a Peruvian medicinal plant used for the treatment of diabetes. Biol. Pharm. Bull. 1999, 22, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Cecilia, L.; Faini, F.; Joseph, C.; Hoshph, D.C. Bakuchiol derivatives from the leaves of Psoalea grandulosa. Phytochemistry 1996, 42, 1299–1303. [Google Scholar]
- Wang, D.; Li, F.; Jiang, Z. Osteoblastic proliferation stimulating activity of Psoralea corylifolia extracts and two of its flavonoids. Planta Med. 2001, 67, 748–749. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, H.; Goyal, R.K.; Cheema, S.K. Anti-diabetic activity of swertiamarin is due to an active metabolite, gentianine, that upregulates PPAR-γ gene expression in 3T3-L1 cells. Phytother. Res. 2013, 27, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Waki, H.; Murakami, K.; Motojima, K.; Komeda, K.; Ide, T.; Kubota, N.; Terauchi, Y.; Tobe, K.; et al. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor γ (PPARγ) deficiency and PPARγ agonist improve insulin resistance. J. Biol. Chem. 2001, 276, 41245–41254. [Google Scholar] [CrossRef] [PubMed]
- Chopra, B.; Dhingra, A.K.; Dhar, K.L. Psoralea corylifolia L. (Buguchi)—Folklore to modern evidence: Review. Fitoterapia 2013, 90, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Feng, L.; Yang, Z.; Shi, J.; Huang, C.; Guo, F.; Li, B.; Zhu, W.; Li, Y. Separation and peroxisome proliferator-activated receptor-γ agonist activity evaluation of synthetic racemic bavachinin enantiomers. Bioorg. Med. Chem. Lett. 2015, 25, 2579–2583. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.W.; Lee, O.H.; Banz, W.J.; Moustaid-Moussa, N.; Shay, N.F.; Kim, Y.C. Daidzein and the daidzein metabolite, equol, enhance adipocyte differentiation and PPARγ transcriptional activity. J. Nutr. Biochem. 2010, 21, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. CCAAT/enhancer-binding protein β is required for mitotic clonal expansion during adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Leonardini, A.; Laviola, L.; Perrini, S.; Natalicchio, A.; Giorgino, F. Cross-talk between PPARγ and insulin signaling and modulation of insulin sensitivity. PPAR Res. 2009, 2009, 818945. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.J.; Eckhardt, M.; Gagen, K.; Dong, M.; Chen, W.; Laurent, D.; Burkey, B.F. Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes 2001, 50, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Takamura, T.; Nohara, E.; Nagai, Y.; Kobayashi, K. Stage-specific effects of a thiazolidinedione on proliferation, differentiation and PPARγ mRNA expression in 3T3-L1 adipocytes. Eur. J. Pharmacol. 2001, 422, 23–29. [Google Scholar] [CrossRef]
- Skrzypski, M.; Kaczmarek, P.; Le, T.T.; Wojciechowicz, T.; Pruszynska-Oszmalek, E.; Szczepankiewicz, D.; Sassek, M.; Arafat, A.; Wiedenmann, B.; Nowak, K.W.; et al. Effects of orexin A on proliferation, survival, apoptosis and differentiation of 3T3-L1 preadipocytes into mature adipocytes. FEBS Lett. 2012, 586, 4157–4164. [Google Scholar] [CrossRef] [PubMed]
- Skrzypski, M.; Le, T.T.; Kaczmarek, P.; Pruszynska-Oszmalek, E.; Pietrzak, P.; Szczepankiewicz, D.; Kolodziejski, P.A.; Sassek, M.; Arafat, A.; Wiedenmann, B.; et al. Orexin A stimulates glucose uptake, lipid accumulation and adiponectin secretion from 3T3-L1 adipocytes and isolated primary rat adipocytes. Diabetologia 2011, 54, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Kopp, C.; Hosseini, A.; Singh, S.P.; Regenhard, P.; Khalilvandi-Behroozyar, H.; Sauerwein, H.; Mielenz, M. Nicotinic acid increases adiponectin secretion from differentiated bovine preadipocytes through G-protein coupled receptor signaling. Int. J. Mol. Sci. 2014, 15, 21401–21418. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, J.P.; Richards, A.A.; Hickman, I.J.; Macdonald, G.A.; Prins, J.B. Adiponectin—A key adipokine in the metabolic syndrome. Diabetes Obes. Metab. 2006, 8, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Litherland, G.J.; Hajduch, E.; Hundal, H.S. Intracellular signalling mechanisms regulating glucose transport in insulin-sensitive tissues (review). Mol. Membr. Biol. 2001, 18, 195–204. [Google Scholar] [PubMed]
- Sano, H.; Eguez, L.; Teruel, M.N.; Fukuda, M.; Chuang, T.D.; Chavez, J.A.; Lienhard, G.E.; McGraw, T.E. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 2007, 5, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Luo, N.; Klein, R.L.; Garvey, W.T. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid Res. 2005, 46, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Eguez, L.; Lee, A.; Chavez, J.A.; Miinea, C.P.; Kane, S.; Lienhard, G.E.; McGraw, T.E. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab. 2005, 2, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Ha do, T.; Trung, T.N.; Hien, T.T.; Dao, T.T.; Yim, N.; Ngoc, T.M.; Oh, W.K.; Bae, K. Selected compounds derived from Moutan Cortex stimulated glucose uptake and glycogen synthesis via AMPK activation in human HepG2 cells. J. Ethnopharmacol. 2010, 131, 417–424. [Google Scholar] [PubMed]
- Lee, M.S.; Hwang, J.T.; Kim, S.H.; Yoon, S.; Kim, M.S.; Yang, H.J.; Kwon, D.Y. Ginsenoside Rc, an active component of Panax ginseng, stimulates glucose uptake in C2C12 myotubes through an AMPK-dependent mechanism. J. Ethnopharmacol. 2010, 127, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L.; Russo, M.; Ungaro, P. AMP-activated protein kinase: A target for old drugs against diabetes and cancer. Biochem. Pharmacol. 2013, 86, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, M.; Giampieri, F.; Alvarez Suarez, J.M.; Mazzoni, L.; Forbes Hernandez, T.Y.; Quiles, J.L.; Bullon, P.; Battino, M. AMPK as a new attractive therapeutic target for disease prevention: The role of dietary compounds. Curr. Drug Targets 2015, 16. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.Y.; Ryu, J.H. Prenylflavones from Psoralea corylifolia inhibit nitric oxide synthase expression through the inhibition of I-κB-α degradation in activated microglial cells. Biol. Pharm. Bull. 2005, 28, 2253–2257. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Yun, B.R.; Kim, M.H.; Park, C.S.; Lee, W.S.; Oh, H.M.; Rho, M.C. Phenolic compounds isolated from Psoralea corylifolia inhibit IL-6-induced STAT3 activation. Planta Med. 2012, 78, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Ngadjui, B.T.; Watchueng, J.; Keumedjio, F.; Ngameni, B.; Simo, I.K.; Abegaz, B.M. Prenylated chalcones, flavone and other constituents of the twigs of Dorstenia angusticornis and Dorstenia barteri var. subtriangularis. Phytochemistry 2005, 66, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Lee, S.J.; Yoo, M.; Go, G.Y.; Lee da, Y.; Kim, Y.K.; Seo, D.W.; Kang, J.S.; Ryu, J.H.; Bae, G.U. Kazinol-P from Broussonetia kazinoki enhances skeletal muscle differentiation via p38MAPK and MyoD. Biochem. Biophys. Res. Commun. 2015, 456, 471–475. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Forward Primer (5–3) | Reverse Primer (5–3) | Accession Number |
|---|---|---|---|
| PPARγ2 | AACTCTGGGAGATTCTCCTGTTGA | GAAGTGCTCATAGGCAGTGCAT | EF062476 |
| C/EBPα | TGCTGGAGTTGACCAGTAC | AAACCATCCTCTGGGTCTCC | NM_001287523 |
| Adiponectin | TGTAGGATTGTCAGTGGATCTG | GCTCTTCAGTTGTAGTAACGTCATC | AY749429 |
| GLUT4 | GGGTCCTTACGTCTTCCTTCT | CCTCTGGTTTCAGGCACTTT | NM_009204 |
| GAPDH | TGCACCACCAACTGCTTAG | GGCATGGACTGTGGTCATGAG | BC096042 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Li, H.; Noh, M.; Ryu, J.-H. Bavachin from Psoralea corylifolia Improves Insulin-Dependent Glucose Uptake through Insulin Signaling and AMPK Activation in 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2016, 17, 527. https://doi.org/10.3390/ijms17040527
Lee H, Li H, Noh M, Ryu J-H. Bavachin from Psoralea corylifolia Improves Insulin-Dependent Glucose Uptake through Insulin Signaling and AMPK Activation in 3T3-L1 Adipocytes. International Journal of Molecular Sciences. 2016; 17(4):527. https://doi.org/10.3390/ijms17040527
Chicago/Turabian StyleLee, Hyejin, Hua Li, Minsoo Noh, and Jae-Ha Ryu. 2016. "Bavachin from Psoralea corylifolia Improves Insulin-Dependent Glucose Uptake through Insulin Signaling and AMPK Activation in 3T3-L1 Adipocytes" International Journal of Molecular Sciences 17, no. 4: 527. https://doi.org/10.3390/ijms17040527
APA StyleLee, H., Li, H., Noh, M., & Ryu, J.-H. (2016). Bavachin from Psoralea corylifolia Improves Insulin-Dependent Glucose Uptake through Insulin Signaling and AMPK Activation in 3T3-L1 Adipocytes. International Journal of Molecular Sciences, 17(4), 527. https://doi.org/10.3390/ijms17040527