Bird Integumentary Melanins: Biosynthesis, Forms, Function and Evolution
Abstract
:1. Introduction
2. Cell Biology of Bird Melanization: Avian Melanosomes
3. Feather Melanin Isolation and Methods for Structural Determination
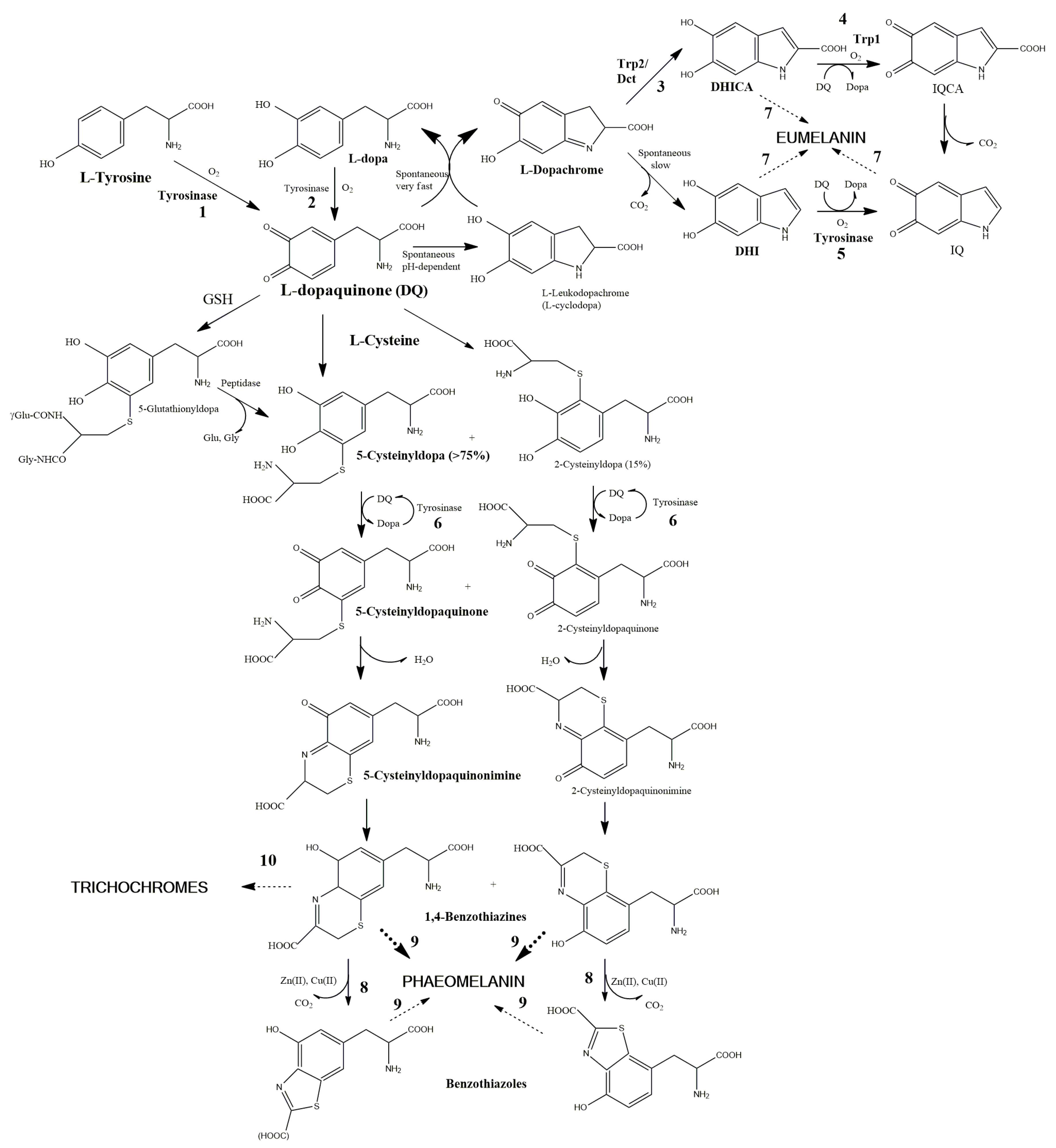
4. Melanin Synthesis Pathway with Special Reference to Avian Melanin
5. Types and Building Blocks in Bird Melanin
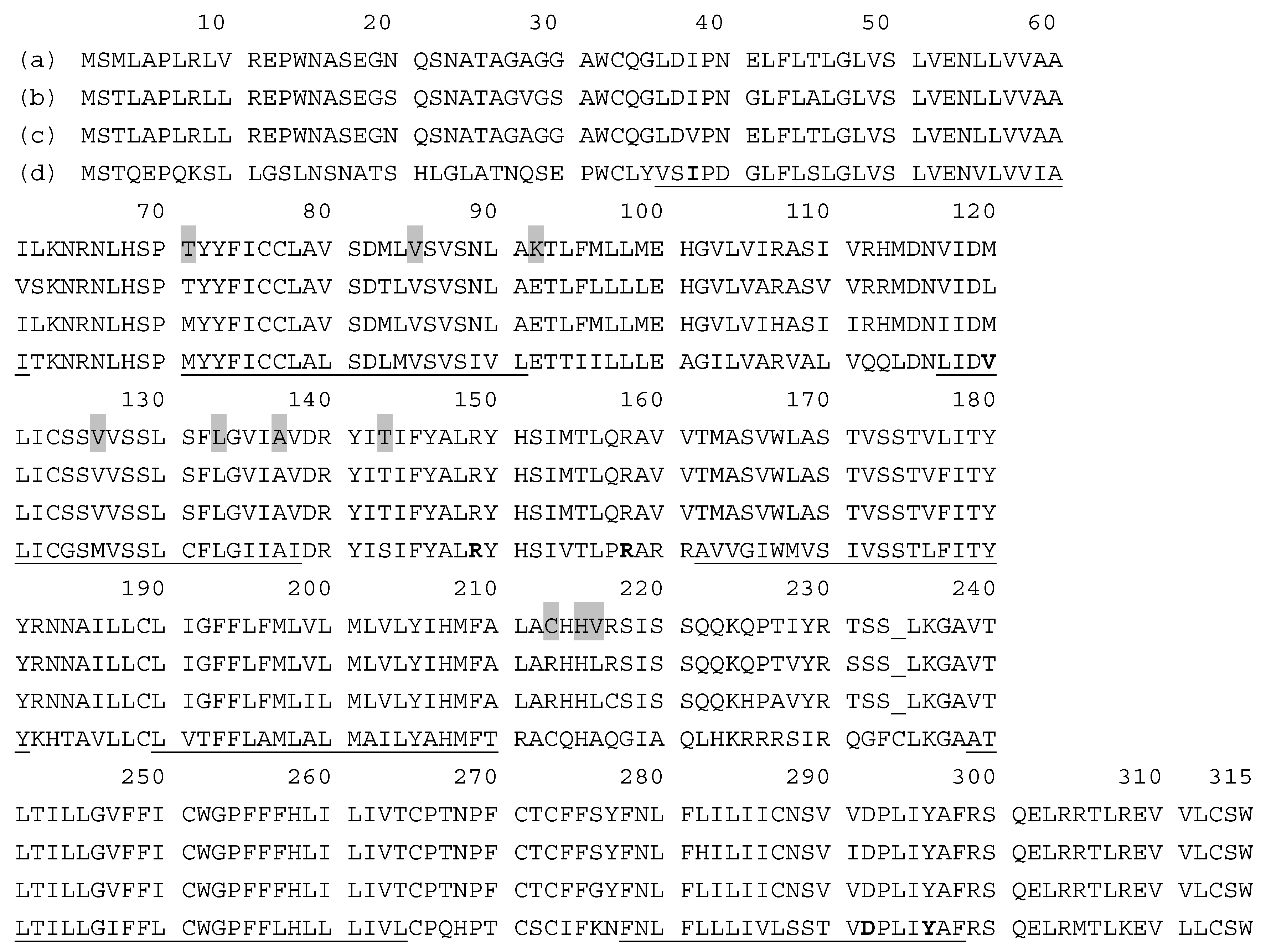
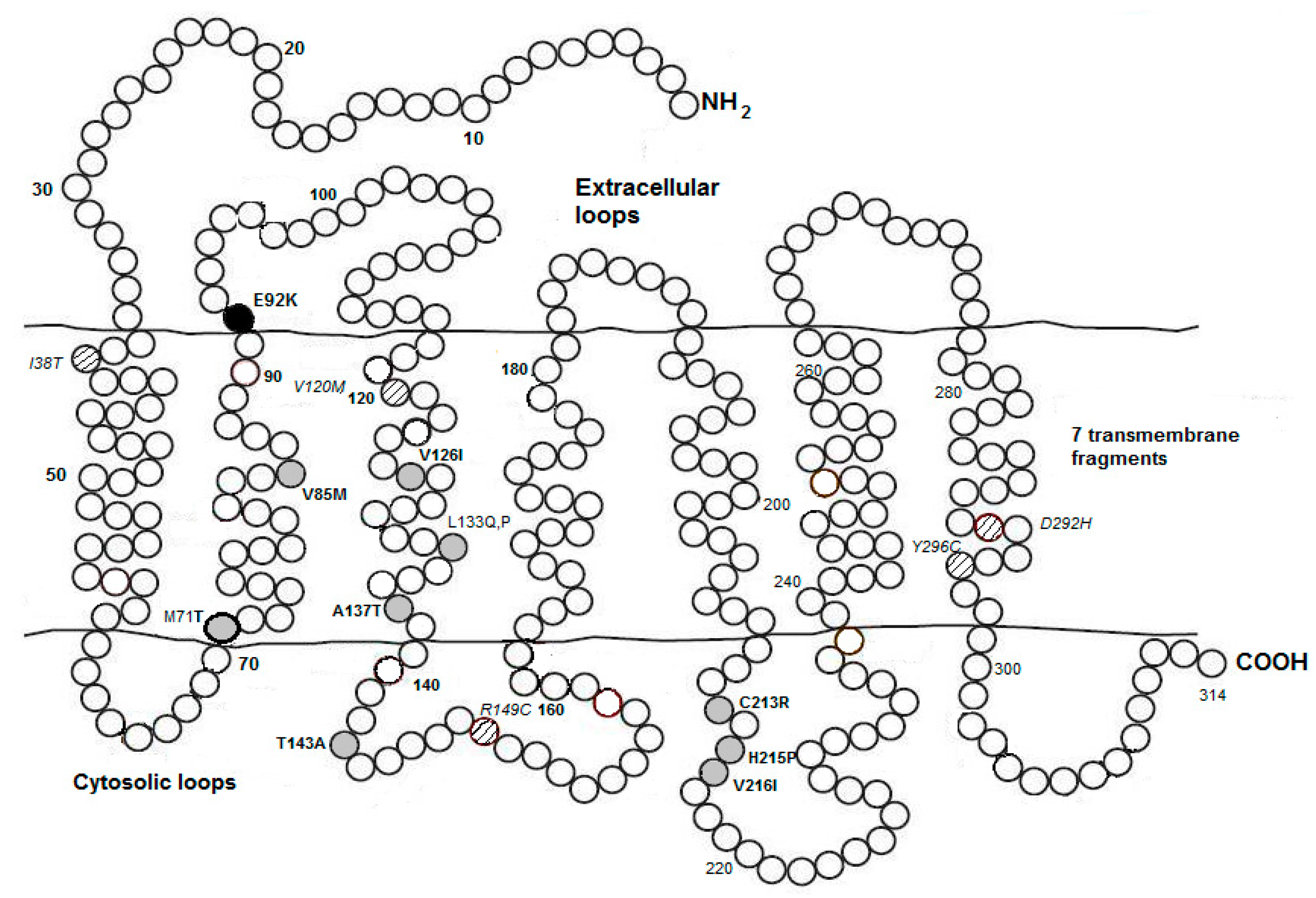
6. Environmental and Genetic Control on Avian Melanogenesis
7. Function and Evolution of Bird Melanins
7.1. Protection against UV Radiation
7.2. Protection against Mechanical Damage
7.3. Thermoregulation
7.4. Signaling
8. The Challenge of Pheomelanin Evolution
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Darwin, C. The Descent of Man, and Selection in Relation to Sex; Murray: London, UK, 1871. [Google Scholar]
- Cott, H.B. Adaptive Coloration in Animals; Methuen, Oxford University Press: London, UK, 1940. [Google Scholar]
- Hoekstra, H.E.; Hirschmann, R.J.; Bundey, R.A.; Insel, P.A.; Crossland, J.P. A single amino acid mutation contributes to adaptive beach mouse color pattern. Science 2006, 313, 101–104. [Google Scholar] [CrossRef] [PubMed]
- D’Alba, L.; Kieffer, L.; Shawkey, M.D. Relative contributions of pigments and biophotonic nanostructures to natural color production: A case study in budgerigar (Melopsittacus. undulatus) feathers. J. Exp. Biol. 2012, 215, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Negro, J.J.; Blasco, R.; Rosell, J.; Finlayson, C. Potential exploitation of avian resources by fossil hominins: An overview from ethnographic and historical data. Quat. Int. 2016. [Google Scholar] [CrossRef]
- McGraw, K.J. Mechanics of uncommon colors: Pterins, porphyrins, and psittacofulvins. In Bird Coloration; Hill, G.H., McGraw, K.J., Eds.; Harvard University Press: Harvard, MS, USA, 2006; Volume 1, pp. 354–398. [Google Scholar]
- Delhey, K. The colour of an avifauna: A quantitative analysis of the colour of Australian birds. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- McGraw, K.J.; Safra, R.J.; Evans, M.R.; Wakamatsu, K. European barn swallows use melanin pigments to color their feathers brown. Behav. Ecol. 2004, 15, 889–891. [Google Scholar]
- Solano, F. Melanins: Skin Pigments and Much More. Types, Structural Models, Biological Functions and Formation Routes. New J. Sci. 2014, 1, 1–28. [Google Scholar]
- Carr, J.G. Internal structure of avian melanin granules: An electron microscope study. J. Cell. Sci. 1957, 98, 159–162. [Google Scholar]
- Brumbaugh, J.A. Ultrastructure differences between forming eumelanin and pheomelanin as revealed by the pink-eye mutation in the fowl. Dev. Biol. 1968, 18, 375–390. [Google Scholar] [CrossRef]
- Brumbaugh, J.A.; Lee, K.W. Types of genetic mechanisms controlling melanogenesis in the fowl. Pigment Cell 1976, 3, 165–176. [Google Scholar]
- Maul, G.G.; Brumbaugh, J.A. On the possible function of coated vesicles in melanogenesis of the regenerating fowl feather. J. Cell Biol. 1971, 48, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Jimbow, K.; Takeuchi, T. Ultrastructural comparison of pheo- and eumelanogenesis in animals. Pigment Cell 1979, 4, 308–317. [Google Scholar]
- Tarafder, A.K.; Bolasco, G.; Correia, M.S.; Pereira, F.J.; Iannone, L.; Hume, A.N.; Kirkpatrick, N.; Picardo, M.; Torrisi, M.R.; Rodrigues, I.P.; et al. Rab11b mediates melanin transfer between donor melanocytes and acceptor keratinocytes via coupled exo/endocytosis. J. Investig. Dermatol. 2014, 134, 1056–1066. [Google Scholar] [PubMed]
- Lin, S.J.; Foley, J.; Jiang, T.X.; Yeh, C.Y.; Wu, P.; Foley, A.; Yen, C.M.; Huang, Y.C.; Cheng, H.C.; Chen, C.F.; et al. Topology of feather melanocyte progenitor niche allows complex pigment patterns to emerge. Science 2013, 340, 1442–1445. [Google Scholar]
- Zhang, F.; Kearns, S.L.; Orr, P.J.; Benton, M.J.; Zhou, Z.; Johnson, D.; Xu, X.; Wang, X. Fossilized melanosomes and the colour of Cretaceous dinosaurs and birds. Nature 2010, 463, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Wogelius, R.A.; Manning, P.L.; Barden, H.E.; Edwards, N.P.; Webb, S.M.; Sellers, W.I.; Taylor, K.G.; Larson, P.L.; Dodson, P.; You, H.; et al. Trace metals as biomarkers for eumelanin pigment in the fossil record. Science 2011, 333, 1622–1626. [Google Scholar] [PubMed]
- Liu, Y.; Hong, L.; Wakamatsu, K.; Ito, S.; Adhyaru, B.; Cheng, C.Y.; Bowers, C.R.; Simon, J.D. Comparison of structural and chemical properties of black and red human hair melanosomes. Photochem. Photobiol. 2005, 81, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Colleary, C.; Dolocan, A.; Gardner, J.; Singh, S.; Wuttke, M.; Rabenstein, R.; Habersetzer, J.; Schaal, S.; Feseha, M.; Clemens, M.; et al. Chemical, experimental, and morphological evidence for diagenetically altered melanin in exceptionally preserved fossils. Proc. Natl. Acad. Sci. USA 2015, 112, 12592–12597. [Google Scholar] [PubMed]
- Clarke, J.A.; Ksepka, D.T.; Salas-Gismondi, R.; Altamirano, A.J.; Shawkey, M.D.; d’Alba, L.; Vinther, J.; DeVries, T.J.; Baby, P. Fossil evidence for evolution of the shape and color of penguin feathers. Science 2010, 330, 954–957. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gao, K.Q.; Vinther, J.; Shawkey, M.D.; Clarke, J.A.; d’alba, L.; Meng, Q.; Briggs, D.E.; Prum, R.O. Plumage color patterns of an extinct dinosaur. Science 2010, 327, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Clarke, J.A.; Gao, K.Q.; Zhou, C.F.; Meng, Q.; Li, D.; D’Alba, L.; Shawkey, M.D. Melanosome evolution indicates a key physiological shift within feathered dinosaurs. Nature 2014, 507, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Carney, R.M.; Vinther, J.; Shawkey, M.D.; D’Alba, L.; Ackermann, J. New evidence on the colour and nature of the isolated Archaeopteryx feather. Nat. Commun. 2012, 3, 637. [Google Scholar] [CrossRef] [PubMed]
- Vinther, J. Fossil melanosomes or bacteria? A wealth of findings favours melanosomes. BioEssays 2016, 38, 220–225. [Google Scholar] [PubMed]
- McGraw, K.J.; Wakamatsu, K.; Ito, S.; Nolan, P.M.; Jouventin, P.; Dobson, F.S.; Austic, R.E.; Safran, R.J.; Siefferman, L.M.; Hill, G.E.; et al. You can’t judge a pigment by its color: Carotenoids and melanin content of yellow and brown feathers in swallows, bluebirds, penguins, and domestic chickens. Condor 2004, 106, 390–395. [Google Scholar]
- Ito, S.; Fujita, K. Microanalysis of eumelanin and pheomelanin in hair and melanomas by chemical degradation and liquid chromatography. Anal. Biochem. 1985, 144, 527–536. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: A comparative review. Pigment Cell Res. 2003, 16, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K.; d’Ischia, M.; Napolitano, A.; Pezzella, A. Structure of melanins. In Melanins and Melanosomes: Biosynthesis, Structure, Physiological and Pathological Functions; Borovansky, J., Riley, P.A., Eds.; Willey-Blackwell: Weinheim, Germany, 2011; pp. 167–185. [Google Scholar]
- Ito, S.; Wakamatsu, K.; Glass, K.; Simon, J.D. High-performance liquid chromatography estimation of cross-linking of dihydroxyindole moiety in eumelanin. Anal. Biochem. 2013, 434, 221–225. [Google Scholar] [CrossRef] [PubMed]
- D’Ischia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J.C.; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; et al. Melanins and melanogenesis: Methods, standards, protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar] [PubMed]
- Liu, S.Y.; Shawkey, M.D.; Parkinson, D.; Tyler, P.; Troy, T.P.; Ahmed, M. Elucidation of the chemical composition of avian melanin. RSC Adv. 2014, 4, 40396–40399. [Google Scholar] [CrossRef]
- Dernroth, D.N.; Rundström, A.A.; Kågedal, B. Gas chromatography–mass spectrometry analysis of pheomelanin degradation products. J. Chromatog. A 2009, 1216, 5730–5739. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.R.; Jiang, B.; Zheng, J.X.; Xu, G.Y.; Li, J.Y.; Yang, N. Isolation and characterization of natural melanin derived from silky fowl (Gallus gallus domesticus Brisson). Food Chem. 2008, 111, 745–749. [Google Scholar] [CrossRef]
- Galván, I.; Solano, F. Melanin chemistry and the ecology of stress. Physiol. Biochem. Zool. 2015, 88, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Galván, I.; Jorge, A. Dispersive Raman spectroscopy allows the identification and quantification of melanin types. Ecol. Evol. 2015, 5, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Raper, H.S. The aerobic oxidases. Physiol. Rev. 1928, 8, 245–282. [Google Scholar]
- Mason, H.S. The chemistry of melanin III. Mechanism of the oxidation of dihydroxyphenylalanine by tyrosinase. J. Biol. Chem. 1948, 172, 83–99. [Google Scholar] [PubMed]
- Prota, G.; Nicolaus, R.A. On the biogenesis of phaeomelanins. In The Pigmentary System; Montagna, W., Flu, F., Eds.; Pergamon Press: New York, NY, USA, 1967; Volume 8, pp. 323–328. [Google Scholar]
- Agrup, G.; Falck, B.; Kennedy, B.M.; Rorsman, H.; Rosengren, A.M.; Rosengren, E. Formation of cysteinyldopa from glutathionyldopa in melanoma. Acta Dermatol. Venereol. (Stockholm) 1975, 55, 1–3. [Google Scholar]
- Rorsman, H.; Agrup, G.; Hansson, C.; Rosengren, A.M.; Rosengren, E. Detection of Phaeomelanins. Pigment Cell 1979, 4, 244–252. [Google Scholar]
- Olivares, C.; Solano, F. New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins. Pigment Cell Melanoma Res. 2009, 22, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Chemistry of mixed melanogenesis—Pivotal roles of dopaquinone. Photochem. Photobiol. 2008, 84, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Ancans, J.; Tobin, D.J.; Hoogduijn, M.J.; Smit, N.P.; Wakamatsu, K.; Thody, A.J. Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells. Exp. Cell Res. 2001, 268, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Aroca, P.; García-Borrón, J.C.; Solano, F.; Lozano, J.A. Regulation of distal mammalian melanogenesis. I: Partial purification and characterization of a dopachrome converting factor: Dopachrome tautomerase. Biochim. Biophys. Acta 1990, 1035, 266–275. [Google Scholar] [PubMed]
- Aroca, P.; Solano, F.; García-Borrón, J.C.; Salinas, C.; Lozano, J.A. Regulation of the final phase of mammalian melanogenesis. Eur. J. Biochem. 1992, 208, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Solano, F.; Martínez-Liarte, J.H.; Jiménez-Cervantes, C.; García-Borrón, J.C.; Lozano, J.A. Dopachrome tautomerase is a zinc-containing enzyme. Biochem. Biophys. Res. Commun. 1994, 204, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Solano, F.; Misuraca, G.; Aroca, P.; Garcia-Borron, J.C.; Lozano, J.A.; Prota, G. Comparative action of dopachrome tautomerase and metal ions on the rearrangement of dopachrome. Biochim. Biophys. Acta 1991, 1115, 1–5. [Google Scholar] [CrossRef]
- Nadeau, N.J.; Burke, T.; Mundy, N.I. Evolution of an avian pigmentation gene correlates with a measure of sexual selection. Proc. R. Soc. B 2007, 274, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Cervantes, C.; Solano, F.; Kobayashi, T.; Urabe, K.; Hearing, V.; Lozano, J.A.; García-Borrón, J.C. A new enzymatic function in the melanogenic pathway. The 5,6-dihydroxyindole-2-carboxylic acid oxidase activity of tyrosinase related protein-1. J. Biol. Chem. 1994, 269, 17993–18001. [Google Scholar] [PubMed]
- Olivares, C.; Jimenez-Cervantes, C.; Lozano, J.A.; Solano, F.; Garcia-Borron, J.C. The 5,6-dihydroxyindole-2-carboxylic acid (DHICA) oxidase activity of human tyrosinase. Biochem. J. 2001, 354, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Jara, J.R.; Aroca, P.; Solano, F.; Martínez, J.H.; Lozano, J.A. The role of sulfhydryl compounds in mammalian melanogenesis: The effect of cysteine and glutathione upon tyrosinase and the intermediates of the pathway. Biochim. Biophys. Acta 1988, 967, 296–303. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Ohtara, K.; Ito, S. Chemical analysis of late stages of pheomelanogenesis, conversion of dihydrobenzothiazine to a benzothiazole structure. Pigment Cell Melanoma Res. 2009, 22, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Nakanishi, Y.; Valenzuela, R.K.; Brilliant, M.H.; Kolbe, L.; Wakamatsu, K. Usefulness of alkaline hydrogen peroxide oxidation to analyze eumelanin and pheomelanin in various tissue samples: Application to chemical analysis of human hair melanins. Pigment Cell Melanoma Res. 2011, 24, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Sato, C.; Ito, S.; Takeuchi, T. Enhancement of pheomelanogenesis by l-dopa in the mouse melanocyte cell line, TM10, in vitro. J. Cell Sci. 1987, 87, 507–512. [Google Scholar] [PubMed]
- Napolitano, A.; Costantini, C.; Crescenzi, O.; Prota, G. Characterisation of 1,4-benzothiazine intermediates in the oxidative conversion of 5-S-cysteinyldopa to pheomelanins. Tetrahedron Lett. 1994, 35, 6365–6368. [Google Scholar] [CrossRef]
- Napolitano, A.; di Donato, P.; Prota, G.; Land, E.J. Transient quinonimines and 1,4-benzothiazines of pheomelanogenesis: New pulse radiolytic and spectrophotometric evidence. Free Radic. Biol. Med. 1999, 27, 521–528. [Google Scholar] [CrossRef]
- Napolitano, A.; di Donato, P.; Prota, G. New regulatory mechanisms in the biosynthesis of pheomelanins: Rearrangement vs. redox exchange reaction routes of a transient 2H-1,4-benzothiazine-o-quinonimine intermediate. Biochim. Biophys. Acta 2000, 1475, 47–54. [Google Scholar] [PubMed]
- Napolitano, A.; Vicensi, M.R.; d’Ischia, M.; Prota, G. A new benzothiazole derivative by degradation of pheomelanins with alkaline hydrogen peroxide. Tetrahedron Lett. 1996, 37, 6799–6802. [Google Scholar] [CrossRef]
- Tesema, Y.T.; Pham, D.M.; Franz, K.J. Counterions Influence Reactivity of Metal Ions with Cysteinyldopa Model Compounds. Inorg. Chem. 2008, 47, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Greco, G.; Panzella, L.; Verotta, L.; d’Ischia, M.; Napolitano, A. Uncovering the structure of human red hair pheomelanin: Benzothiazolylthiazinodihydroisoquinolines as key building blocks. J. Nat. Prod. 2011, 74, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, P.; Napolitano, A.; Prota, G. Metal ions as potential regulatory factors in the biosynthesis of red hair pigments: A new benzothiazole intermediate in the iron or copper assisted oxidation of 5-S-cysteinyldopa. Biochim. Biophys. Acta 2002, 1571, 157–166. [Google Scholar] [CrossRef]
- Napolitano, A.; Panzella, L.; Leone, L.; d’Ischia, M. Red hair benzothiazines and benzothiazoles: Mutation-inspired chemistry in the quest for functionality. Acc. Chem. Res. 2013, 46, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; di Donato, P.; Prota, G. Zinc-catalyzed oxidation of 5-S-Cysteinyldopa to 2,2′-Bi(2H-1,4-benzothiazine): Tracking the biosynthetic pathway of trichochromes, the characteristic pigments of red hair. J. Org. Chem. 2001, 66, 6958–6966. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Wang, Y.; Chan, H.W.; Wang, L.; Chan, W. Mass spectrometric and spectrophotometric analyses reveal an alternative structure and a new formation mechanism for melanin. Anal. Chem. 2015, 87, 7958–7963. [Google Scholar] [CrossRef] [PubMed]
- Galván, I.; Wakamatsu, K.; Alonso-Alvarez, C.; Solano, F. Buthionine sulfoximine diverts the melanogenesis pathway toward the production of more soluble and degradable pigments. Bioorg. Med. Chem. Lett. 2014, 24, 2150–2154. [Google Scholar] [CrossRef] [PubMed]
- Hudon, J. Considerations in the conservation of feathers and hair, particularly their pigments. In Proceedings of the CAC/ACCR 31st Annual Conference, Jasper, AB, Canada, May 2005; pp. 127–147.
- Prum, R.O. Anatomy, physics, and evolution of avian structural colors. In Bird Coloration; Hill, G.E., McGraw, K.J., Eds.; Harvard University Press: Cambridge, MA, USA, 2006; Volume 1, pp. 295–353. [Google Scholar]
- García-Borrón, J.C.; Saura, M.D.; Solano, F.; Iborra, J.L.; Lozano, J.A. Water and protein interactions with model melanins. In Biological, Molecular and Clinical Aspects of Pigmentation; Bagnara, J., Klaus, S.N., Paul, E., Schartl, M., Eds.; University of Tokyo Press: Tokyo, Japan, 1984; pp. 91–95. [Google Scholar]
- Salinas, C.; García-Borrón, J.C.; Solano, F.; Lozano, J.A. Dopachrome tautomerase decreases the binding of indolic melanogenesis intermediates to proteins. Biochim. Biophys. Acta 1994, 1204, 53–60. [Google Scholar] [CrossRef]
- Poston, J.P.; Hasselquist, D.; Stewart, I.R.K.; Westneat, D.F. Dietary amino acids influence plumage traits and immune responses of male house sparrows, Passer domesticus, but not as expected. Anim. Behav. 2005, 70, 1171–1181. [Google Scholar] [CrossRef]
- Galván, I.; Bijlsma, R.G.; Negro, J.J.; Jarén, M.; Garrido-Fernández, J. Environmental constraints for plumage melanization in the northern goshawk Accipiter gentilis. J. Avian Biol. 2010, 41, 523–531. [Google Scholar] [CrossRef]
- Galván, I.; Alonso-Alvarez, C. The expression of melanin-based plumage is separately modulated by exogenous oxidative stress and a melanocortin. Proc. R. Soc. Lond. B: Biol. Sci. 2009, 276, 3089–3097. [Google Scholar] [CrossRef] [PubMed]
- Galván, I.; Solano, F. The evolution of eu- and pheomelanic traits may respond to an economy of pigments related to environmental oxidative stress. Pigment Cell Melanoma Res. 2009, 22, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Chintala, S.; Li, W.; Lamoreux, M.L.; Ito, S.; Wakamatsu, K.; Sviderskaya, E.V.; Bennett, D.C.; Park, Y.M.; Gahl, W.A.; Huizing, M.; et al. Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc. Natl. Acad. Sci. USA 2005, 102, 10964–10969. [Google Scholar] [PubMed]
- Van Grouw, H. What colour is that bird? The causes and recognition of common colour aberrations in birds. Brit. Birds 2013, 106, 17–29. [Google Scholar]
- Nadeau, N.J.; Minvielle, F.; Ito, S.; Inoue-Murayama, M.; Gourichon, D.; Follett, S.A.; Burke, T.; Mundy, N.I. Characterization of Japanese quail yellow as a genomic deletion upstream of the avian homolog of the mammalian ASIP (agouti) gene. Genetics 2008, 178, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Mundy, N.I. A window on the genetics of evolution: MC1R and plumage colouration in birds. Proc. R. Soc. Lond. B: Biol. Sci. 2005, 272, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Suzuki, H.; Yabuuchi, M.; Takahashi, S. A possible involvement of melanocortin 1-receptor in regulating feather color pigmentation in the chicken. Biochim. Biophys. Acta 1996, 1308, 164–168. [Google Scholar] [CrossRef]
- Theron, E.; Hawkins, K.; Bermingham, E.; Ricklefs, R.E.; Mundy, N.I. The molecular basis of an avian plumage polymorphism in the wild: A melanocortin-1-receptor point mutation is perfectly associated with the melanic plumage morph of the bananaquit, Coereba flaveola. Curr. Biol. 2001, 11, 550–557. [Google Scholar] [CrossRef]
- Guernsey, M.W.; Ritscher, L.; Miller, M.A.; Smith, D.A.; Schöneberg, T.; Shapiro, M.D. A Val85Met mutation in melanocortin-1 receptor is associated with reductions in eumelanic pigmentation and cell surface expression in domestic rock pigeons (Columba livia). PLoS ONE 2013, 8, e74475. [Google Scholar] [CrossRef] [PubMed]
- San-José, L.M.; Ducrest, A.L.; Ducret, V.; Béziers, P.; Simon, C.; Wakamatsu, K.; Roulin, A. Effect of the MC1R gene on sexual dimorphism in melanin-based colorations. Mol. Ecol. 2015, 24, 2794–2808. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.J.; Dixon, A.; Fox, N.C.; Bruford, M.W. Missense SNP of the MC1R gene is associated with plumage variation in the Gyrfalcon (Falco rusticolus). Anim. Genet. 2012, 43, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Dávila, S.G.; Gil, M.G.; Resino-Talaván, P.; Campo, J.L. Association between polymorphism in the melanocortin 1 receptor gene and E locus plumage color phenotype. Poult. Sci. 2014, 3, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Esparza, M.; Jiménez-Cervantes, C.; Bennett, D.C.; Lozano, J.A.; Solano, F.; García-Borrón, J.C. The murine silver locus: Coding and expression of a single transcript truncated by the silver mutation. Mamm. Genome 1999, 10, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Mochii, M.; Agata, M.; Eguchi, G. Complete sequence and expression of a cDNA encoding a chicken 115-kda melanosomal matrix protein. Pigment Cell Res. 1991, 4, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Theos, A.C.; Truschel, S.T.; Raposo, G.; Marks, M.S. The Silver locus product Pmel17/gp100/Silv/ME20: Controversial in name and in function. Pigment Cell Melanoma Res. 2005, 18, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Kerje, S.; Sharma, P.; Gunnarsson, U.; Kim, H.; Bagchi, S.; Fredriksson, R.; Schütz, K.; Jensen, P.; von Heijne, G.; Okimoto, R.; et al. The Dominant white, Dun and Smoky color variants in chicken are associated with insertion/deletion polymorphisms in the PMEL17 gene. Genetics 2004, 168, 1507–1518. [Google Scholar] [PubMed]
- Keeling, L.; Andersson, L.; Schütz, K.E.; Kerje, S.; Fredriksson, R.; Carlborg, O.; Cornwallis, C.K.; Pizzari, T.; Jensen, P. Chicken genomics: Feather-pecking and victim pigmentation. Nature 2004, 431, 645–646. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Brumbaugh, J.A. Purification and isoelectric heterogeneity of chicken tyrosinase. Biochim. Biophys. Acta 1984, 800, 282–290. [Google Scholar] [CrossRef]
- April, C.S.; Jackson, I.J.; Kidson, S.H. Molecular cloning and sequence analysis of a chicken cDNA encoding tyrosinase-related protein-2/DOPAchrome tautomerase. Gene 1998, 219, 45–53. [Google Scholar] [CrossRef]
- April, C.S.; Jackson, I.J.; Kidson, S.H. The cloning and sequencing of a cDNA coding for chick tyrosinase-related protein-1. Biochim. Biophys. Acta 1998, 1395, 7–12. [Google Scholar] [CrossRef]
- Liu, W.B.; Chen, S.R.; Zheng, J.X.; Qu, L.J.; Xu, G.Y.; Yang, N. Developmental phenotypic-genotypic associations of tyrosinase and melanocortin 1 receptor genes with changing profiles in chicken plumage pigmentation. Poult. Sci. 2010, 89, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Domyan, E.T.; Guernsey, M.W.; Kronenberg, Z.; Krishnan, S.; Boissy, R.E.; Vickrey, A.I.; Rodgers, C.; Cassidy, P.; Leachman, S.A.; Fondon, J.W.; et al. Epistatic and combinatorial effects of pigmentary gene mutations in the domestic pigeon. Curr. Biol. 2014, 24, 459–464. [Google Scholar]
- Wang, Y.; Li, S.M.; Huang, J.; Chen, S.Y.; Liu, Y.P. Mutations of TYR and MITF genes are associated with plumage colour phenotypes in geese. Asian Austral. J. Anim. Sci. 2014, 27, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, X.H.; Pang, Y.Z. Association of tyrosinase (TYR) and tyrosinase related protein 1 (TYRP1) with melanic plumage color in Korean quails (Coturnix. coturnix). Asian Austral. J. Anim. Sci. 2013, 26, 1518–1522. [Google Scholar] [CrossRef] [PubMed]
- Sultana, H.; Seo, D.W.; Park, H.B.; Cahyadi, M.; Jin, S.; Hoque, R.; Kim, Y.S.; Heo, K.N.; Jo, C.; Gotoh, T.; Lee, J.H. Identification of polymorphisms in plumage color related genes in Korean native ducks. J. Fac. Agric. Kyushu Univ. 2015, 60, 119–126. [Google Scholar]
- Chiaverini, C.; Sillard, L.; Flori, E.; Ito, S.; Briganti, S.; Wakamatsu, K.; Fontas, E.; Berard, E.; Cailliez, M.; Cochat, P.; et al. Cystinosin is a melanosomal protein that regulates melanin synthesis. FASEB J. 2012, 26, 3779–3789. [Google Scholar] [PubMed]
- Liu, X.F.; Luo, J.; Hu, X.X.; Yang, H.; Lv, X.Q.; Feng, C.G.; Tong, J.; Wang, Y.Q.; Wang, S.H.; Liu, X.J.; et al. Repression of Slc24a5 can reduce pigmentation in chicken. Front. Biosci. 2011, 3, 158–165. [Google Scholar]
- Straniero, L.; Rimoldi, V.; Solda, G.; Mauri, L.; Manfredini, E.; AndreuccI, E.; Bargiacchi, S.; Penco, S.; Giovanni, P.; Gesu, G.P.; et al. Two novel splicing mutations in the SLC45A2 gene cause Oculocutaneous Albinism Type IV by unmasking cryptic splice sites. J. Hum. Gen. 2015, 60, 467–471. [Google Scholar]
- Gunnarsson, U.; Hellström, A.R.; Tixier-Boichard, M.; Minvielle, F.; Bed’Hom, B.; Ito, S.; Jensen, P.; Rattink, A.; Vereijken, A.; Andersson, L. Mutations in SLC45A2 cause plumage color variation in chicken and Japanese quail. Genetics 2007, 175, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.; Schwabl, H. Yolk testosterone organizes behavior and male plumage coloration in house sparrows (Passer domesticus). Behav. Ecol. Sociobiol. 2004, 56, 491–497. [Google Scholar] [CrossRef]
- Eising, C.M.; Müller, W.; Groothuis, T.G.G. Avian mothers create different phenotypes by hormone deposition in their eggs. Biol. Lett. 2006, 2, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Galván, I.; Alonso-Alvarez, C. Yolk testosterone shapes the expression of a melanin-based signal in great tits: an antioxidant-mediated mechanism? J. Exp. Biol. 2010, 213, 3127–3130. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.; Eens, M. Elevated yolk androgen levels and the expression of multiple sexually selected male characters. Horm. Behav. 2009, 55, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F. Effect of selenium and vitamin E content of the maternal diet on the antioxidant system of the yolk and the developing chick. Br. Poult. Sci. 2000, 41, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Roulin, A.; Dijkstra, C. Genetic and environmental components of variation in eumelanin and phaeomelanin sex-traits in the barn owl. Heredity 2003, 90, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, G.R. Natural selection and coloration: Protection, concealment, advertisement, or deception? In Bird Coloration; Hill, G.E., McGraw, K.J., Eds.; Harvard University Press: Cambridge, MS, USA, 2006; Volumn 2, pp. 3–35. [Google Scholar]
- Dillberger, J.E.; Citino, S.B.; Altman, N.H. Four cases of neoplasia in captive wild birds. Avian Dis. 1987, 31, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Moses, D.N.; Mattoni, M.A.; Slack, N.L.; Waite, J.H.; Zok, F.W. Role of melanin in mechanical properties of Glycera jaws. Acta Biomater. 2006, 2, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Bonser, R.H.C. Melanin and the abrasion resistance of feathers. Condor 1995, 97, 590–591. [Google Scholar]
- Schreiber, R.W.; Schreiber, E.; Peele, A.M.; Burtt, E.H., Jr. Pattern of damage to albino Great Frigatebird flight feathers supports hypothesis of abrasion by airborne particles. Condor 2006, 108, 736–741. [Google Scholar] [CrossRef]
- Pannkuk, E.L.; Siefferman, L.M.; Butts, J.A. Colour phases of the eastern screech owl: A comparison of biomechanical variables of body contour feathers. Funct. Ecol. 2010, 24, 347–353. [Google Scholar] [CrossRef]
- Burtt, E.H., Jr.; Ichida, J.M. Occurrence of feather-degrading bacilli in the plumage of birds. Auk 1999, 116, 364–372. [Google Scholar] [CrossRef]
- Mackintosh, J.A. The antimicrobial properties of melanocytes, melanosomes and melanin and the evolution of black skin. J. Theor. Biol. 2001, 211, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Grande, J.M.; Negro, J.J.; Torres, M.J. The evolution of bird plumage colouration: A role for feather-degrading bacteria? Ardeola 2004, 51, 375–383. [Google Scholar]
- Gunderson, A.R.; Frame, A.M.; Swaddle, J.P.; Forsyth, M.H. Resistance of melanized feathers to bacterial degradation: Is it really so black and white? J. Avian Biol. 2008, 39, 539–545. [Google Scholar] [CrossRef]
- Bush, S.E.; Kim, D.; Moyer, B.R.; Lever, J.; Clayton, D.H. Is melanin a defense against feather-feeding lice? Auk 2006, 123, 153–161. [Google Scholar] [CrossRef]
- Vágási, C.I. The origin of feather holes: A word of caution. J. Avian Biol. 2014, 45, 431–436. [Google Scholar] [CrossRef]
- Clusella-Trullas, S.; van Wyk, J.H.; Spotila, J.R. Thermal melanism in ectotherms. J. Therm. Biol. 2007, 32, 235–245. [Google Scholar] [CrossRef]
- Bech, C.; Præsteng, K.E. Thermoregulatory use of heat increment of feeding in the tawny owl (Strix. aluco). J. Therm. Biol. 2004, 29, 649–654. [Google Scholar] [CrossRef]
- Margalida, A.; Negro, J.J.; Galván, I. Melanin-based color variation in the bearded vulture suggests a thermoregulatory function. Comp. Biochem. Physiol. A 2008, 149, 87–91. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Negro, J.J.; Sarasola, J.H.; Fariñas, F.; Zorrilla, I. Function and occurrence of facial flushing in birds. Comp. Biochem. Physiol. A 2006, 143, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Bamford, A.J.; Monadjem, A.; Hardy, I.C. Associations of avian facial flushing and skin colouration with agonistic interaction outcomes. Ethology 2010, 116, 1163–1170. [Google Scholar] [CrossRef]
- Koskenpato, K.; Ahola, K.; Karstinen, T.; Karell, P. Is the denser contour feather structure in pale grey than in pheomelanic brown tawny owls Strix aluco an adaptation to cold environments? J. Avian Biol. 2016, 47, 1–6. [Google Scholar] [CrossRef]
- Karell, P.; Ahola, K.; Karstinen, T.; Valkama, J.; Brommer, J.E. Climate change drives microevolution in a wild bird. Nat. Commun. 2011, 2, 208. [Google Scholar] [CrossRef] [PubMed]
- Gümüşlü, S.; Sarikçioğlu, S.B.; Sahin, E.; Yargiçoğlu, P.; Ağar, A. Influences of different stress models on the antioxidant status and lipid peroxidation in rat erythrocytes. Free Radic. Res. 2002, 36, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Caro, T.I.M. The adaptive significance of coloration in mammals. BioScience 2005, 55, 125–136. [Google Scholar] [CrossRef]
- Searcy, W.A.; Nowicki, S. The Evolution of Animal Communication: Reliability and Deception in Signaling Systems; Princeton University Press: Princeton, NJ, USA, 2006. [Google Scholar]
- Hasson, O. Towards a general theory of biological signaling. J. Theor. Biol. 1997, 185, 139–156. [Google Scholar] [CrossRef] [PubMed]
- McGraw, K.J. An update on the honesty of melanin-based color signals in birds. Pigment Cell Melanoma Res. 2008, 21, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Guindre-Parker, S.; Love, O.P. Revisiting the condition-dependence of melanin-based plumage. J. Avian Biol. 2014, 45, 29–33. [Google Scholar] [CrossRef]
- Zahavi, A. Mate selection-a selection for a handicap. J. Theor. Biol. 1975, 53, 205–214. [Google Scholar] [CrossRef]
- Chaine, A.S.; Lyon, B.E. Adaptive plasticity in female mate choice dampens sexual selection on male ornaments in the lark bunting. Science 2008, 319, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Galván, I.; Sanz, J.J. The cheek plumage patch is an amplifier of dominance in great tits. Biol. Lett. 2008, 4, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Galván, I.; Sanz, J.J. Cheek plumage uniformity as a social status signal in great tits. Ann. Zool. Fenn. 2009, 46, 271–282. [Google Scholar] [CrossRef]
- Galván, I. The importance of white on black: Unmelanized plumage proportion predicts display complexity in birds. Behav. Ecol. Sociobiol. 2008, 63, 303–311. [Google Scholar] [CrossRef]
- Fargallo, J.A.; Velando, A.; López-Rull, I.; Gañán, N.; Lifshitz, N.; Wakamatsu, K.; Torres, R. Sex-specific phenotypic integration: Endocrine profiles, coloration, and behavior in fledgling boobies. Behav. Ecol. 2014, 25, 76–87. [Google Scholar] [CrossRef]
- Galván, I.; Wakamatsu, K.; Camarero, P.R.; Mateo, R.; Alonso-Alvarez, C. Low-quality birds do not display high-quality signals: The cysteine-pheomelanin mechanism of honesty. Evolution 2015, 69, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Galván, I.; Bonisoli-Alquati, A.; Jenkinson, S.; Ghanem, G.; Wakamatsu, K.; Mousseau, T.A.; Møller, A.P. Chronic exposure to low-dose radiation at Chernobyl favours adaptation to oxidative stress in birds. Funct. Ecol. 2014, 28, 1387–1403. [Google Scholar] [CrossRef]
- Hill, H.Z.; Hill, G.J. UVA, pheomelanin and the carcinogenesis of melanoma. Pigment Cell Res. 2000, 13, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Galván, I.; Ghanem, G.; Møller, A.P. Has removal of excess cysteine led to the evolution of pheomelanin? BioEssays 2012, 34, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Galván, I.; Møller, A.P. Pheomelanin-based plumage coloration predicts survival rates in birds. Physiol. Biochem. Zool. 2013, 86, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Galván, I.; Møller, A.P. Brain size and the expression of pheomelanin-based color in birds. J. Evol. Biol. 2011, 24, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Almasi, B.; Roulin, A.; Jenni-Eiermann, S.; Jenni, L. Parental investment and its sensitivity to corticosterone is linked to melanin-based coloration in barn owls. Horm. Behav. 2008, 54, 217–223. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galván, I.; Solano, F. Bird Integumentary Melanins: Biosynthesis, Forms, Function and Evolution. Int. J. Mol. Sci. 2016, 17, 520. https://doi.org/10.3390/ijms17040520
Galván I, Solano F. Bird Integumentary Melanins: Biosynthesis, Forms, Function and Evolution. International Journal of Molecular Sciences. 2016; 17(4):520. https://doi.org/10.3390/ijms17040520
Chicago/Turabian StyleGalván, Ismael, and Francisco Solano. 2016. "Bird Integumentary Melanins: Biosynthesis, Forms, Function and Evolution" International Journal of Molecular Sciences 17, no. 4: 520. https://doi.org/10.3390/ijms17040520
APA StyleGalván, I., & Solano, F. (2016). Bird Integumentary Melanins: Biosynthesis, Forms, Function and Evolution. International Journal of Molecular Sciences, 17(4), 520. https://doi.org/10.3390/ijms17040520







