Intervention of Grape Seed Proanthocyanidin Extract on the Subchronic Immune Injury in Mice Induced by Aflatoxin B1
Abstract
:1. Introduction
2. Results
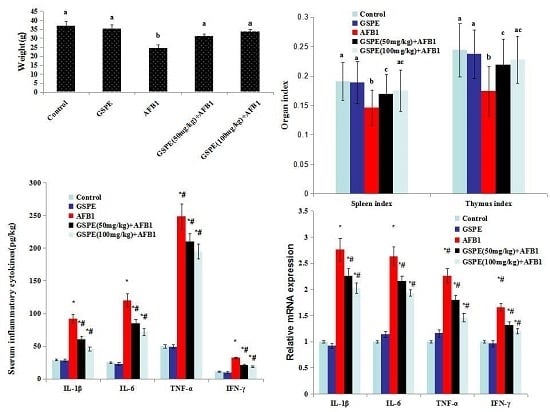
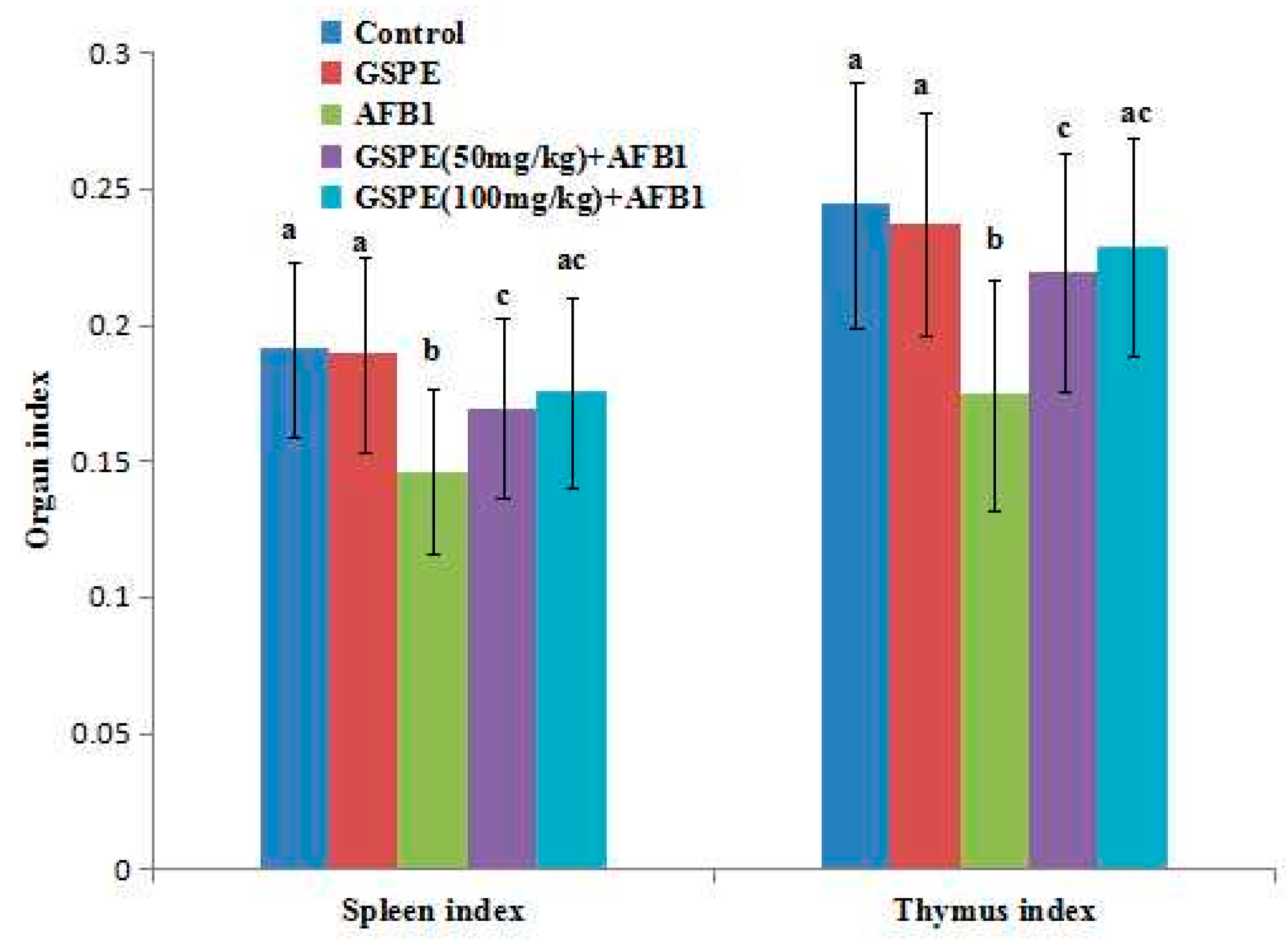
2.1. Effects on Body Weight and Organ Index
2.2. Effect on Contents of Serum IgA, IgG, and IgM
2.3. Effect on Antioxidant of Spleen
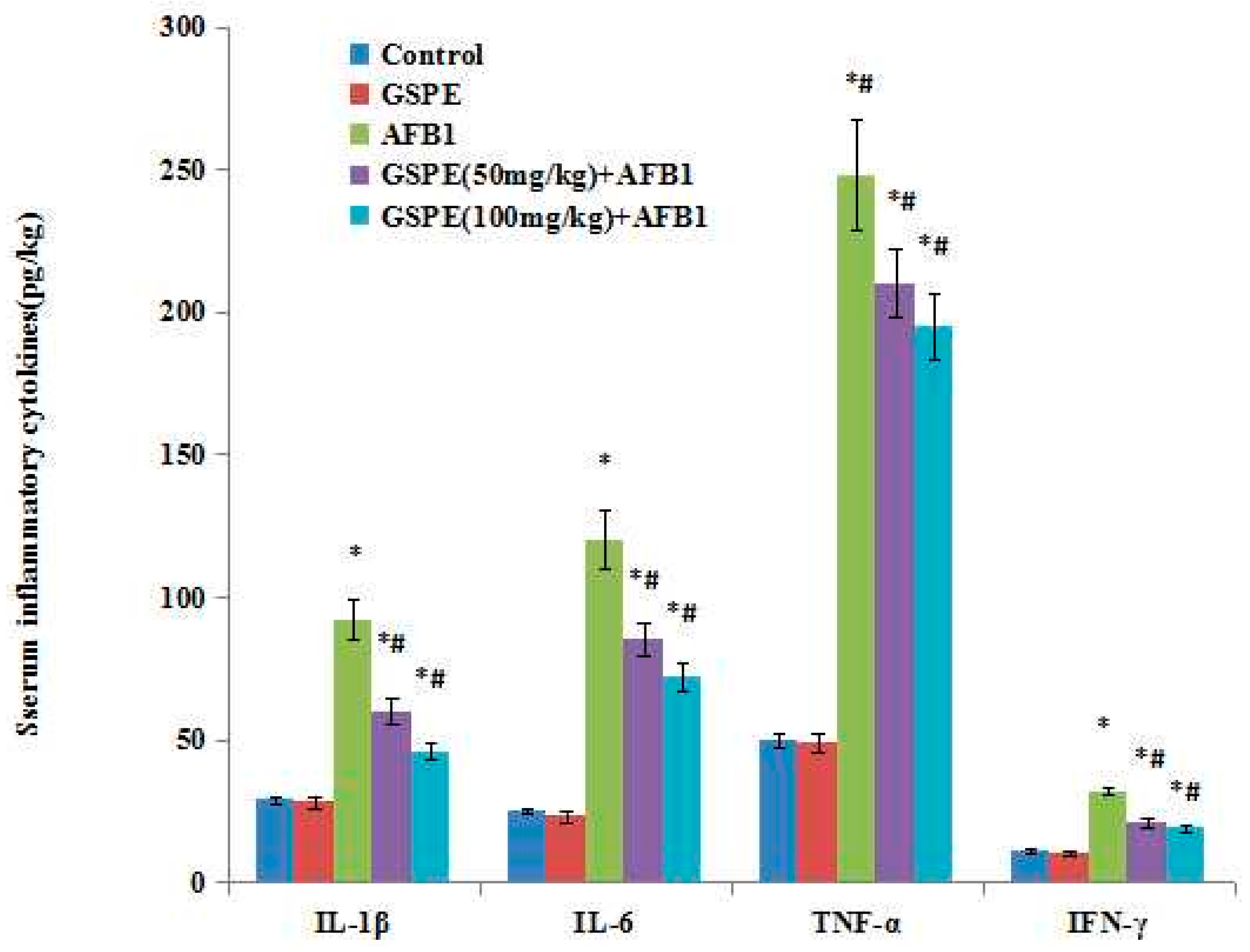
2.4. Effect on Serum Inflammatory Cytokines
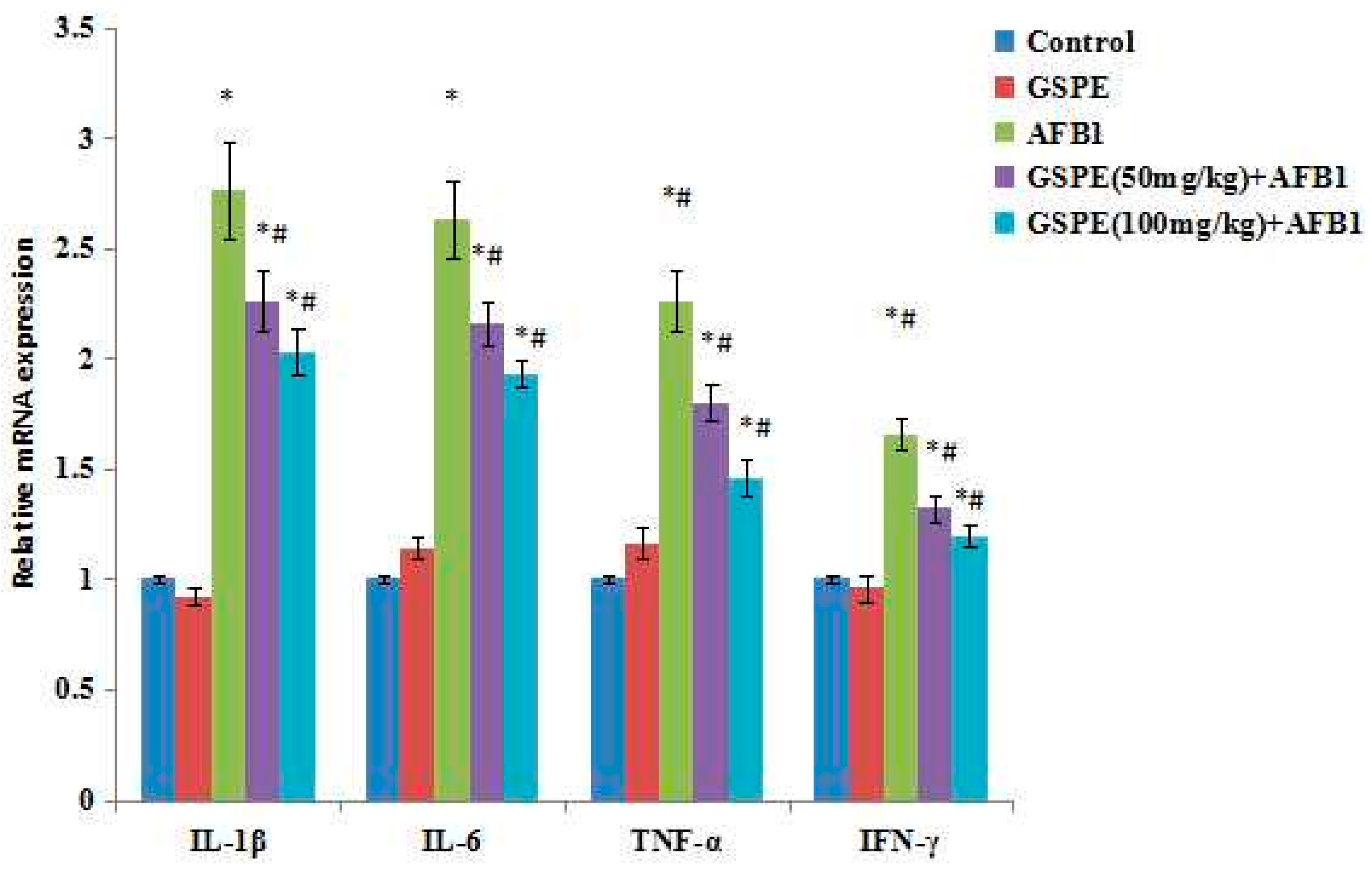
2.5. Effect on Gene mRNA Expression
3. Discussion
4. Experimental Section
4.1. Animals
4.2. Chemicals
4.3. Experimental Design
4.4. Parameter Analysis
4.5. Gene Expression Analyses
4.6. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ma, Q.; Li, Y.; Fan, Y.; Zhao, L.; Wei, H.; Ji, C.; Zhang, J. Molecular mechanisms of lipoic acid protection against aflatoxin B1-induced liver oxidative damage and inflammatory responses in broilers. Toxins (Basel) 2015, 7, 5435–5447. [Google Scholar] [CrossRef] [PubMed]
- Hinton, D.M.; Myers, M.J.; Raybourne, R.A.; Francke, C.S.; Sotomayor, R.E.; Shaddock, J.; Warbritton, A.; Chou, M.W. Immunotoxicity of aflatoxin B1 in rats: Effects on lymphocytes and the inflammatory response in a chronic intermittent dosing study. Toxicol. Sci. 2003, 73, 362–377. [Google Scholar] [CrossRef] [PubMed]
- Magnoli, A.P.; Monge, M.P.; Miazzo, R.D.; Cavaglieri, L.R.; Magnoli, C.E.; Merkis, C.I.; Cristofolini, A.L.; Dalcero, A.M.; Chiacchiera, S.M. Effect of low levels of aflatoxin B1 on performance, biochemical parameters, and aflatoxin B1 in broiler liver tissues in the presence of monensin and sodium bentonite. Poult. Sci. 2011, 90, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Sirajudeen, M.; Gopi, K.; Tyagi, J.S.; Moudgal, R.P.; Mohan, J.; Singh, R. Protective effects of melatonin in reduction of oxidative damage and immunosuppression induced by aflatoxin B1-contaminated diets in young chicks. Environ. Toxicol. 2011, 26, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F. Natural antioxidants and mycotoxins. In Natural Antioxidants in Avian Nutrition and Reproduction, 1st ed.; Nottingham University Press: Nottingham, UK, 2002; pp. 455–509. [Google Scholar]
- Shen, H.M.; Shi, C.Y.; Lee, H.P.; Ong, C.N. Aflatoxin B1 induced lipid peroxidation in rat liver. Toxicol. Appl. Pharmacol. 1994, 127, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahhab, M.A.; Aly, S.E. Antioxidants and radical scavenging properties of vegetable extracts in rats fed aflatoxin-contaminated diet. J. Agric. Food Chem. 2003, 51, 2409–2414. [Google Scholar] [CrossRef] [PubMed]
- Galvano, F.; Piva, A.; Ritieni, A.; Galvano, G. Dietary strategies to counteract the effects of mycotoxins: A review. J. Food Prot. 2001, 64, 120–131. [Google Scholar]
- Mansouri, E.; Panahi, M.; Ghaffari, M.A.; Ghorbani, A. Effects of grape seed proanthocyanidin extract on oxidative stress induced by diabetes in rat kidney. Iran. Biomed. J. 2011, 15, 100–106. [Google Scholar] [PubMed]
- Ouedraogo, M.; Charles, C.; Ouedraogo, M.; Guissou, I.P.; Stevigny, C.; Duez, P. An overview of cancer chemopreventive potential and safety of proanthocyanidins. Nutr. Cancer 2011, 63, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Zou, Y.; Zhu, L.; Wang, H.-F.; Dai, M.-G. Antioxidant properties of proanthocyanidins attenuate carbon tetrachloride (CCl4)–induced steatosis and liver injury in rats via CYP2E1 regulation. J. Med. Food 2014, 17, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-C.; Yin, J.; Zhou, B.; Liu, Y.-T.; Yu, Y.; Li, G.-Q. Grape seed proanthocyanidin protects liver against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress. World J. Gastroenterol. 2015, 21, 7468–7477. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qiu, J.; Zhao, S.; You, B.; Ji, X.; Wang, Y.; Cui, X.; Wang, Q.; Gao, H. Grape seed proanthocyanidin extract alleviates ouabain-induced vascular remodeling through regulation of endothelial function. Mol. Med. Rep. 2012, 6, 949–954. [Google Scholar] [PubMed]
- Gao, Z.; Liu, G.; Hu, Z.; Li, X.; Yang, X.; Jiang, B.; Li, X. Grape seed proanthocyanidin extract protects from cisplatin-induced nephrotoxicity by inhibiting endoplasmic reticulum stress-induced apoptosis. Mol. Med. Rep. 2014, 9, 801–807. [Google Scholar] [PubMed]
- Chu, H.; Tang, Q.; Huang, H.; Hao, W.; Wei, X. Grape-seed proanthocyanidins inhibit the lipopolysaccharide-induced inflammatory mediator expression in RAW264.7 macrophages by suppressing MAPK and NF-κb signal pathways. Environ. Toxicol. Pharmacol. 2016, 41, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fang, W.; Xiao, W.; Lu, L.; Jia, X. Protective role of oligomeric proanthocyanidin complex against hazardous nodularin-induced oxidative toxicity in Carassius auratus lymphocytes. J. Hazard. Mater. 2014, 274, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Dinh, J.; Angeloni, J.T.; Pederson, D.B.; Wang, X.; Cao, M.; Dong, Y. Cranberry extract standardized for proanthocyanidins promotes the immune response of Caenorhabditis elegans to Vibrio cholerae through the p38 MAPK pathway and HSF-1. PLoS ONE 2014, 9, e103290. [Google Scholar]
- Zhou, D.Y.; Du, Q.; Li, R.R.; Huang, M.; Zhang, Q.; Wei, G.Z. Grape seed proanthocyanidin extract attenuates airway inflammation and hyperresponsiveness in a murine model of asthma by downregulating inducible nitric oxide synthase. Planta Med. 2011, 77, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Park, M.K.; Oh, H.J.; Woo, Y.J.; Lim, M.A.; Lee, J.H.; Ju, J.H.; Jung, Y.O.; Lee, Z.H.; Park, S.H.; et al. Grape-seed proanthocyanidin extract as suppressors of bone destruction in inflammatory autoimmune arthritis. PLoS ONE 2012, 7, e51377. [Google Scholar] [CrossRef] [PubMed]
- Banlunara, W.; Bintvihok, A.; Kumagai, S. Immunohistochemical study of proliferating cell nuclear antigen (PCNA) in duckling liver fed with aflatoxin B1 and esterified glucomannan. Toxicon 2005, 46, 954–957. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Shi, D.; Liao, S.; Su, R.; Lin, Y.; Pan, J.; Tang, Z. Influence of selenium on body weights and immune organ indexes in ducklings intoxicated with aflatoxin B1. Biol. Trace Elem. Res. 2012, 146, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Krithika, R.; Manjeet, D.; Verma, R.J. Protective effect of black tea infusion on aflatoxin-induced hepatotoxicity in mice. J. Clin. Exp. Hepatol. 2013, 3, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Abbès, S.; Ben Salah-Abbès, J.; Jebali, R.; Younes, R.B.; Oueslati, R. Interaction of aflatoxin B1 and fumonisin B1 in mice causes immunotoxicity and oxidative stress: Possible protective role using lactic acid bacteria. J. Immunotoxicol. 2016, 13, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D.J.; Huff, W.E.; Hamilton, P.B.; Burmeister, H.R. Comparison of ochratoxin, aflatoxin, and T-2 toxin for their effects on selected parameters related to digestion and evidence for specific metabolism of carotenoids inchickens. Poult. Sci. 1982, 61, 1646–1652. [Google Scholar] [CrossRef]
- Leeson, S.; Diaz, G.J.; Summers, J.D. Poultry Metabolic Disorders and Mycotoxins; University Books: Montreal, QC, Canada, 1995. [Google Scholar]
- Kodama, M.; Inoue, F.; Akao, M. Enzymatic and non-enzymatic formation of free radicals from aflatoxin B1. Free Radic. Res. Commun. 1990, 10, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.C.; Lee, B.S.; Chung, W.T.; Choi, M.S.; Lee, J.C. Protective effects of apigenin and quercetin on aflatoxin B1-induced immunotoxicity in mice. Food Sci. Biotechnol. 2010, 19, 987–992. [Google Scholar] [CrossRef]
- Naaz, F.; Abdin, M.Z.; Javed, S. Protective effect of esculin against prooxidant aflatoxin B1-induced nephrotoxicity in mice. Mycotoxin Res. 2014, 30, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Yener, Z.; Celik, I.; Ilhan, F.; Bal, R. Effects of Urtica dioica L. seed on lipid peroxidation, antioxidants and liver pathology in aflatoxin-induced tissue injury in rats. Food Chem. Toxicol. 2009, 47, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Meissonnier, G.M.; Pinton, P.; Laffitte, J.; Cossalter, A.M.; Gong, Y.Y.; Wild, C.P.; Bertin, G.; Galtier, P.; Oswald, I.P. Immunotoxicity of aflatoxin B1: Impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicol. Appl. Pharmacol. 2008, 231, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Taranu, I.; Bunaciu, R.P.; Pascale, F.; Tudor, D.S.; Avram, N.; Sarca, M.; Cureu, I.; Criste, R.D.; Suta, V.; et al. Changes in performance, blood parameters, humoral and cellular immune responses in weanling piglets exposed to low doses of aflatoxin. J. Anim. Sci. 2002, 80, 1250–1257. [Google Scholar] [PubMed]
- Turner, P.C.; Moore, S.E.; Hall, A.J.; Prentice, A.M.; Wild, C.P. Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environ. Health Perspect. 2003, 111, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, Q.G.; Zhao, L.H.; Wei, H.; Duan, G.X.; Zhang, J.Y.; Ji, C. Effects of lipoic acid on immune function, the antioxidant defense system, and inflammation-related genes expression of broiler chickens fed aflatoxin contaminated diets. Int. J. Mol. Sci. 2014, 15, 5649–5662. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Kwon, H.S.; Bang, B.R.; Lee, Y.S.; Park, M.Y.; Moon, K.A.; Kim, T.B.; Lee, K.Y.; Moon, H.B.; Cho, Y.S. Grape seed proanthocyanidin extract attenuates allergic inflammation in murine models of asthma. J. Clin. Immunol. 2012, 32, 1292–1304. [Google Scholar] [CrossRef] [PubMed]
- Subarnas, A.; Wagner, H. Analgesic and anti-inflammatory activity of the proanthocyanidin shellegueain A from Polypodium feei METT. Phytomedicine 2000, 7, 401–405. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Zoheir, K.M.; Abdel-Hamied, H.E.; Attia, S.M.; Bakheet, S.A.; Ashour, A.E.; Abd-Allah, A.R. Grape seed proanthocyanidin extract protects against carrageenan-induced lung inflammation in mice through reduction of pro-inflammatory markers and chemokine expressions. Inflammation 2014, 37, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Park, I.J.; Cha, S.Y.; Kang, M.; Jang, H.K. Immunomodulatory effect of a proanthocyanidin-rich extract from Pinus radiata bark by dosing period in chickens. Poult. Sci. 2013, 92, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Choy, Y.Y.; Quifer-Rada, P.; Holstege, D.M.; Frese, S.A.; Calvert, C.C.; Mills, D.A.; Lamuela-Raventos, R.M.; Waterhouse, A.L. Phenolic metabolites and substantial microbiome changes in pig feces by ingesting grape seed proanthocyanidins. Food Funct. 2014, 5, 2298–2308. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Garg, A.; Krohn, R.L.; Bagchi, M.; Bagchi, D.J.; Balmoori, J.; Stohs, S.J. Protective effects of grape seed proanthocyanidins and selected antioxidants against TPA-induced hepatic and brain lipid peroxidation and DNA fragmentation, and peritoneal macrophage activation in mice. Gen. Pharmacol. 1998, 30, 771–776. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Xing, B.S.; Zhu, C.C.; Shen, M.; Yu, F.X.; Liu, H.L. Protective effect of proanthocyanidin against oxidative ovarian damage induced by 3-nitropropionic acid in mice. Genet. Mol. Res. 2015, 14, 2484–2494. [Google Scholar] [CrossRef] [PubMed]

| Group | IgG (mg/mL) | IgA (mg/mL) | IgM (mg/mL) |
|---|---|---|---|
| Control | 15.96 ± 2.31 a | 0.54 ± 0.15 a | 2.39 ± 0.36 a |
| GSPE (100 mg/kg) | 16.20 ± 2.54 a | 0.59 ± 0.22 a | 2.36 ± 0.32 a |
| AFB1 (100 μg/kg) | 14.13 ± 2.42 a | 0.50 ± 0.28 a | 2.30 ± 0.40 a |
| GSPE (50 mg/kg) + AFB1 100 μg/kg | 15.67 ± 2.32 a | 0.55 ± 0.29 a | 2.35 ± 0.36 a |
| GSPE (100 mg/kg) + AFB1 100 μg/kg | 15.06 ± 2.24 a | 0.53 ± 0.24 a | 2.32 ± 0.30 a |
| Group | MDA nmol/mgprot | CAT U/mgprot | GSH U/mgprot | GSH-PX U/mgprot | SOD U/mgprot |
|---|---|---|---|---|---|
| Control | 2.82 ± 0.36 c | 56.32 ± 3.16 c | 279.64 ± 18.14 c | 14.60 ± 1.725 b | 156.34 ± 23.16 b |
| GSPE (100 mg/kg) | 2.15 ± 0.31 c | 75.14 ± 2.96 d | 396.74 ± 20.63 d | 19.74 ± 1.654 c | 214.96 ± 22.34 d |
| AFB1 (100 μg/kg) | 9.87 ± 0.42 a | 28.17 ± 2.14 a | 196.66 ± 15.43 a | 8.842 ± 0.475 a | 84.36 ± 20.47 a |
| GSPE (50 mg/kg) + AFB1 100 μg/kg | 5.34 ± 0.38 b | 38.75 ± 2.66 b | 234.28 ± 18.64 b | 12.11 ± 0.362 b | 109.14 ± 19.21 bc |
| GSPE (100 mg/kg) + AFB1 100 μg/kg | 4.12 ± 0.29 b | 43.23 ± 3.41 b | 256.54 ± 19.87 b | 13.46 ± 0.330 b | 123.45 ± 18.36 b |
| Gene | Primer Sequences (5′→3′) | Accession No. | Product Size/bp |
|---|---|---|---|
| β-actin | F: GTGCTATGTTGCTCTAGACTTCG | NM_007393.5 | 174 |
| R: ATGCCACAGGATTCCATACC | |||
| IL-1β | F: GAGCACCTTCTTTTCCTTCATCTT | NM_000836 | 84 |
| R: TCACACACCAGCAGGTTATCATC | |||
| IL-6 | F: GAGGATACCACTCCCAACAGACC | NM_031168 | 141 |
| R: AAGTGTATCATCGTTGTTCATACA | |||
| TNF-α | F: ATCCGCGACGTGGAACTG | NM_013693 | 70 |
| R: ACCGCCTGGAGTTCTGGAA | |||
| IFN-γ | F: CCATCGGCTGACCTAGAGAA | NM_008337 | 131 |
| R: GATGCAGTGTGTAGCGTTCA |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, M.; Zhang, Y.; Li, P.; Yang, S.-H.; Zhang, W.-K.; Han, J.-X.; Wang, Y.; He, J.-B. Intervention of Grape Seed Proanthocyanidin Extract on the Subchronic Immune Injury in Mice Induced by Aflatoxin B1. Int. J. Mol. Sci. 2016, 17, 516. https://doi.org/10.3390/ijms17040516
Long M, Zhang Y, Li P, Yang S-H, Zhang W-K, Han J-X, Wang Y, He J-B. Intervention of Grape Seed Proanthocyanidin Extract on the Subchronic Immune Injury in Mice Induced by Aflatoxin B1. International Journal of Molecular Sciences. 2016; 17(4):516. https://doi.org/10.3390/ijms17040516
Chicago/Turabian StyleLong, Miao, Yi Zhang, Peng Li, Shu-Hua Yang, Wen-Kui Zhang, Jian-Xin Han, Yuan Wang, and Jian-Bin He. 2016. "Intervention of Grape Seed Proanthocyanidin Extract on the Subchronic Immune Injury in Mice Induced by Aflatoxin B1" International Journal of Molecular Sciences 17, no. 4: 516. https://doi.org/10.3390/ijms17040516
APA StyleLong, M., Zhang, Y., Li, P., Yang, S.-H., Zhang, W.-K., Han, J.-X., Wang, Y., & He, J.-B. (2016). Intervention of Grape Seed Proanthocyanidin Extract on the Subchronic Immune Injury in Mice Induced by Aflatoxin B1. International Journal of Molecular Sciences, 17(4), 516. https://doi.org/10.3390/ijms17040516