Overexpression of G0/G1 Switch Gene 2 in Adipose Tissue of Transgenic Quail Inhibits Lipolysis Associated with Egg Laying
Abstract
1. Introduction
2. Results
2.1. Overexpression of G0S2 Delays Onset of Laying and Reduces Egg Production
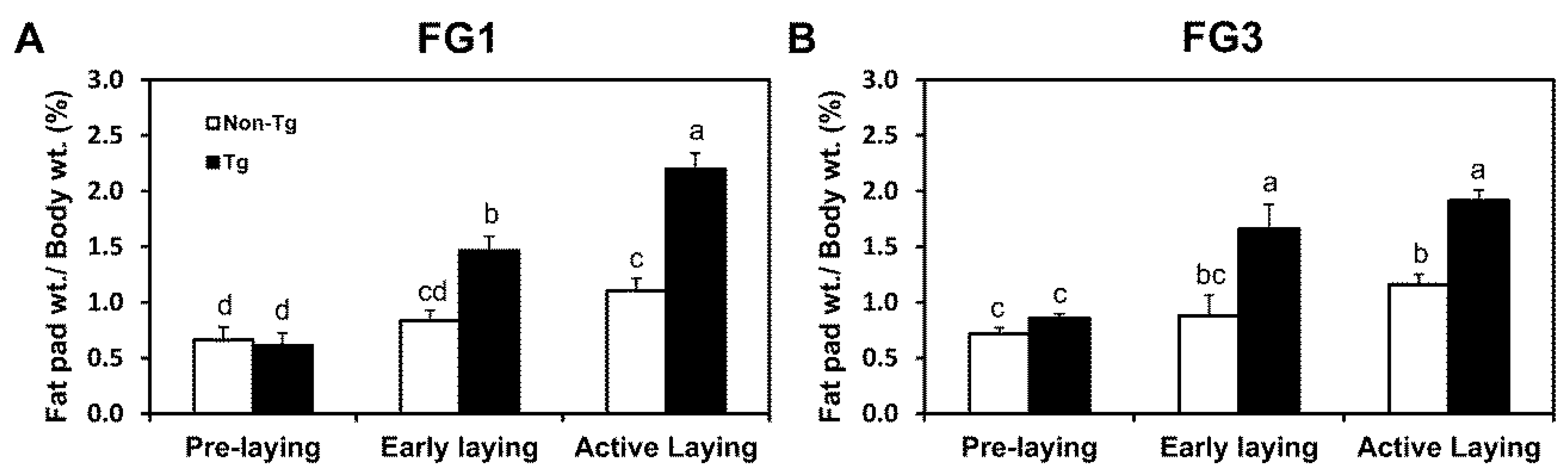
2.2. G0S2 Transgenic Quail Gain Adipose Mass throughout Stages of Laying
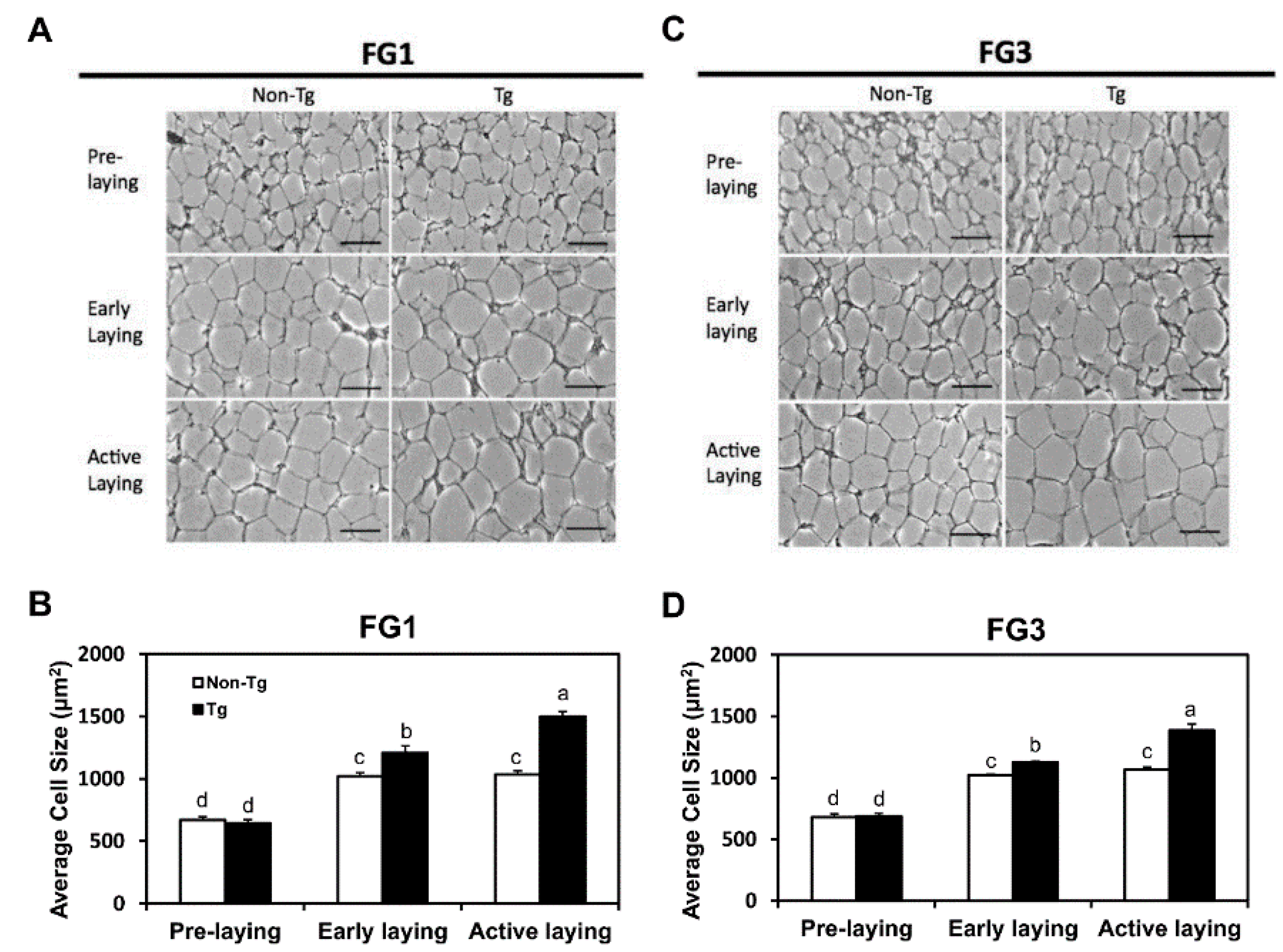
2.3. Adipocyte Hypertrophy Caused by G0S2 Overexpression after Onset of Laying
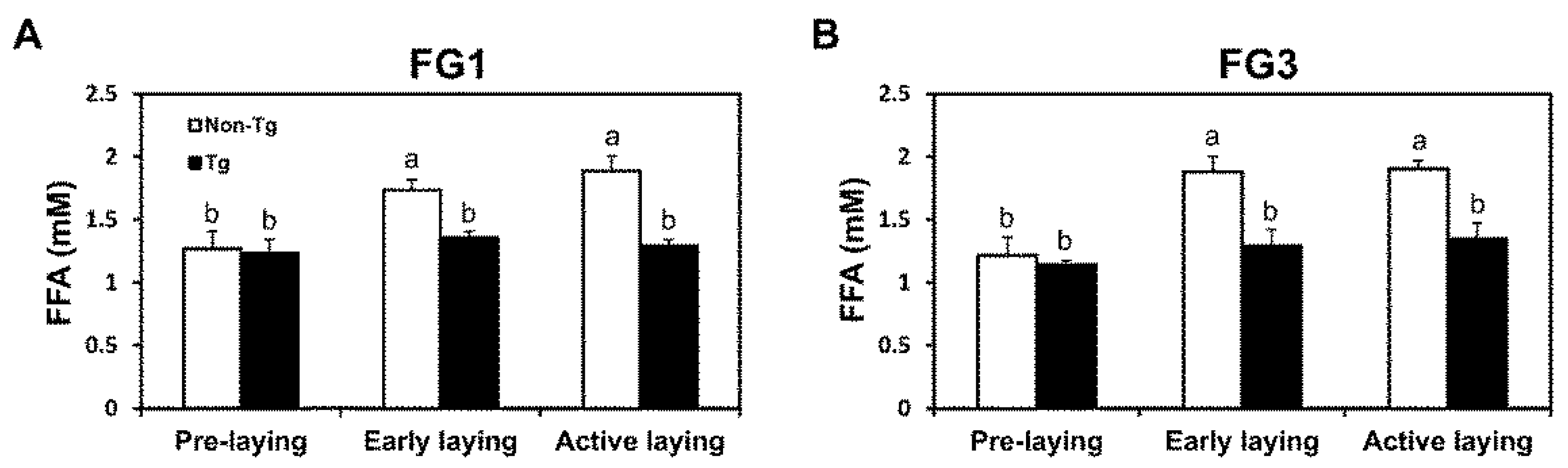
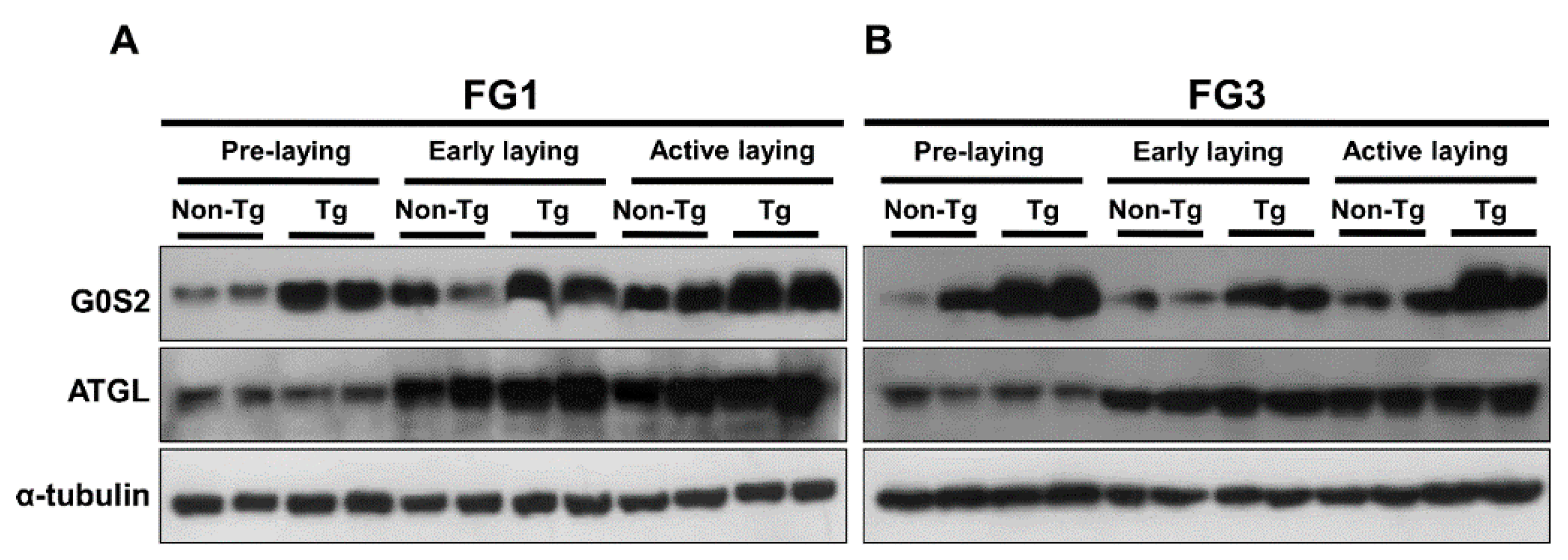
2.4. Lipolysis Initiated by Egg Laying Is Inhibited by G0S2
3. Discussion
4. Experimental Section
4.1. Animal Use and Ethics Statement
4.2. Detection of G0S2 Transgene by PCR
4.3. Egg Production and Tissue Collection
4.4. Histological Processing for Determining Cell Size
4.5. NEFA Assay
4.6. Western Blotting
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TAG | triacylglycerol |
| NEFA | nonesterified fatty acid |
| ATGL | adipose triglyceride lipase |
| G0S2 | G0/G1 switch gene 2 |
| FABP4 | fatty acid binding protein 4 |
References
- Zimmermann, R.; Strauss, J.G.; Haemmerle, G.; Schoiswohl, G.; Birner-Gruenberger, R.; Riederer, M.; Lass, A.; Neuberger, G.; Eisenhaber, F.; Hermetter, A.; et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 2004, 306, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Suh, Y.; Choi, Y.M.; Shin, S.; Han, J.Y.; Lee, K. Loss of fat with increased adipose triglyceride lipase-mediated lipolysis in adipose tissue during laying stages in quail. Lipids 2013, 48, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, X.; Lombes, M.; Rha, G.B.; Chi, Y.I.; Guerin, T.M.; Smart, E.J.; Liu, J. The G0/G1 switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010, 11, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Oh, S.A.; Suh, Y.; Moeller, S.J.; Lee, K. Porcine G0/G1 switch gene 2 (G0S2) expression is regulated during adipogenesis and short-term in vivo nutritional interventions. Lipids 2013, 48, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yang, X.; Liu, J. Differential control of ATGL-mediated lipid droplet degradation by CGI-58 and G0S2. Cell Cycle 2010, 9, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.A.; Suh, Y.; Pang, M.G.; Lee, K. Cloning of avian G0/G1 switch gene 2 genes and developmental and nutritional regulation of G0/G1 switch gene 2 in chicken adipose tissue. J. Anim. Sci. 2011, 89, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.S.; Vendelbo, M.H.; Jessen, N.; Pedersen, S.B.; Jorgensen, J.O.; Lund, S.; Moller, N. Fasting, but not exercise, increases adipose triglyceride lipase (ATGL) protein and reduces G0/G1 switch gene 2 (G0S2) protein and mRNA content in human adipose tissue. J. Clin. Endocrinol. Metab. 2011, 96, E1293–E1297. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, M.; Paar, M.; Eder, C.; Brandis, J.; Moser, E.; Gorkiewicz, G.; Grond, S.; Radner, F.P.; Cerk, I.; Cornaciu, I.; et al. G0/G1 switch gene-2 regulates human adipocyte lipolysis by affecting activity and localization of adipose triglyceride lipase. J. Lipid Res. 2012, 53, 2307–2317. [Google Scholar] [CrossRef] [PubMed]
- Zandbergen, F.; Mandard, S.; Escher, P.; Tan, N.S.; Patsouris, D.; Jatkoe, T.; Rojas-Caro, S.; Madore, S.; Wahli, W.; Tafuri, S.; et al. The G0/G1 switch gene 2 is a novel PPAR target gene. Biochem. J. 2005, 392, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Noble, R.C.; Cocchi, M. Lipid metabolism and the neonatal chicken. Prog. Lipid Res. 1990, 29, 107–140. [Google Scholar] [CrossRef]
- Romanoff, A.L. The Avian Embryo: Structural and Functional Development; Macmillan: New York, NY, USA, 1960; p. 1305. [Google Scholar]
- Shin, S.; Choi, Y.M.; Han, J.Y.; Lee, K. Inhibition of lipolysis in the novel transgenic quail model overexpressing G0/G1 switch gene 2 in the adipose tissue during feed restriction. PLoS ONE 2014, 9, e100905. [Google Scholar] [CrossRef] [PubMed]
- Chwalibog, A.; Baldwin, R.L. Systems to predict the energy and protein-requirements of laying fowl. World. Poult. Sci. J. 1995, 51, 187–196. [Google Scholar] [CrossRef]
- Reid, B.L.; Valencia, M.E.; Maiorino, P.M. Energy utilization by laying hens. I. Energetic efficiencies of maintenance and production. Poult. Sci. 1978, 57, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Kingori, A.M.; Wachira, A.M.; Tuitoek, J.K. Influence of energy intake on egg production and weight in indigenous chickens of Kenya. Int. J. Poult. Sci. 2014, 13, 151–155. [Google Scholar] [CrossRef]
- Pearson, R.A. Influence of nutritional factors on hatchability. In Recent Developments in Poultry Nutrition; Butterworths: London, UK & Boston, MA, USA, 1989; pp. 276–291. [Google Scholar]
- Bernstein, L.; Pike, M.C.; Ross, R.K.; Henderson, B.E. Age at menarche and estrogen concentrations of adult women. Cancer Causes Control 1991, 2, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Emaus, A.; Espetvedt, S.; Veierod, M.B.; Ballard-Barbash, R.; Furberg, A.S.; Ellison, P.T.; Jasienska, G.; Hjartaker, A.; Thune, I. 17-β-estradiol in relation to age at menarche and adult obesity in premenopausal women. Hum. Reprod. 2008, 23, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Chen, D.; Moy, I.; Brooks, D.C.; Zhao, H. Aromatase, breast cancer and obesity: A complex interaction. Trends Endocrinol. Metab. 2012, 23, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Leszczynski, D.E.; Hagan, R.C.; Bitgood, J.J.; Kummerow, F.A. Relationship of plasma estradiol and progesterone levels to egg productivity in domestic chicken hens. Poult. Sci. 1985, 64, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Walzem, R.L.; Hansen, R.J.; Williams, D.L.; Hamilton, R.L. Estrogen induction of VLDLy assembly in egg-laying hens. J. Nutr. 1999, 129, 467S–472S. [Google Scholar] [PubMed]
- Garnica, A.D.; Chan, W.Y. The role of the placenta in fetal nutrition and growth. J. Am. Coll. Nutr. 1996, 15, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Suh, Y.; Choi, Y.M.; Shin, S.; Lee, K. Developmental regulation of adipose tissue growth through hyperplasia and hypertrophy in the embryonic Leghorn and broiler. Poult. Sci. 2014, 93, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Suh, Y.; Kim, E.; Moeller, S.J.; Lee, K. Alternative splicing and developmental and hormonal regulation of porcine comparative gene identification-58 (CGI-58) mRNA. J. Anim. Sci. 2012, 90, 4346–4354. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.R.; Shin, S.; Choi, Y.M.; Kim, E.; Han, J.Y.; Lee, K. Overexpression of G0/G1 Switch Gene 2 in Adipose Tissue of Transgenic Quail Inhibits Lipolysis Associated with Egg Laying. Int. J. Mol. Sci. 2016, 17, 384. https://doi.org/10.3390/ijms17030384
Chen PR, Shin S, Choi YM, Kim E, Han JY, Lee K. Overexpression of G0/G1 Switch Gene 2 in Adipose Tissue of Transgenic Quail Inhibits Lipolysis Associated with Egg Laying. International Journal of Molecular Sciences. 2016; 17(3):384. https://doi.org/10.3390/ijms17030384
Chicago/Turabian StyleChen, Paula Renee, Sangsu Shin, Young Min Choi, Elizabeth Kim, Jae Yong Han, and Kichoon Lee. 2016. "Overexpression of G0/G1 Switch Gene 2 in Adipose Tissue of Transgenic Quail Inhibits Lipolysis Associated with Egg Laying" International Journal of Molecular Sciences 17, no. 3: 384. https://doi.org/10.3390/ijms17030384
APA StyleChen, P. R., Shin, S., Choi, Y. M., Kim, E., Han, J. Y., & Lee, K. (2016). Overexpression of G0/G1 Switch Gene 2 in Adipose Tissue of Transgenic Quail Inhibits Lipolysis Associated with Egg Laying. International Journal of Molecular Sciences, 17(3), 384. https://doi.org/10.3390/ijms17030384






