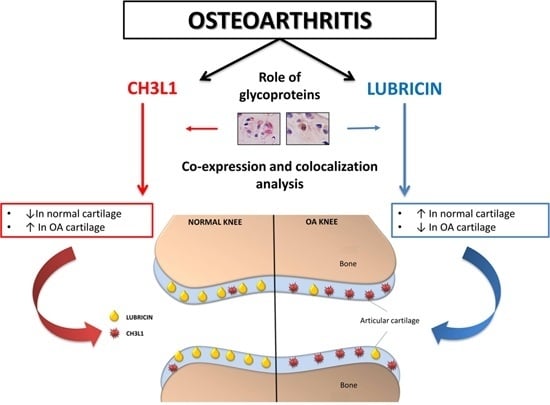
Co-Expression and Co-Localization of Cartilage Glycoproteins CHI3L1 and Lubricin in Osteoarthritic Cartilage: Morphological, Immunohistochemical and Gene Expression Profiles
Abstract
:1. Introduction
2. Results
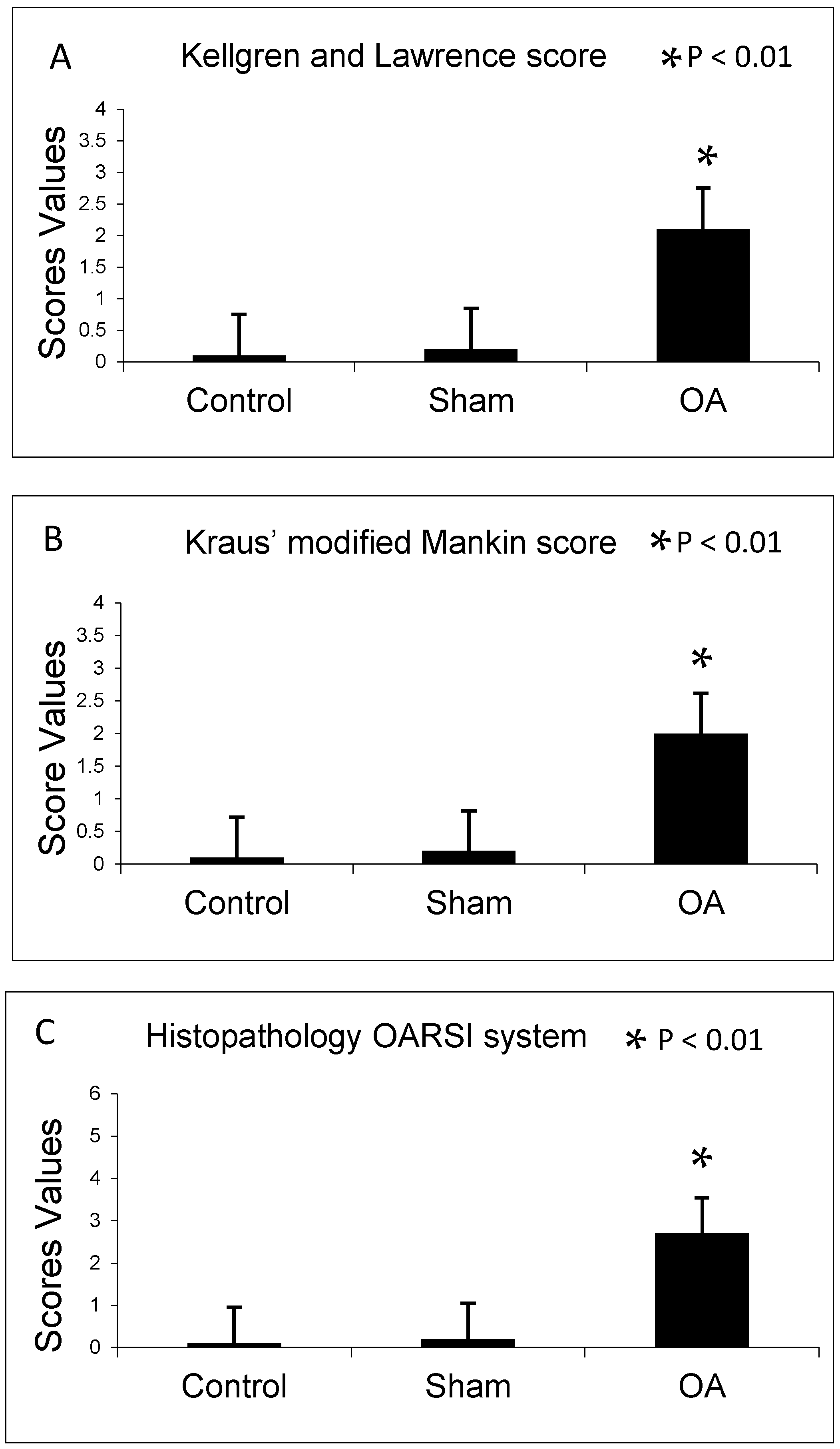
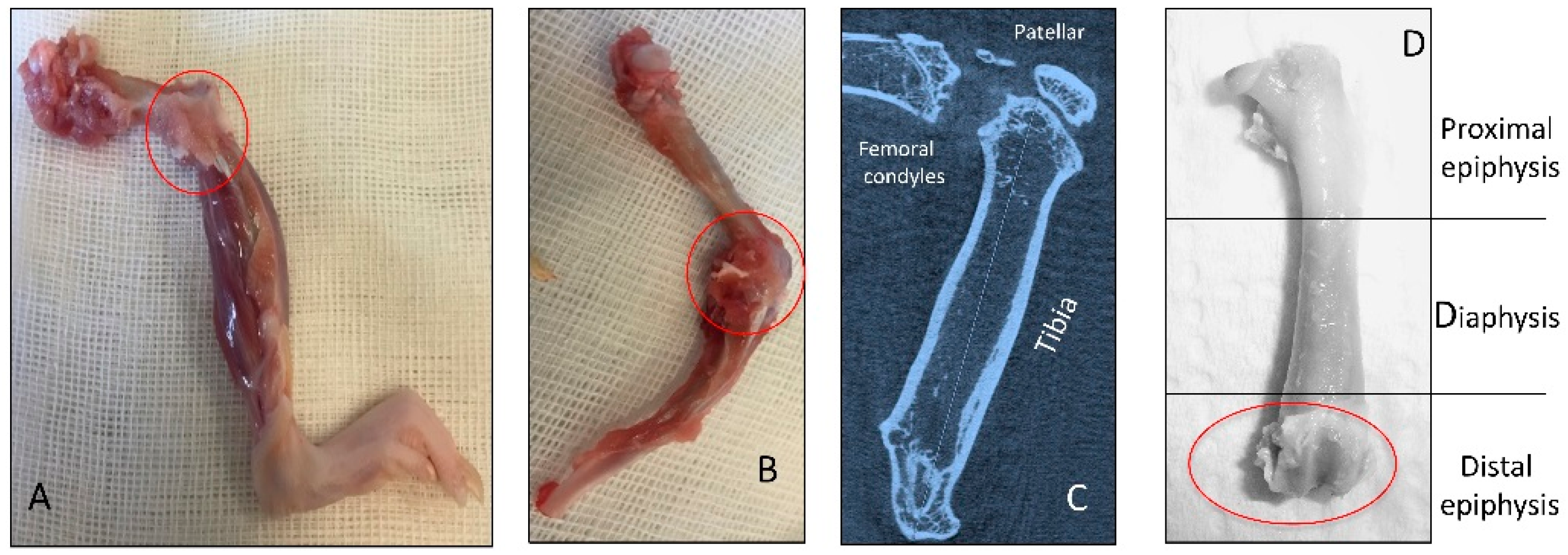
2.1. Radiographic Analysis
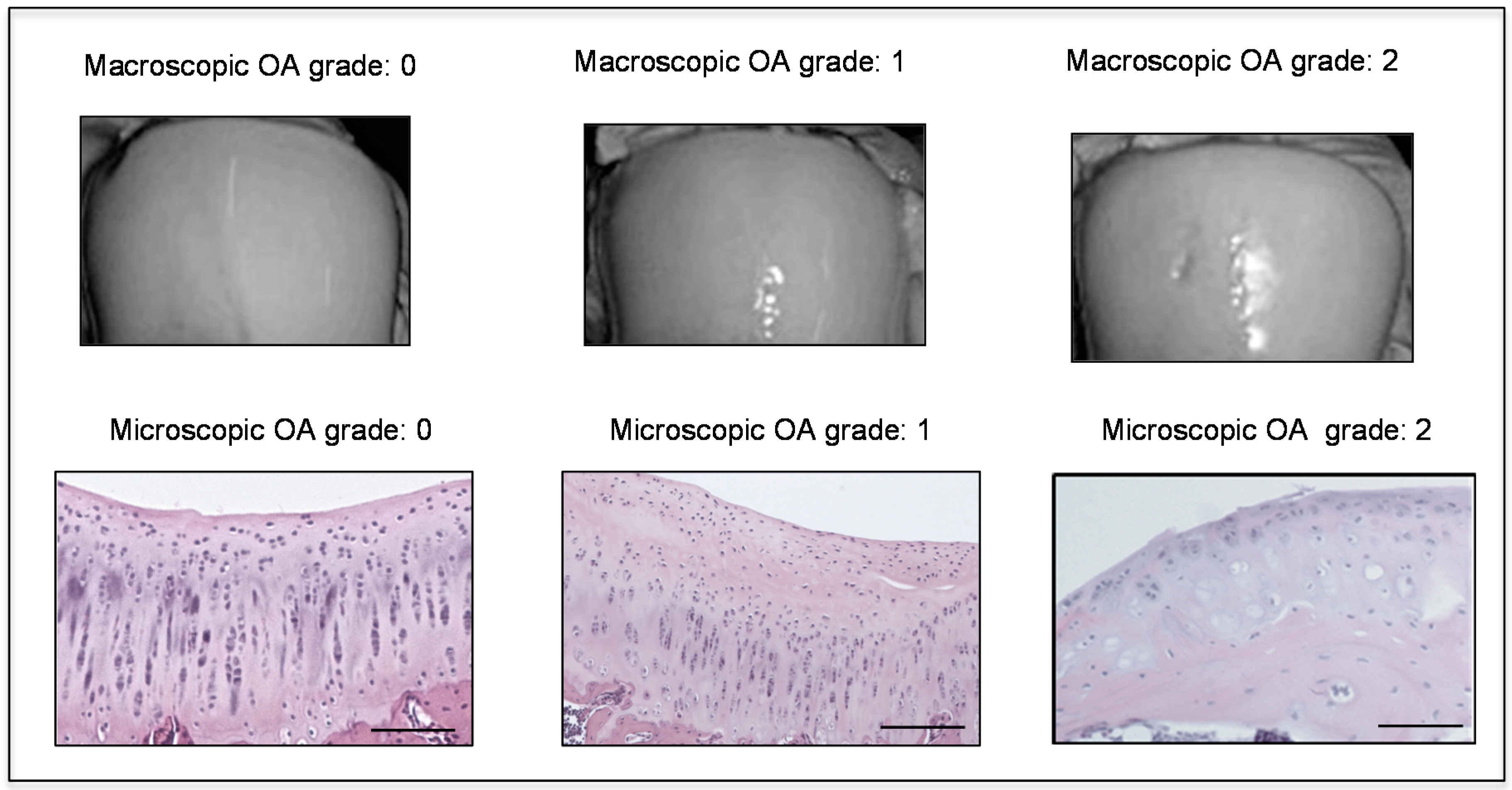
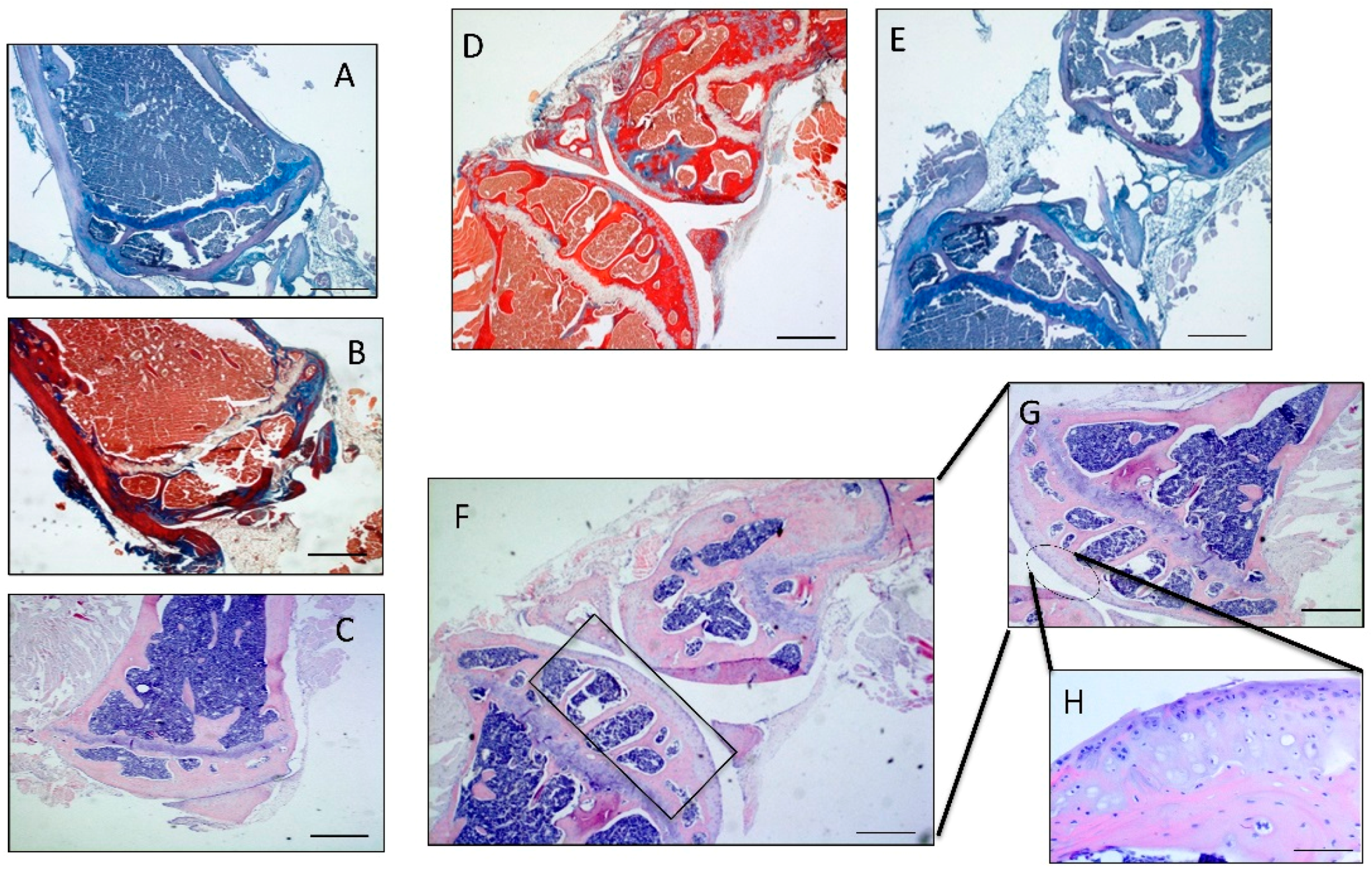
2.2. Histomorphometric Analyses
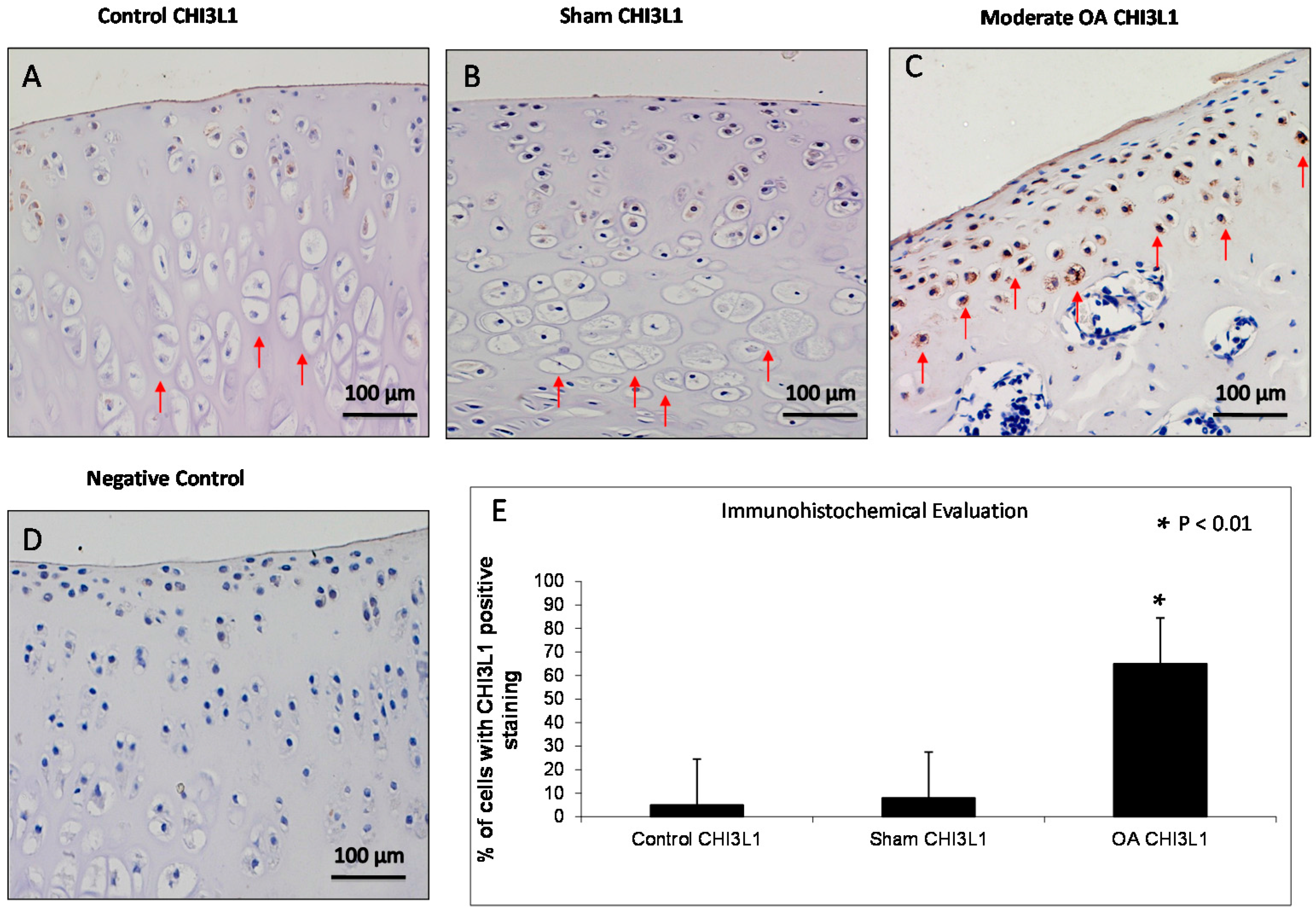
2.3. Immunohistochemistry (IHC) Observations
2.4. Double Immunostaining Observations
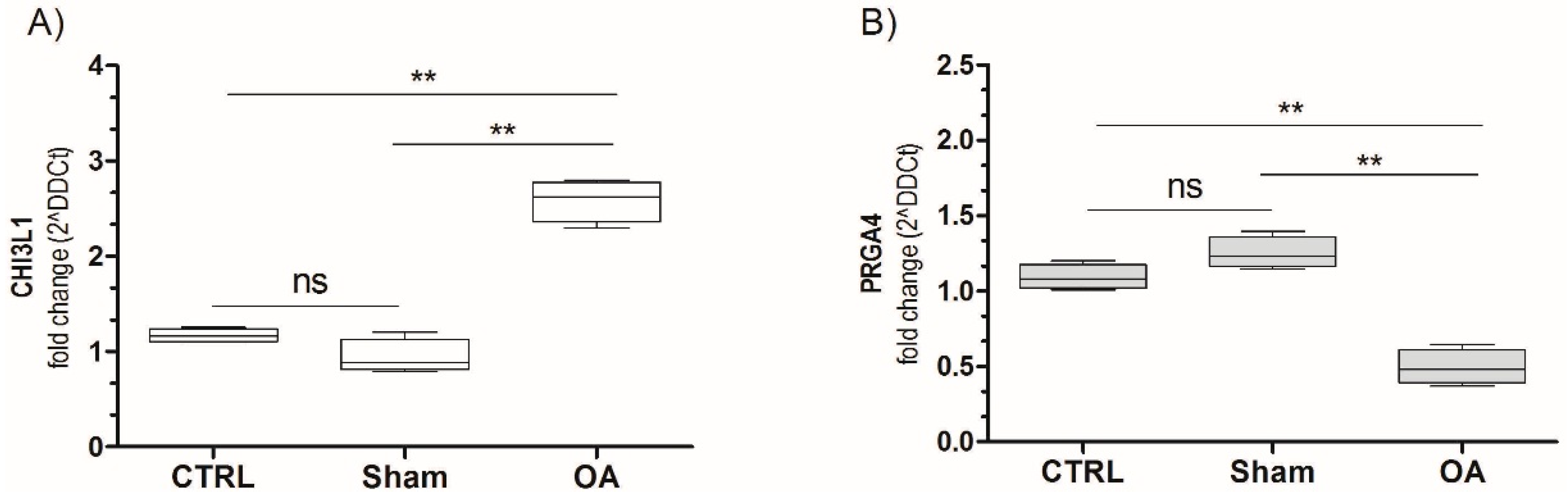
2.5. Chitinase 3-Like-1 (CHI3L1) and Lubricin mRNA Expression in Osteoarthritic Rat Cartilage Model
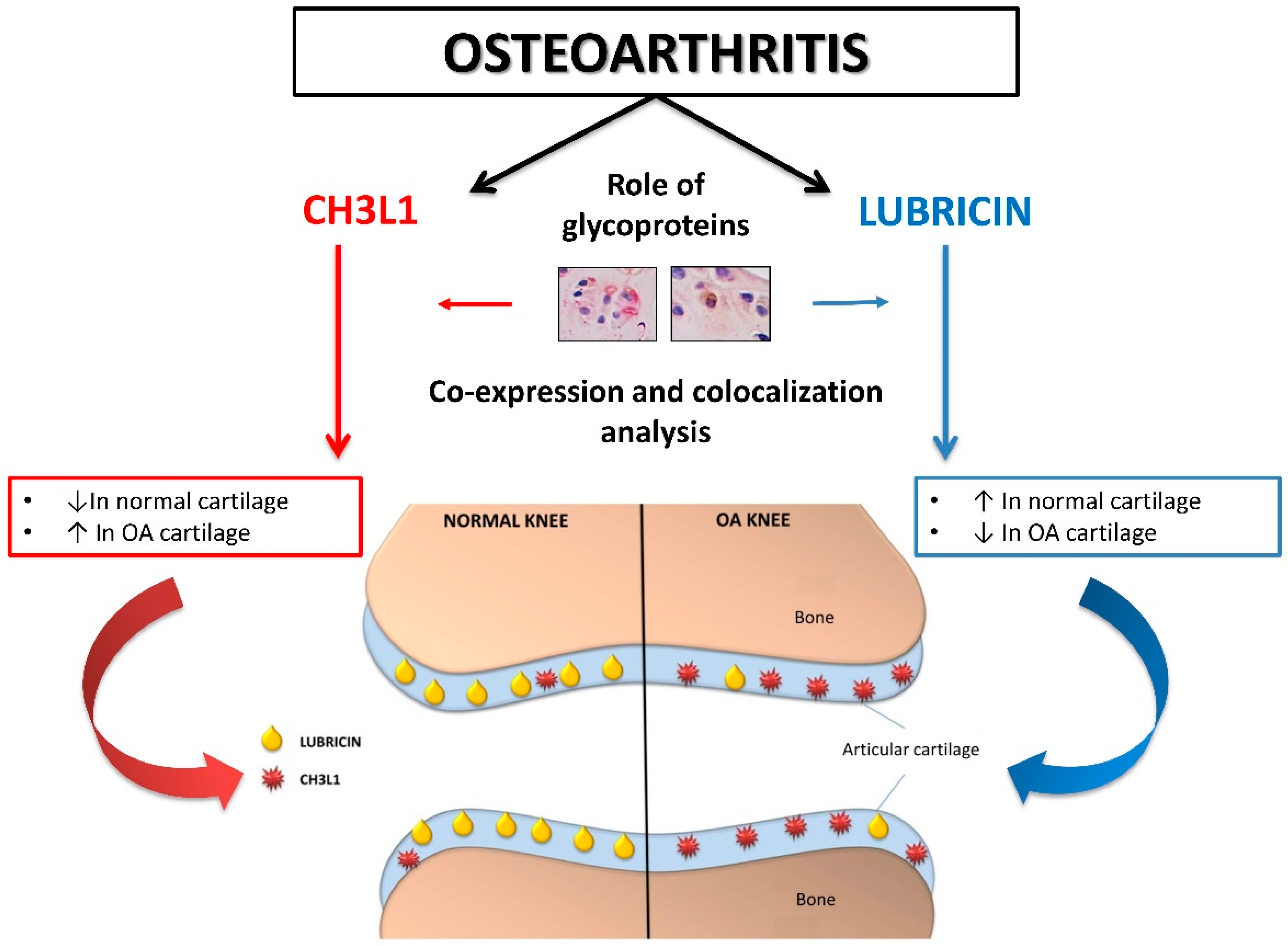
3. Discussion
4. Materials and Methods
4.1. Breeding and Housing of Animals
4.2. Radiographic Analysis
4.3. Histomorphometric Analysis
4.4. Histology and Histochemistry Analysis
4.5. Immunohistochemistry (IHC) Analysis
4.6. Double Immuno-Staining Analysis
4.7. Evaluation of Immunohistochemistry
4.8. Computerized Morphometric Measurements and Image Analysis
4.9. RNA Isolation and Preparation
4.10. Gene Expression Analysis by Real-Time PCR (qRT-PCR)
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| OA | Osteoarthritis |
| GP-39 | Glycoprotein 39 |
| CHI3L1 YKL-40 | Chitinase 3-like-1 |
| SF | Synovial fluid |
| SZP | Superficial zone protein |
| PRG4 | Proteoglycan 4 |
| ACLT | Anterior cruciate ligament transection |
References
- Giunta, S.; Castorina, A.; Marzagalli, R.; Szychlinska, M.A.; Pichler, K.; Mobasheri, A.; Musumeci, G. Ameliorative effects of PACAP against cartilage degeneration. Morphological, immunohistochemical and biochemical evidence from in vivo and in vitro models of rat osteoarthritis. Int. J. Mol. Sci. 2015, 16, 5922–5944. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Matta, C.; Zákány, R.; Musumeci, G. Chondrosenescence: Definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas 2015, 80, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Aiello, F.C.; Szychlinska, M.A.; di Rosa, M.; Castrogiovanni, P.; Mobasheri, A. Osteoarthritis in the XXIst century: Risk factors and behaviours that influence disease onset and progression. Int. J. Mol. Sci. 2015, 16, 6093–6112. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Szychlinska, M.A.; Mobasheri, A. Age-related degeneration of articular cartilage in the pathogenesis of osteoarthritis: Molecular markers of senescent chondrocytes. Histol. Histopathol. 2015, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.; Malaguarnera, G.; de Gregorio, C.; D’Amico, F.; Mazzarino, M.C.; Malaguarnera, L. Modulation of chitotriosidase during macrophage differentiation. Cell. Biochem. Biophys. 2013, 66, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.; de Gregorio, C.; Malaguarnera, G.; Tuttobene, M.; Biazzo, F.; Malaguarnera, L. Evaluation of AMCase and CHIT-1 expression in monocyte macrophages lineage. Mol. Cell. Biochem. 2013, 374, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.; Malaguarnera, G.; de Gregorio, C.; Drago, F.; Malaguarnera, L. Evaluation of CHI3L-1 and CHIT-1 expression in differentiated and polarized macrophages. Inflammation 2013, 36, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Väänänen, T.; Koskinen, A.; Paukkeri, E.L.; Hämäläinen, M.; Moilanen, T.; Moilanen, E.; Vuolteenaho, K. YKL-40 as a novel factor associated with inflammation and catabolic mechanisms in osteoarthritic joints. Mediat. Inflamm. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.; Szychlinska, M.A.; Tibullo, D.; Malaguarnera, L.; Musumeci, G. Expression of CHI3L1 and CHIT1 in osteoarthritic rat cartilage model. A morphological study. Eur. J. Histochem. 2014, 58. [Google Scholar] [CrossRef] [PubMed]
- Jay, G.D.; Fleming, B.C.; Watkins, B.A.; McHugh, K.A.; Anderson, S.C.; Zhang, L.X.; Teeple, E.; Waller, K.A.; Elsaid, K.A. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2010, 62, 2382–2391. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, K.A.; Zhang, L.; Waller, K.; Tofte, J.; Teeple, E.; Fleming, B.C.; Jay, G.D. The impact of forced joint exercise on lubricin biosynthesis from articular cartilage following ACL transection and intra-articular lubricin’s effect in exercised joints following ACL transection. Osteoarthr. Cartil. 2012, 20, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R.; Musumeci, G.; Sicurezza, E.; Loreto, C. Lubricin in human temporomandibular joint disc: An immunohistochemical study. Arch. Oral Biol. 2012, 57, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Loreto, C.; Carnazza, M.L.; Strehin, I.; Elisseeff, J. OA cartilage derived chondrocytes encapsulated in poly(ethylene glycol) diacrylate (PEGDA) for the evaluation of cartilage restoration and apoptosis in an in vitro model. Histol. Histopathol. 2011, 26, 1265–1278. [Google Scholar] [PubMed]
- Loeser, R.F. Osteoarthritis year in review 2013: Biology. Osteoarthr. Cartil. 2013, 21, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Ruan, M.Z.; Erez, A.; Guse, K.; Dawson, B.; Bertin, T.; Chen, Y.; Jiang, M.M.; Yustein, J.; Gannon, F.; Lee, B.H. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci. Transl. Med. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.; Alini, M.; Cucchiarini, M.; Dodge, G.R.; Eglin, D.; Guilak, F.; Madry, H.; Mata, A.; Mauck, R.L.; Semino, C.E.; et al. Tissue engineering for articular cartilage repair-the state of the art. Eur. Cell Mater. 2013, 2, 248–267. [Google Scholar]
- Musumeci, G.; Loreto, C.; Carnazza, M.L.; Coppolino, F.; Cardile, V.; Leonardi, R. Lubricin is expressed in chondrocytes derived from osteoarthritic cartilage encapsulated in poly(ethylene glycol) diacrylate scaffold. Eur. J. Histochem. 2011, 55, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Mazzone, V.; Szychlinska, M.A.; Castorina, S.; Loreto, C. Histochemistry as a unique approach for investigating normal and osteoarthritic cartilage. Eur. J. Histochem. 2014, 58, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, I.; Akagi, M.; Inoue, S.; Yamagishi, K.; Mori, S.; Asada, S. Expressions of local renin-angiotensin system components in chondrocytes. Eur. J. Histochem. 2014, 58. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Sakamoto, Y.; Baba, O.; Qin, C.; Murakami, G.; Cho, B.H. An immunohistochemical study of matrix proteins in the craniofacial cartilage in midterm human fetuses. Eur. J. Histochem. 2013, 57. [Google Scholar] [CrossRef] [PubMed]
- Loreto, C.; Leonardi, R.; Musumeci, G.; Pannone, G.; Castorina, S. An ex vivo study on immunohistochemical localization of MMP-7 and MMP-9 in temporomandibular joint discs with internal derangement. Eur. J. Histochem. 2013, 57. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Martin, F.; Mannigel, K.; Kaltschmidt, K.; Sack, U.; Anderer, U. Three dimensional scaffold-free fusion culture: The way to enhance chondrogenesis of in vitro propagated human articular chondrocytes. Eur. J. Histochem. 2013, 57. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Weinberg, A.M.; Al-Wasiyah, M.K.; Alqahtani, M.H.; Mobasheri, A. Biomarkers of chondrocyte apoptosis and autophagy in osteoarthritis. Int. J. Mol. Sci. 2015, 16, 20560–20575. [Google Scholar] [CrossRef] [PubMed]
- Halper, J.; Kjaer, M. Basic components of connective tissues and extracellular matrix: Elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv. Exp. Med. Biol. 2014, 802, 31–47. [Google Scholar] [PubMed]
- Olson, S.A.; Horne, P.; Furman, B.; Huebner, J.; Al-Rashid, M.; Kraus, V.B.; Guilak, F. The role of cytokines in posttraumatic arthritis. J. Am. Acad. Orthop. Surg. 2014, 22, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Halper, J. Proteoglycans and diseases of soft tissues. Adv. Exp. Med. Biol. 2014, 802, 49–58. [Google Scholar] [PubMed]
- Daniel, M. Boundary cartilage lubrication: Review of current concepts. Wien. Med. Wochenschr. 2014, 164, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Zivanović, S.; Rackov, L.P.; Vojvodić, D.; Vucetić, D. Human cartilage glycoprotein 39—Biomarker of joint damage in knee osteoarthritis. Int. Orthop. 2009, 33, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Baeten, D.; Steenbakkers, P.G.; Rijnders, A.M.; Boots, A.M.; Veys, E.M.; de Keyser, F. Detection of major histocompatibility complex/human cartilage GP-39 complexes in rheumatoid arthritis synovitis as a specific and independent histologic marker. Arthritis Rheum. 2004, 50, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, J.M.; Bahrke, S.; Sigurdsson, B.T.; Ng, C.H.; Petersen, P.H.; Sigurjonsson, O.E.; Jonsson, H., Jr.; Gislason, J.; Thormodsson, F.R.; Peter, M.G. Partially acetylated chitooligosaccharides bind to YKL-40 and stimulate growth of human osteoarthritic chondrocytes. Biochem. Biophys. Res. Commun. 2013, 434, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Volck, B.; Ostergaard, K.; Johansen, J.S.; Garbarsch, C.; Price, P.A. The distribution of YKL-40 in osteoarthritic and normal human articular cartilage. Scand. J. Rheumatol. 1999, 28, 171–179. [Google Scholar] [PubMed]
- Guan, J.; Liu, Z.; Li, F.; Feng, J.S.; Wang, H.J.; Chu, J.G.; Song, Y.Z.; Xie, L.; Ding, L.B. Increased synovial fluid YKL-40 levels are linked with symptomatic severity in knee osteoarthritis patients. Clin. Lab. 2015, 61, 991–997. [Google Scholar] [PubMed]
- Musumeci, G.; Lo Furno, D.; Loreto, C.; Giuffrida, R.; Caggia, S.; Leonardi, R.; Cardile, V. Mesenchymal stem cells from adipose tissue which have been differentiated into chondrocytes in three-dimensional culture express lubricin. Exp. Biol. Med. 2011, 236, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, T.E.; McAllister, J.R.; Lun, V.; Wiley, J.P.; Schmidt, T.A. Diminished cartilage-lubricating ability of human osteoarthritic synovial fluid deficient in proteoglycan 4: Restoration through proteoglycan 4 supplementation. Arthritis Rheum. 2012, 64, 3963–3971. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Loreto, C.; Leonardi, R.; Castorina, S.; Giunta, S.; Carnazza, M.L.; Trovato, F.M.; Pichler, K.; Weinberg, A.M. The effects of physical activity on apoptosis and lubricin expression in articular cartilage in rats with glucocorticoid-induced osteoporosis. J. Bone Miner. Metab. 2013, 31, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R.; Loreto, C.; Talic, N.; Caltabiano, R.; Musumeci, G. Immunolocalization of lubricin in the rat periodontal ligament during experimental tooth movement. Acta Histochem. 2012, 114, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R.; Rusu, M.C.; Loreto, F.; Loreto, C.; Musumeci, G. Immunolocalization and expression of lubricin in the bilaminar zone of the human temporomandibular joint disc. Acta Histochem. 2012, 114, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Loreto, C.; Carnazza, M.L.; Cardile, V.; Leonardi, R. Acute injury affects lubricin expression in knee menisci. An immunohistochemical study. Ann. Anat. 2013, 195, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Trovato, F.M.; Pichler, K.; Weinberg, A.M.; Loreto, C.; Castrogiovanni, P. Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model. An “in vivo” and “in vitro” study on lubricin expression. J. Nutr. Biochem. 2013, 24, 2064–2075. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Kozhemyakina, E.; Hung, H.H.; Grodzinsky, A.J.; Lassar, A.B. Mechanical motion promotes expression of Prg4 in articular cartilage via multiple CREB-dependent, fluid flow shear stress-induced signaling pathways. Genes Dev. 2014, 28, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Imbesi, R.; Giunta, S.; Szychlinska, M.A.; Loreto, C.; Castorina, S.; Mobasheri, A. Physical activity ameliorates cartilage degeneration in a rat model of aging: A study on lubricin expression. Scand. J. Med. Sci. Sports 2015, 25, e222–e230. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, P.; Musumeci, G. Which is the Best Physical Treatment for Osteoarthritis? J. Funct. Morphol. Kinesiol. 2016, 1, 54–68. [Google Scholar] [CrossRef]
- Catterall, J.B.; Stabler, T.V.; Flannery, C.R.; Kraus, V.B. Changes in serum and synovial fluid biomarkers after acute injury (NCT00332254). Arthritis Res. Ther. 2010, 12. [Google Scholar] [CrossRef] [PubMed]
- Flannery, C.R. Novel therapies in OA. Curr. Drug Targets 2010, 11, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Flannery, C.R.; Zollner, R.; Corcoran, C.; Jones, A.R.; Root, A.; Rivera-Bermúdez, M.A.; Blanchet, T.; Gleghorn, J.P.; Bonassar, L.J.; Bendele, A.M.; et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009, 60, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, X. Intraarticular treatments for osteoarthritis: New perspectives. Curr. Drug Targets 2010, 11, 546–560. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Xu, C.; Li, X.; Song, J.; Yu, B. Treatment with recombinant lubricin attenuates osteoarthritis by positive feedback loop between articular cartilage and subchondral bone in ovariectomized rats. Bone 2015, 74, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.P.; Chen, W.P.; Wu, L.D. Lubricin: A novel potential biotherapeutic approaches for the treatment of osteoarthritis. Mol. Biol. Rep. 2011, 38, 2879–2885. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Leonardi, R.; Carnazza, M.L.; Cardile, V.; Pichler, K.; Weinberg, A.M.; Loreto, C. Aquaporin 1 (AQP1) expression in experimentally induced osteoarthritic knee menisci: An in vivo and in vitro study. Tissue Cell 2013, 45, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Buck, R.J.; Wirth, W.; Dreher, D.; Nevitt, M.; Eckstein, F. Frequency and spatial distribution of cartilage thickness change in knee osteoarthritis and its relation to clinical and radiographic covariates—Data from the osteoarthritis initiative. Osteoarthr. Cartil. 2013, 21, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Pichler, K.; Loreto, C.; Leonardi, R.; Reuber, T.; Weinberg, A.M.; Musumeci, G. In rat with glucocorticoid-induced osteoporosis, RANKL is downregulated in bone cells by physical activity (treadmill and vibration stimulation training). Histol. Histopathol. 2013, 28, 1185–1196. [Google Scholar] [PubMed]
- Mankin, H.J.; Dorfman, H.; Lippiello, L.; Zarins, A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J. Bone Jt. Surg. 1971, 53, 523–537. [Google Scholar]
- Kraus, V.B.; Huebner, J.L.; Stabler, T.; Flahiff, C.M.; Setton, L.A.; Fink, C.; Vilim, V.; Clark, A.G. Ascorbic acid increase the severity of spontaneous knee osteoarthritis in a guinea pig model. Arthritis Rheum. 2004, 50, 1822–1831. [Google Scholar] [CrossRef] [PubMed]
- Pauli, C.; Grogan, S.P.; Patil, S.; Otsuki, S.; Hasegawa, A.; Koziol, J.; Lotz, M.K.; D’Lima, D.D. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthr. Cartil. 2011, 19, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Loreto, C.; Lo Castro, E.; Musumeci, G.; Loreto, F.; Rapisarda, G.; Rezzani, R.; Castorina, S.; Leonardi, R.; Rusu, M.C. Aquaporin 1 expression in human temporomandibular disc. Acta Histochem. 2012, 114, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, P.; Trovato, F.M.; Szychlinska, M.A.; Loreto, C.; Giunta, S.; Scuderi, S.; Passanisi, R.; Fidone, F.; Fagone, P.; Imbesi, R.; et al. Effects of Synthetic Anti-Inflammatory Sterol in CB3V-Induced Myocarditis: A Morphological Study on Heart Muscle Tissue. J. Funct. Morphol. Kinesiol. 2016, 1, 69–89. [Google Scholar] [CrossRef]
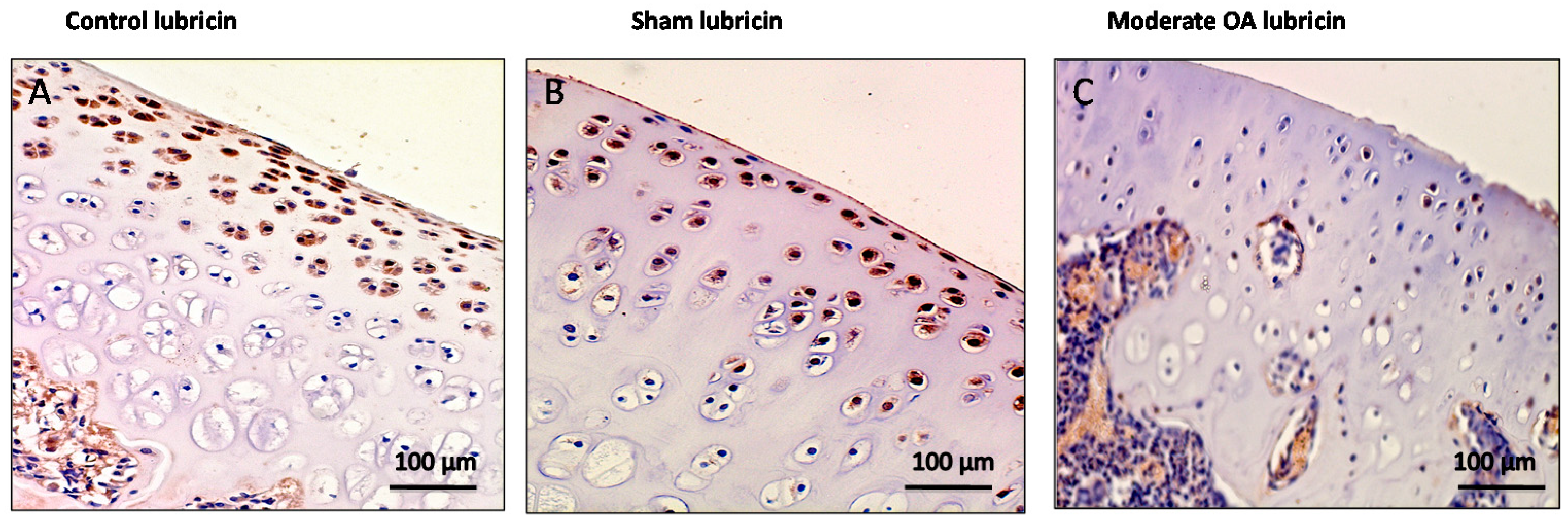

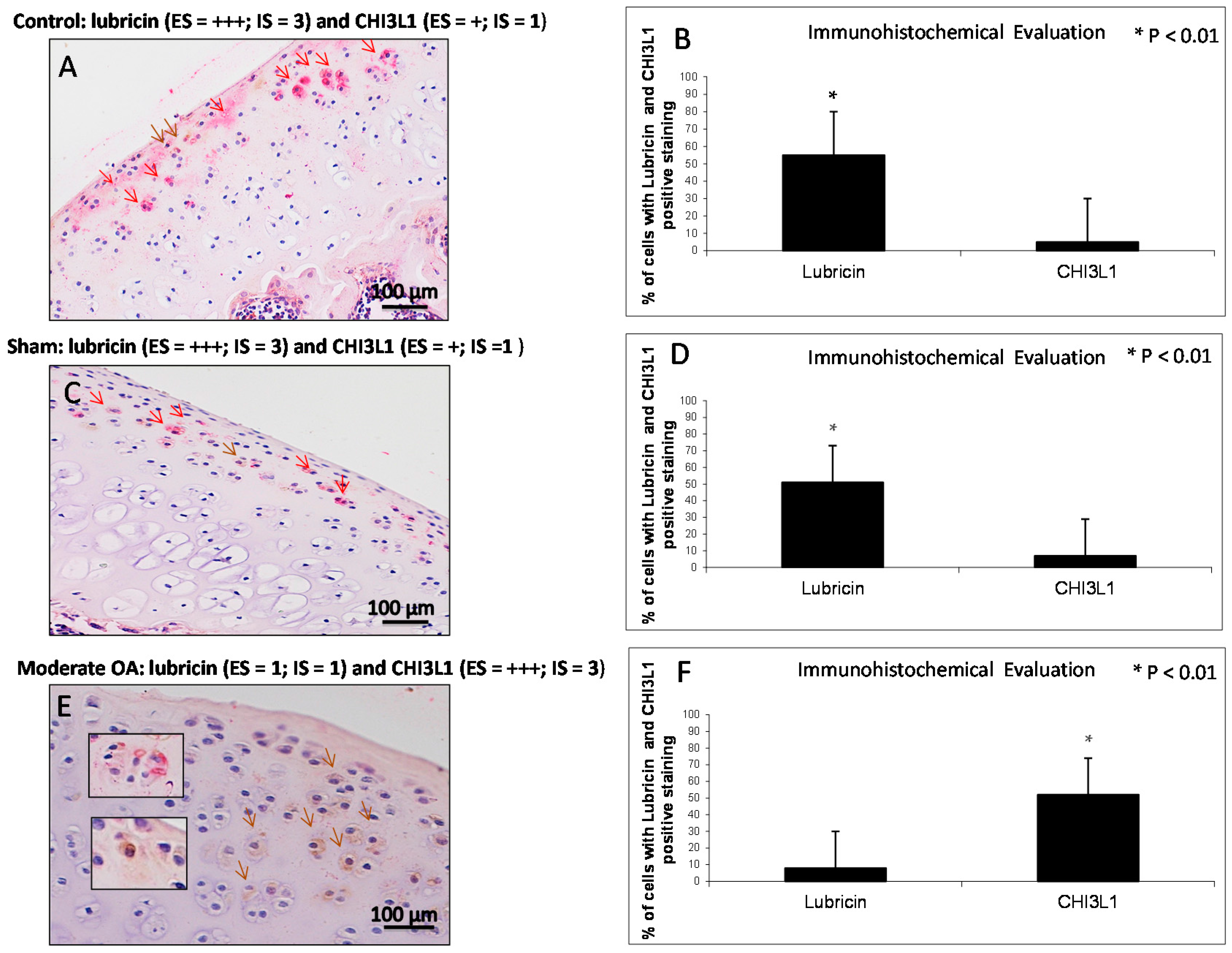
| Groups | Lubricin | CHI3L1 |
|---|---|---|
| Control rats without ACLT | Very strong immunostaining (ES = +++; IS = 4) | Weak/absent immunostaining (ES = +; IS = 1) |
| Sham operated control rats | Very strong immunostaining (ES = +++; IS = 4) | Weak/absent immunostaining (ES = +; IS = 1) |
| Experimental rats with ACLT (OA) | Weak/absent immunostaining (ES = +; IS = 1) | Strong immunostaining (ES = +++; IS = 3) |
| Primers | Forward | Reverse | Ta | Size |
|---|---|---|---|---|
| PRG4 | CTACAACAGCTTCTGCGAAGAA | GATTTGGGTGAACGTTTGGTGG | 60 | 117 |
| CHI3L1 | GAGCTGCTTCCCAGATGCCC | CATGCCATACAGGGTTACGTC | 60 | 121 |
| ACTB | CATGTACGTAGCCATCCAGG | CTCTCAGCTGTGGTGGTGAA | 57 | 225 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szychlinska, M.A.; Trovato, F.M.; Di Rosa, M.; Malaguarnera, L.; Puzzo, L.; Leonardi, R.; Castrogiovanni, P.; Musumeci, G. Co-Expression and Co-Localization of Cartilage Glycoproteins CHI3L1 and Lubricin in Osteoarthritic Cartilage: Morphological, Immunohistochemical and Gene Expression Profiles. Int. J. Mol. Sci. 2016, 17, 359. https://doi.org/10.3390/ijms17030359
Szychlinska MA, Trovato FM, Di Rosa M, Malaguarnera L, Puzzo L, Leonardi R, Castrogiovanni P, Musumeci G. Co-Expression and Co-Localization of Cartilage Glycoproteins CHI3L1 and Lubricin in Osteoarthritic Cartilage: Morphological, Immunohistochemical and Gene Expression Profiles. International Journal of Molecular Sciences. 2016; 17(3):359. https://doi.org/10.3390/ijms17030359
Chicago/Turabian StyleSzychlinska, Marta Anna, Francesca Maria Trovato, Michelino Di Rosa, Lucia Malaguarnera, Lidia Puzzo, Rosy Leonardi, Paola Castrogiovanni, and Giuseppe Musumeci. 2016. "Co-Expression and Co-Localization of Cartilage Glycoproteins CHI3L1 and Lubricin in Osteoarthritic Cartilage: Morphological, Immunohistochemical and Gene Expression Profiles" International Journal of Molecular Sciences 17, no. 3: 359. https://doi.org/10.3390/ijms17030359
APA StyleSzychlinska, M. A., Trovato, F. M., Di Rosa, M., Malaguarnera, L., Puzzo, L., Leonardi, R., Castrogiovanni, P., & Musumeci, G. (2016). Co-Expression and Co-Localization of Cartilage Glycoproteins CHI3L1 and Lubricin in Osteoarthritic Cartilage: Morphological, Immunohistochemical and Gene Expression Profiles. International Journal of Molecular Sciences, 17(3), 359. https://doi.org/10.3390/ijms17030359