Spinal Glutamate Transporters Are Involved in the Development of Electroacupuncture Tolerance
Abstract
:1. Introduction
2. Results
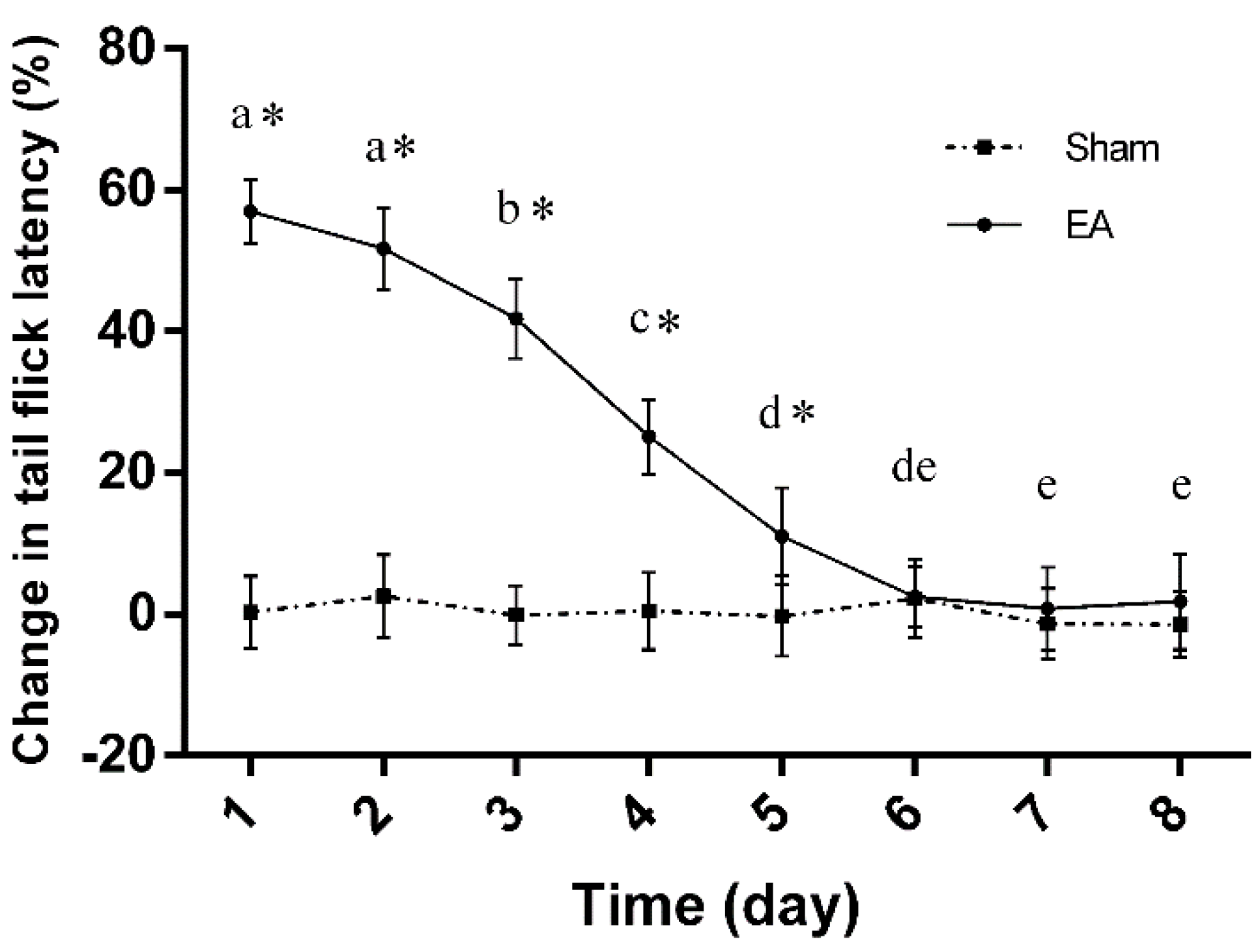
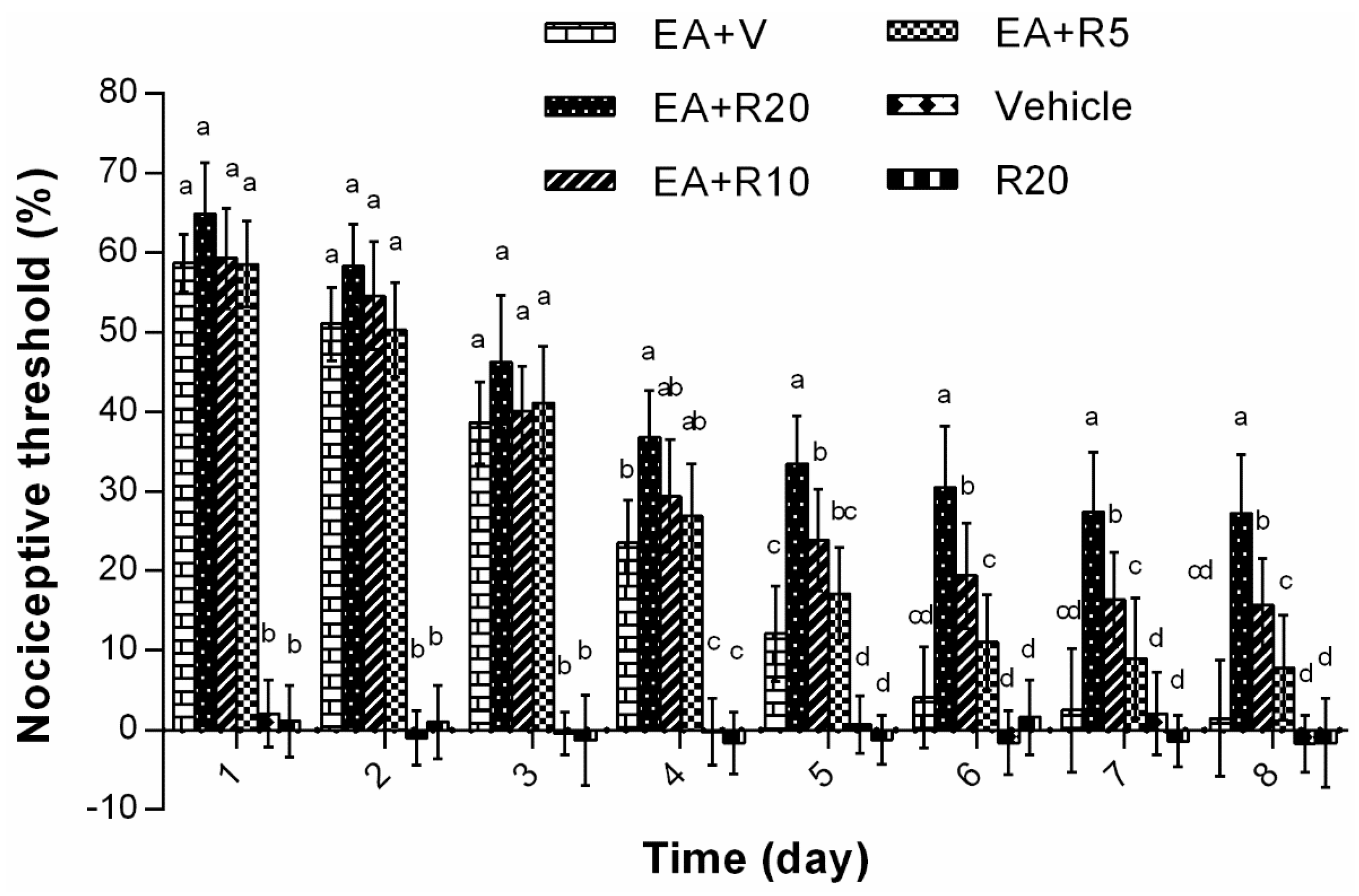
2.1. Dynamic Changes in Tail Flick Latency of Rats with Repeated EA
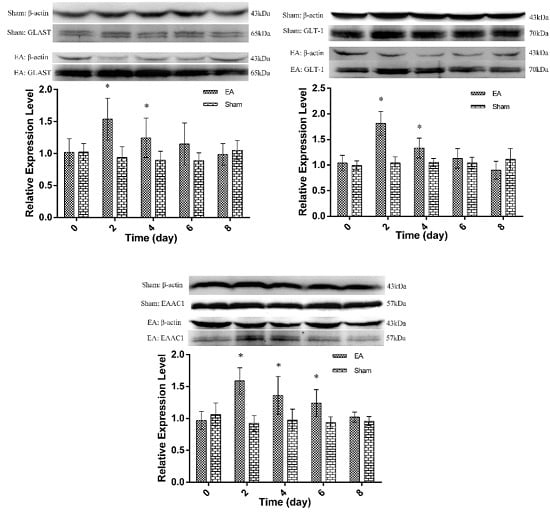
2.2. Expression of Spinal GTs Induced by Single and Repeated EA in Rats
2.3. The Effect of Riluzole on Changes in Tail Flick Latency Induced by Repeated EA
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals
4.3. Experiment Design
4.4. EA Stimulation
4.5. Assay of Tail Flick Latency and Selection of Responders
4.6. RT-PCR
4.7. Western Blot
4.8. Intrathecal Placement
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| EA | Electroacupuncture |
| GT | Glutamate transporter |
| GLAST | l-Glutamate-l-aspartate transporter |
| GLT-1 | Glutamate transporter 1 |
| EAAC1 | Excitatory amino-acid carrier 1 |
| NMDAR | N-Methyl-d-aspartic acid receptor |
| CFA | Complete Freund’s adjuvant |
| i.t. | Intrathecal administration |
| TFL | Tail flick latency |
| bTFL | Basal tail flick latency |
References
- Ko, E.S.; Kim, S.K.; Kim, J.T.; Lee, G.; Han, J.B.; Rho, S.W.; Hong, M.C.; Bae, H.; Min, B. The difference in mRNA expressions of hypothalamic CCK and CCK-A and -B receptors between responder and non-responder rats to high frequency electroacupuncture analgesia. Peptides 2006, 27, 1841–1845. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Kim, Y.R.; Jang, J.Y.; Shin, H.K.; Choi, B.T. Electroacupuncture confers antinociceptive effects via inhibition of glutamate transporter downregulation in complete freund’s adjuvant-injected rats. Evid-Based Complement. Altern. Med. 2012, 2012, 643973. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Tang, J.; Han, J.S. The implication of central serotonin in electro-acupuncture tolerance in the rat. Sci. China Chem. 1982, 12, 256–262. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Chang, J.K.; Han, J.S. Endomorphin and mu-opioid receptors in mouse brain mediate the analgesic effect induced by 2 Hz but not 100 Hz electroacupuncture stimulation. Neurosci. Lett. 2000, 294, 159–162. [Google Scholar] [CrossRef]
- Han, J.S. Acupuncture: Neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003, 26, 17–22. [Google Scholar] [CrossRef]
- Ni, L.; Lv, Z.B.; An, L.; Liu, D.X. The changes of opiate receptor binding in rat brain under electroacupuncture tolerance condition. Acupunct. Res. 1987, 12, 62–65. (In Chinese) [Google Scholar]
- Dong, Y.N.; Mao, M.H.; Wang, X.M.; Han, J.S. Changes in the binding characteristics of central kappa-opioid receptor during the development of 100 Hz electroacupuncture (EA) tolerance in rats. Acta Physiol. Sin. 1998, 50, 663–670. [Google Scholar] [CrossRef]
- Han, J.S. Opioid and antiopioid peptides: A model of Yin-Yang balance in acupuncture mechanisms of pain modulation. In Clinical Acupuncture; Stux, G., Hammerschlag, R., Eds.; Springer: Berlin, Germany; Heidelberg, Germany, 2001; pp. 51–68. [Google Scholar]
- Huang, C.; Long, H.; Shi, Y.; Han, J.; Wan, Y. Ketamine enhances the efficacy to and delays the development of tolerance to electroacupuncture-induced antinociception in rats. Neurosci. Lett. 2005, 375, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.T.; Zhou, Z.F.; Han, J.S. Tolerance to electroacupuncture analgesia was reversed by microinjection of 5-hydroxytryptophan into nuclei accumbens in the rabbit. Int. J. Neurosci. 1982, 17, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.W.; Tang, J.; Han, J.S. Central nrepinephrine and tolerance to electroacupuncture analgesia. Sci. China Ser. B 1984, 14, 818–824. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Z.X.; Zhang, Y.Z.; Jia, S.P.; Lu, X.J.; Yu, R.R.; Wang, X.G.; Chen, R.S. Role of calcium in electro-acupuncture analgesia and the developments of analgesic tolerance to electro-acupuncture and morphine. Sci. China Ser. B 1987, 30, 974–985. [Google Scholar]
- Xie, X.Y. The change of mitochondrial protein boune Ca2+ in some brain regions under the conditions of electroacupuncture or morphine analgesia and the analgesic tolerance (Chinese article). Acta Physiol. Sin. 1988, 40, 553–560. [Google Scholar]
- Lee, A.D.; Hsu, E.S.Z. Mechanisms of acupuncture analgesia. In Acupuncture for Pain Management; Lin, Y.C., Hsu, E.S.Z., Eds.; Springer: New York, NY, USA, 2014; pp. 73–85. [Google Scholar]
- Weng, H.R.; Chen, J.H.; Cata, J.P. Inhibition of glutamate uptake in the spinal cord induces hyperalgesia and increased responses of spinal dorsal horn neurons to peripheral afferent stimulation. Neuroscience 2006, 138, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.X.; Gu, J.; Stephens, R.L., Jr. Role of spinal cord glutamate transporter during normal sensory transmission and pathological pain states. Mol. Pain 2005, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Guillet, B.A.; Velly, L.J.; Canolle, B.; Masmejean, F.M.; Nieoullon, A.L.; Pisano, P. Differential regulation by protein kinases of activity and cell surface expression of glutamate transporters in neuron-enriched cultures. Neurochem. Int. 2005, 46, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.L.; Wang, S.X.; Lim, G.; Sung, B.; Zeng, Q.; Mao, J.R. Inhibition of the ubiquitin-proteasome activity prevents glutamate transporter degradation and morphine tolerance. Pain 2008, 140, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Sung, B.; Ji, R.R.; Lim, G. Chronic morphine induces downregulation of spinal glutamate transporters: Implications in morphine tolerance and abnormal pain sensitivity. J. Neurosci. 2002, 22, 8312–8323. [Google Scholar] [PubMed]
- Nakagawa, T.; Ozawa, T.; Shige, K.; Yamamoto, R.; Minami, M.; Satoh, M. Inhibition of morphine tolerance and dependence by MS-153, a glutamate transporter activator. Eur. J. Pharmacol. 2001, 419, 39–45. [Google Scholar] [CrossRef]
- Han, J.S.; LI, S.J.; Tang, J. Tolerance to electroacupuncture and its cross tolerance to morphine. Neuropharmacology 1981, 20, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.L.; Ding, M.X.; Xiong, C.; Zhou, M.Y.; Qiu, Z.Y.; Wang, Q. Effects of electroacupuncture of different frequencies on the release profile of endogenous opioid peptides in the central nerve system of goats. Evid-Based Complement. Altern. Med. 2012, 2012, 476457. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.L.; Ding, M.X.; Wei, J.; Wu, Y.Q.; Qiu, Z.Y.; Chen, J.G.; Liu, D.M.; Hu, C.M.; Hu, M.L.; Shah, Z.; et al. Electroacupuncture-induced dynamic processes of gene expression levels of endogenous opioid peptide precursors and opioid receptors in the CNS of goats. Evid-Based Complement. Altern. Med. 2013, 2013, 257682. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Z.; Guo, S.Y.; Yin, Q.Z.; Hisamitsu, T.; Jiang, X.H. An individual variation study of Electroacupuncture analgesia in rats using microarray. Am. J. Chin. Med. 2007, 35, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Hu, Z.P.; Jiang, S.Z.; Li, H.T.; Han, J.S.; Wan, Y. CCKB receptor antagonist L365,260 potentiates the efficacy to and reverses chronic tolerance to electroacupuncture-induced analgesia in mice. Brain Res. Bull. 2007, 71, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.Y.; Ding, Y.; Feng, Y.; Chen, S.H.; Xu, Y.Q.; Li, M.; Hu, M.L.; Qiu, Z.Y.; Ding, M.X. MiRNAs are involved in chronic electroacupuncture tolerance in the rat hypothalamus. Mol. Neurobiol. 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.H.; Zhang, W.; Fang, Y.; Xu, W.; Grandy, D.K.; Han, J.S. Endogenous orphanin FQ: Evidence for a role in the modulation of electroacupuncture analgesia and the development of tolerance to analgesia produced by morphine and electroacupuncture. Br. J. Pharmacol. 1998, 124, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Han, J. Effects of 2 Hz and 100 Hz electroacupuncture tolerance on the transcription of CNS kappa opioid receptor mRNA. J. Beijing Med. Univ. 1998, 30, 1–4. [Google Scholar]
- Xi, Q.; Guo, Y.; Guo, Y.; Wang, J. Brief investigation on acupuncture tolerance. J. Clin. Acupunct. Moxib. 2009, 25, 43–44. (In Chinese) [Google Scholar] [CrossRef]
- Liaw, W.J.; Stephens, R.L., Jr.; Binns, B.C.; Chu, Y.; Sepkuty, J.P.; Johns, R.A.; Rothstein, J.D.; Tao, Y.X. Spinal glutamate uptake is critical for maintaining normal sensory transmission in rat spinal cord. Pain 2005, 115, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, S.; Sung, B.; Lim, G.; Mao, J. Morphine induces ubiquitin-proteasome activity and glutamate transporter degradation. J. Biol. Chem. 2008, 283, 21703–21713. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Foster, J.; Lin, C.L. Glutamate transporter EAAT2: Regulation, function, and potential as a therapeutic target for neurological and psychiatric disease. Cell. Mol. Life Sci. 2015, 72, 3489–3506. [Google Scholar] [CrossRef] [PubMed]
- Azbill, R.D.; Mu, X.; Springer, J.E. Riluzole increases high-affinity glutamate uptake in rat spinal cord synaptosomes. Brain Res. 2000, 871, 175–180. [Google Scholar] [CrossRef]
- Trotti, D.; Peng, J.B.; Dunlop, J.; Hediger, M.A. Inhibition of the glutamate transporter EAAC1 expressed in Xenopus oocytes by phorbol esters. Brain Res. 2001, 914, 196–203. [Google Scholar] [CrossRef]
- Fumagalli, E.; Funicello, M.; Rauen, T.; Gobbi, M.; Mennini, T. Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur. J. Pharmacol. 2008, 578, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Hama, A.; Sagen, J. Antinociceptive effect of riluzole in rats with neuropathic spinal cord injury pain. J. Neurotrauma 2011, 28, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Han, J. Rat tail flick acupuncture analgesia model. Chin. Med. J. 1979, 92, 576–582. [Google Scholar]

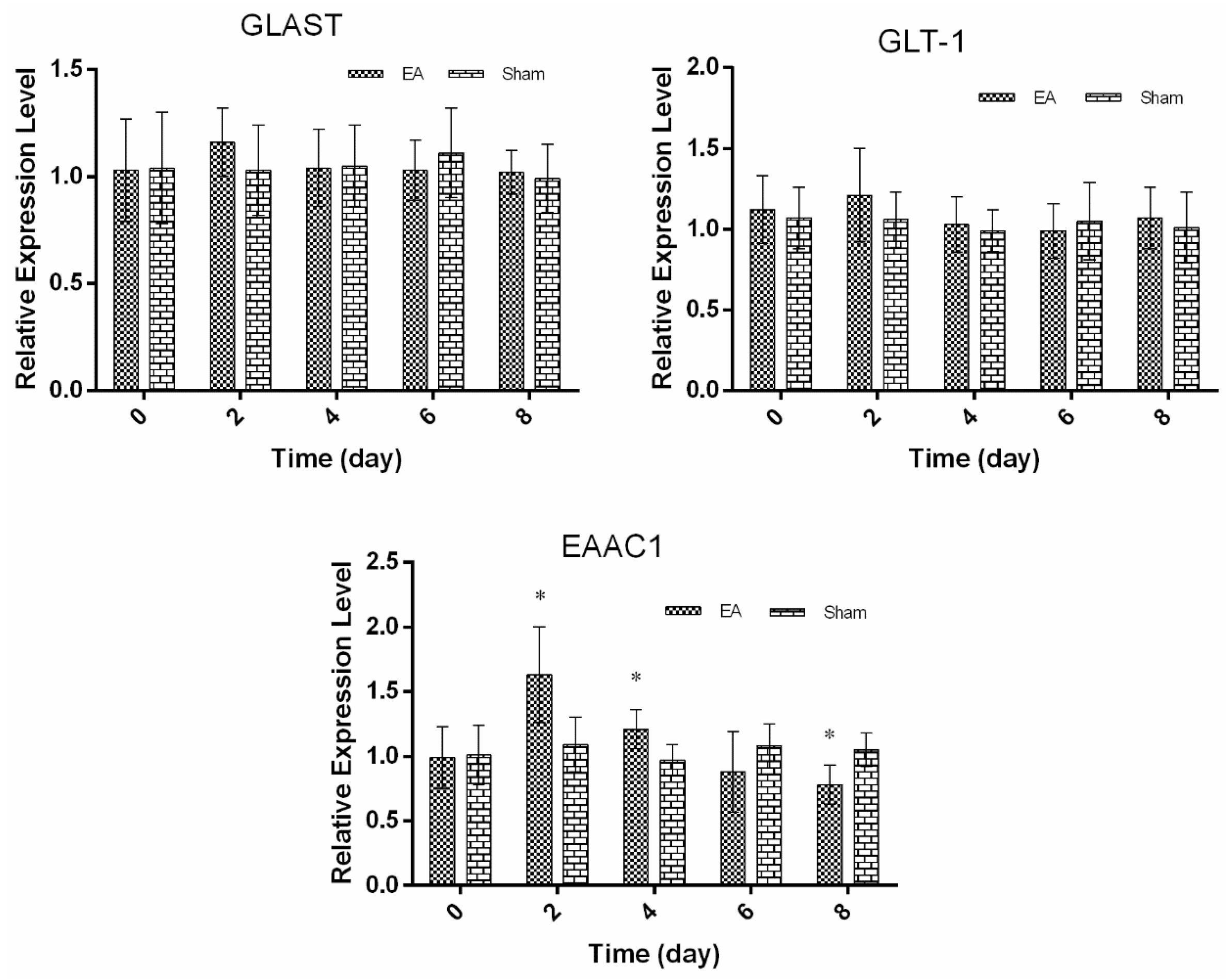
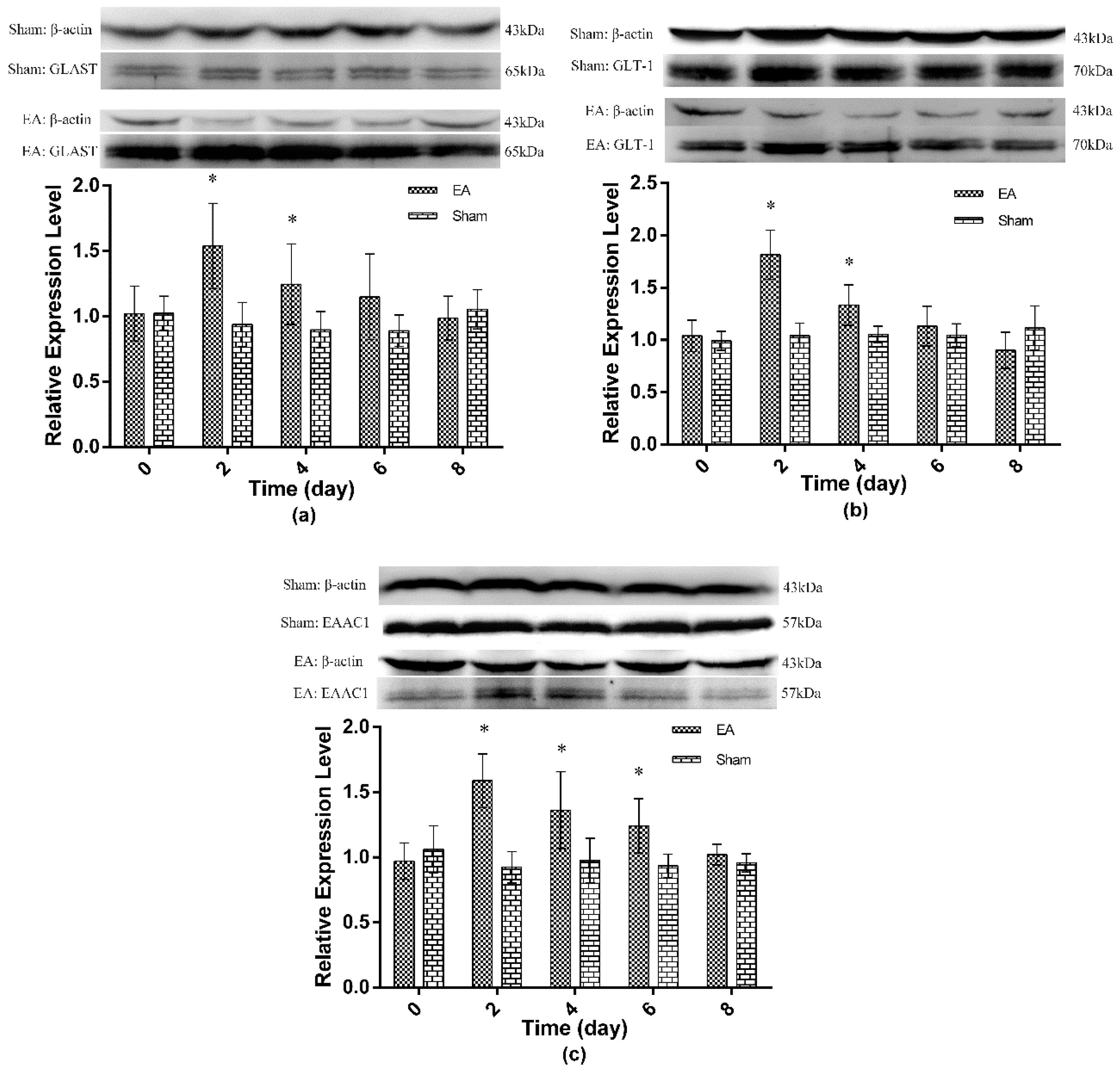
| Name | Accession Number | Primer Sequence |
|---|---|---|
| GLAST | NM_019225.2 | F: 5′-TATACAGTGACAGTCATCGTC-3′ |
| R: 5′-ACAAATCTGGTGATGCGT-3′ | ||
| GLT-1 | NM_017215.2 | F: 5′-TCCTGGATGGAGGTCAGATAG-3′ |
| R: 5′-CTCGGACTTGGAAAGGTGATAG-3′ | ||
| EAAC1 | NM_013032.3 | F: 5′-TACTGGGCATCGTGGTAGGA-3′ |
| R: 5′-TACAGCAATGACGGTGGTGG-3′ |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, L.; Ding, Y.; Zeng, J.; Feng, Y.; Li, M.; Ding, M. Spinal Glutamate Transporters Are Involved in the Development of Electroacupuncture Tolerance. Int. J. Mol. Sci. 2016, 17, 357. https://doi.org/10.3390/ijms17030357
Cui L, Ding Y, Zeng J, Feng Y, Li M, Ding M. Spinal Glutamate Transporters Are Involved in the Development of Electroacupuncture Tolerance. International Journal of Molecular Sciences. 2016; 17(3):357. https://doi.org/10.3390/ijms17030357
Chicago/Turabian StyleCui, Luying, Yi Ding, Jie Zeng, Yan Feng, Meng Li, and Mingxing Ding. 2016. "Spinal Glutamate Transporters Are Involved in the Development of Electroacupuncture Tolerance" International Journal of Molecular Sciences 17, no. 3: 357. https://doi.org/10.3390/ijms17030357
APA StyleCui, L., Ding, Y., Zeng, J., Feng, Y., Li, M., & Ding, M. (2016). Spinal Glutamate Transporters Are Involved in the Development of Electroacupuncture Tolerance. International Journal of Molecular Sciences, 17(3), 357. https://doi.org/10.3390/ijms17030357