Triptolide Combined with Radiotherapy for the Treatment of Nasopharyngeal Carcinoma via NF-κB-Related Mechanism
Abstract
:1. Introduction
2. Results
2.1. Effects of Combined Therapy on CNE Cell Proliferation
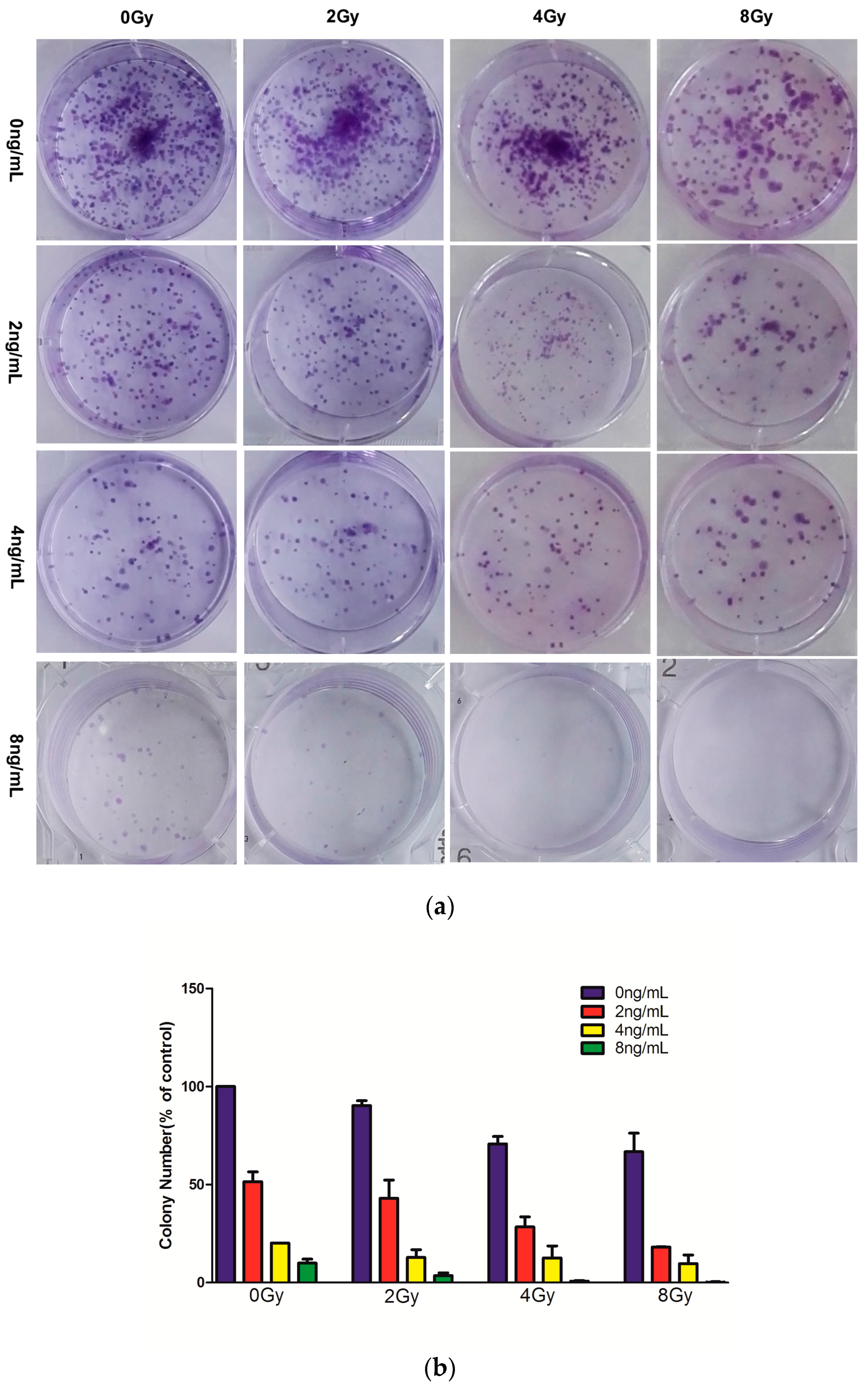
2.2. Effects of Combined Therapy on CNE Cell Clonogenicity
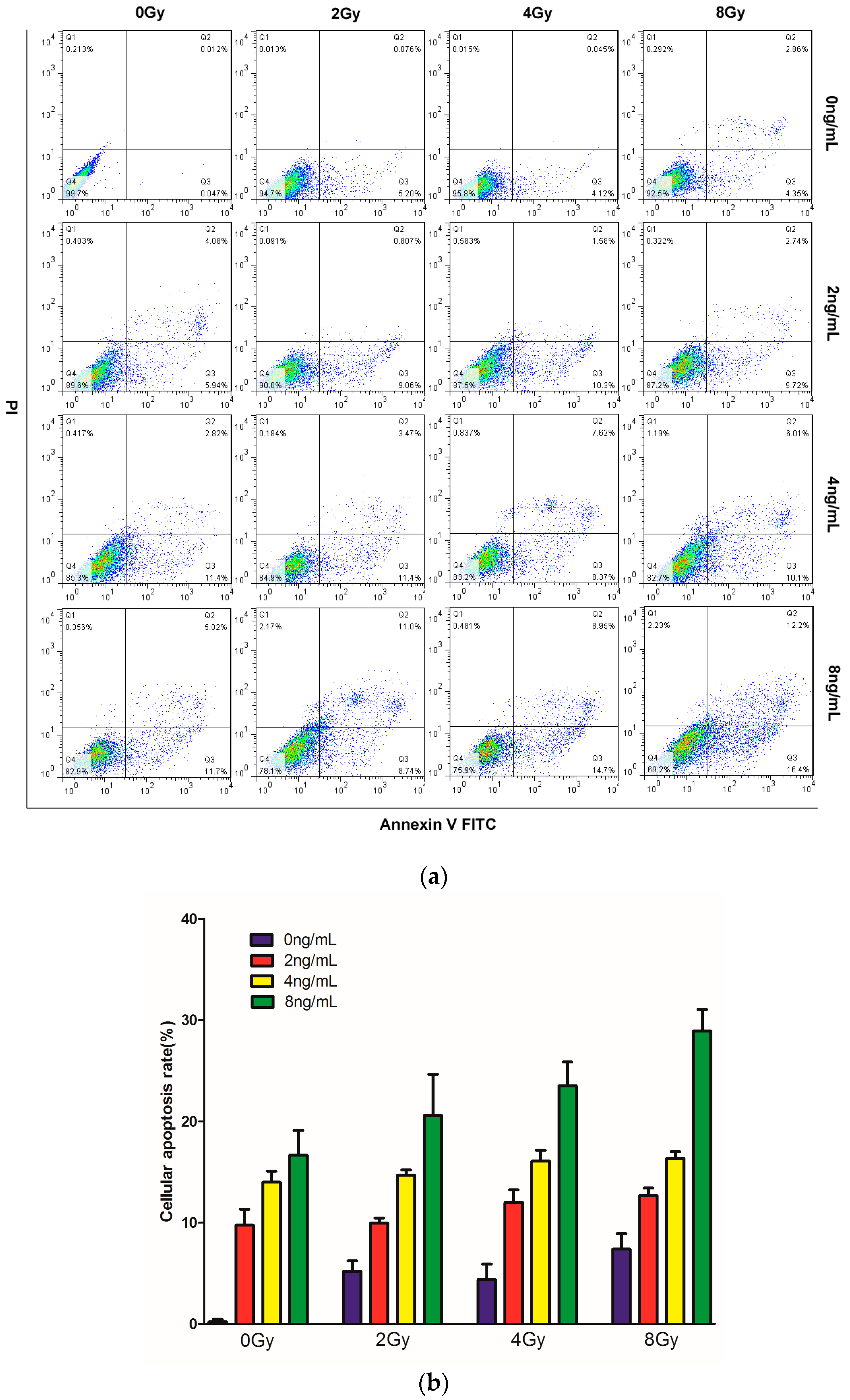
2.3. Effects of TPL Combination Treatments on Apoptotic Process in CNE Cells
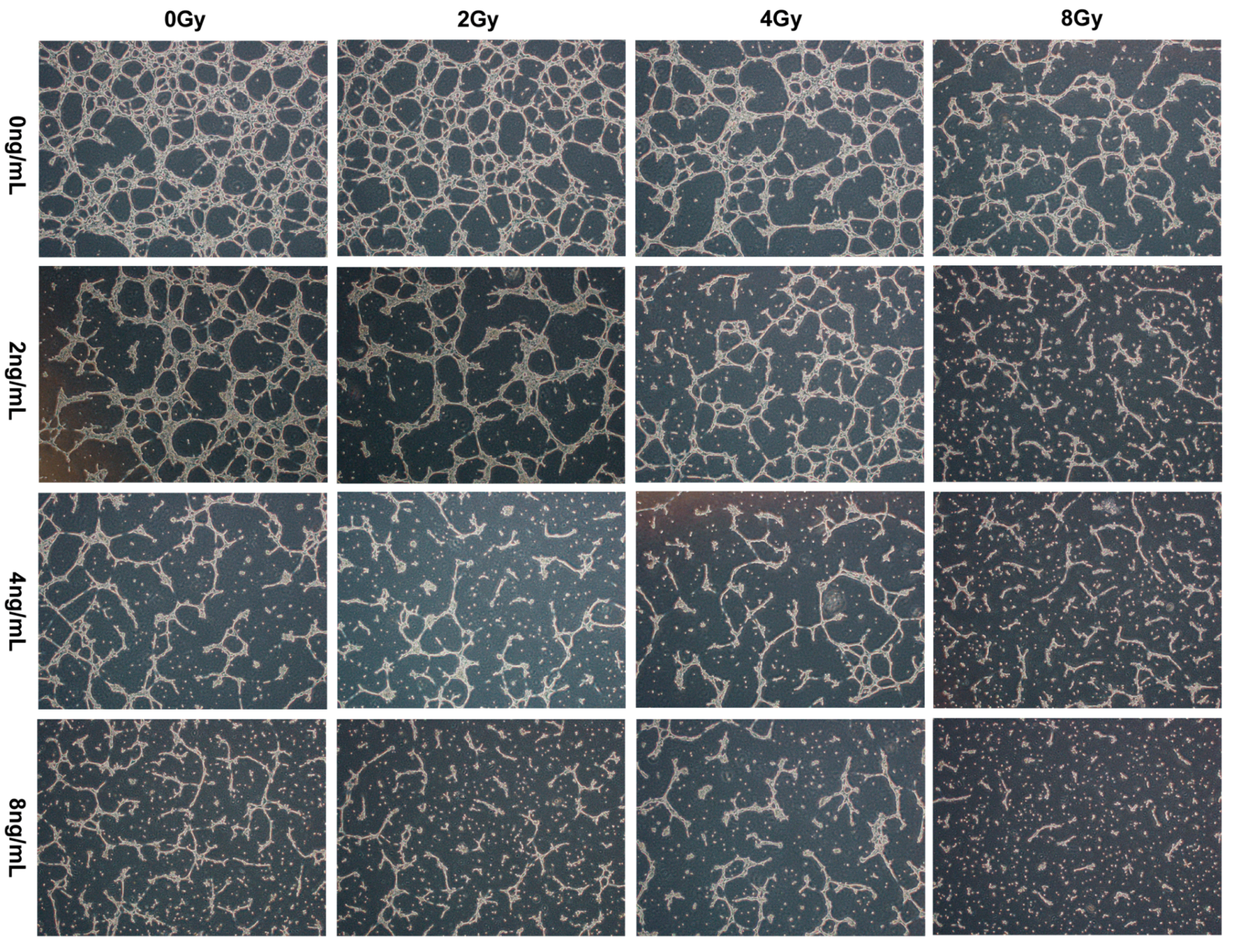
2.4. Effects of TPL Combination Therapy on Tube Formation of HUVECs
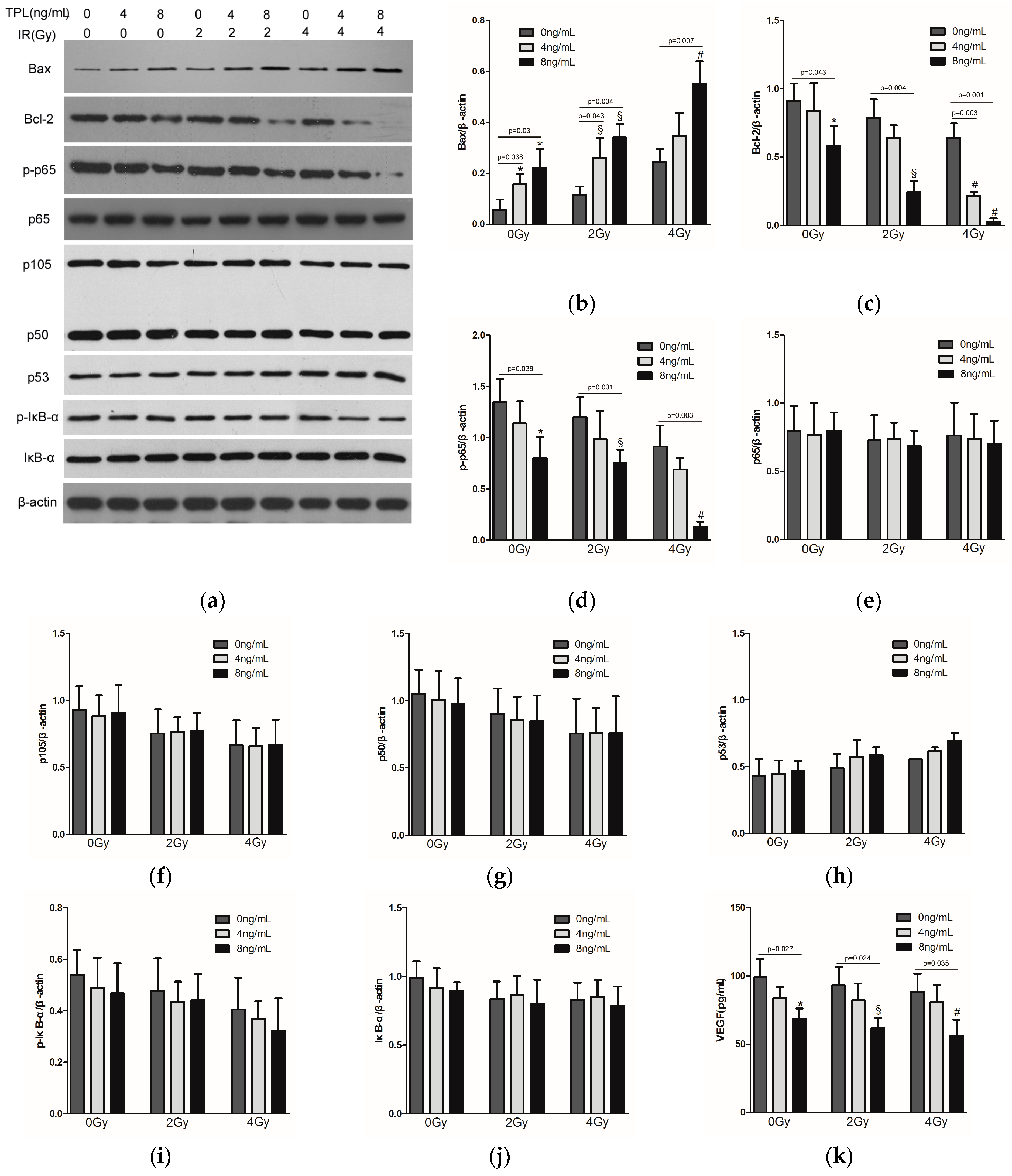
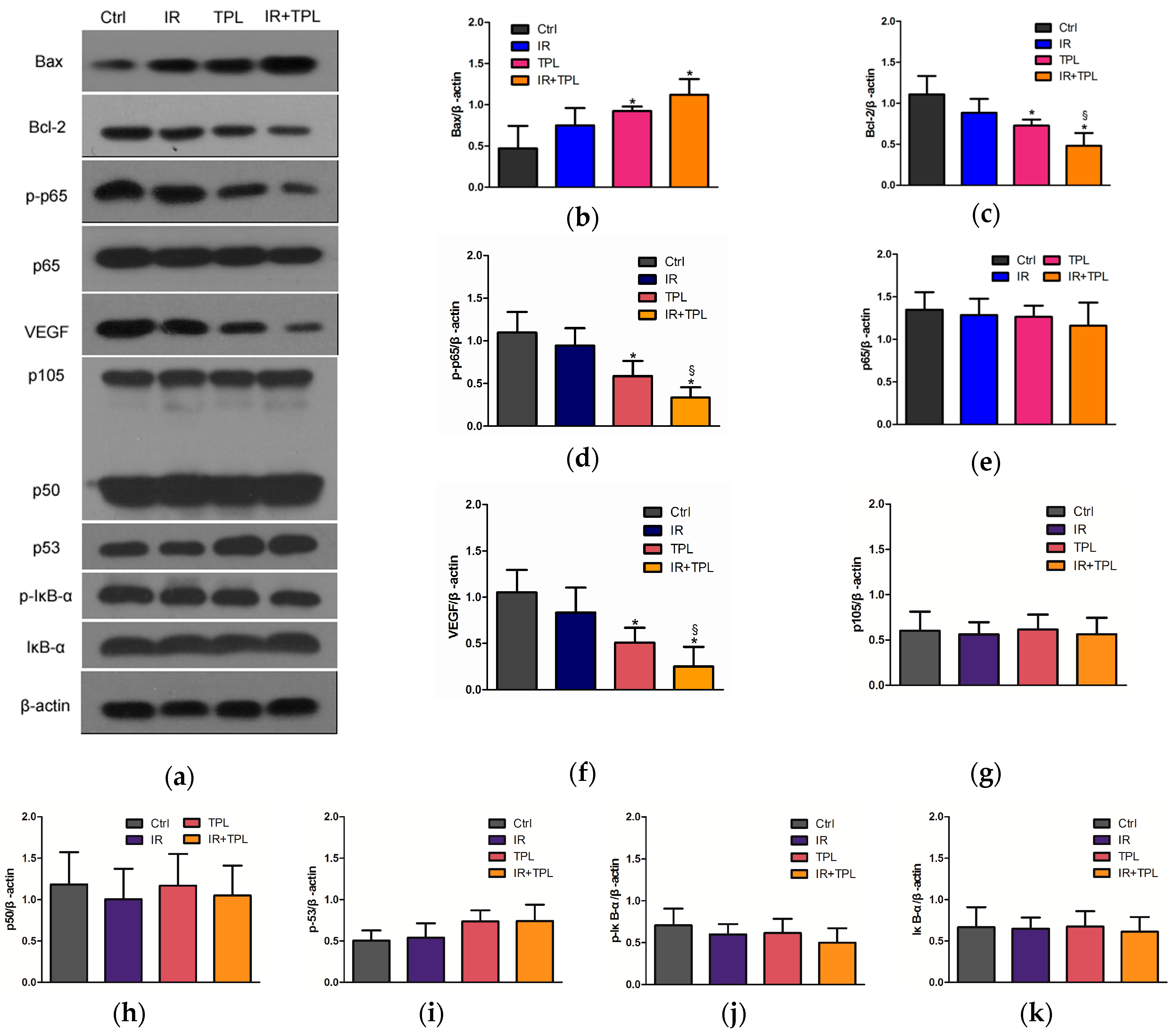
2.5. Effects of TPL Combination Therapy on Related Protein Expression Levels in CNE Cells
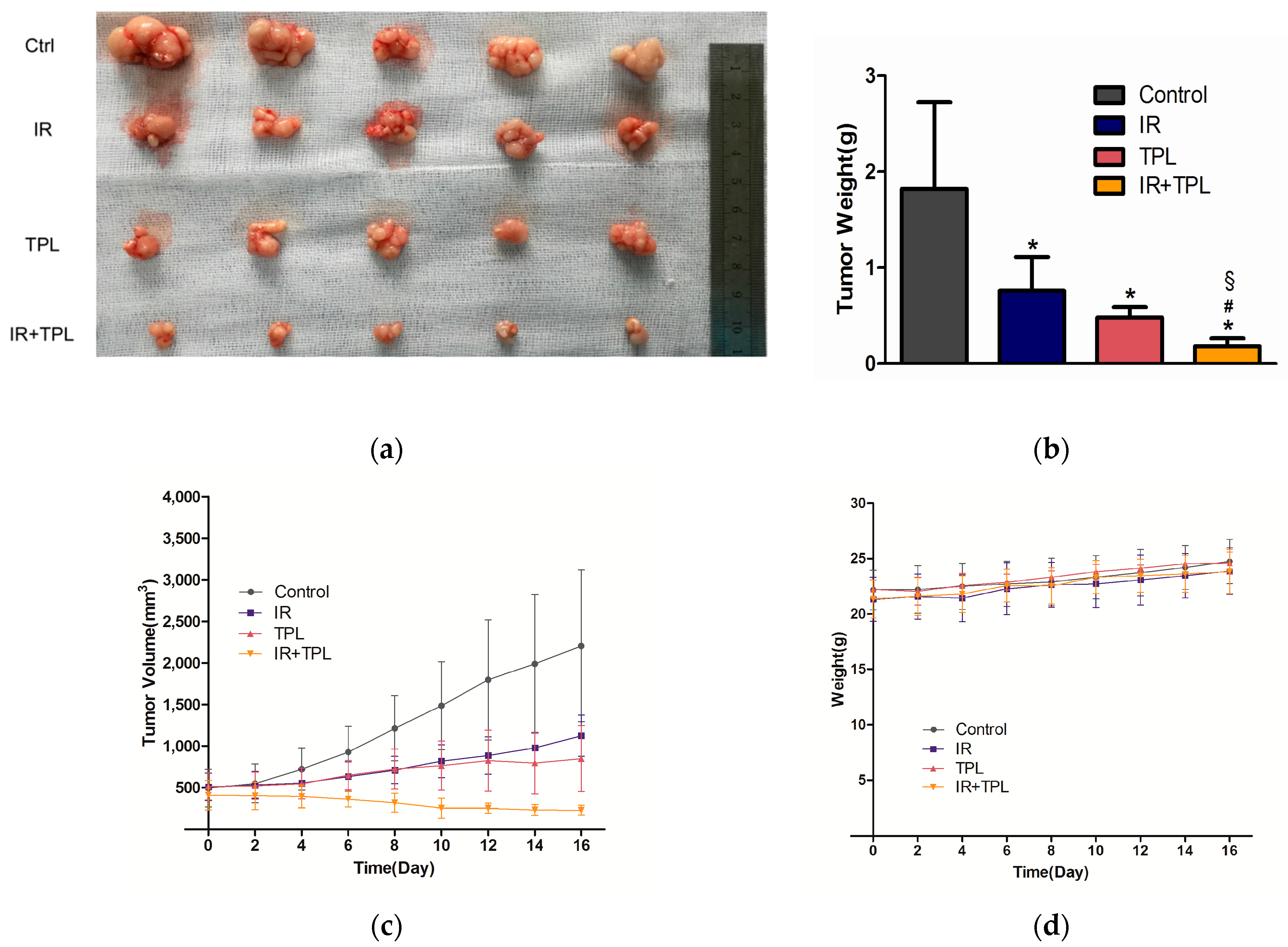
2.6. In Vivo Animal Study
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Drug Treatment
4.2. Cell Proliferation Assay
4.3. Morphological Observation
4.4. Colony Formation Assay
4.5. Cell Apoptosis Assay
4.6. Tube Formation Assay
4.7. Western Blot Analysis and ELISA Analysis
4.8. Xenograft Tumor Model
4.9. Protein Extraction and Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Busson, P.; Keryer, C.; Ooka, T.; Corbex, M. Ebv-associated nasopharyngeal carcinomas: From epidemiology to virus-targeting strategies. Trends Microbiol. 2004, 12, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.; Golitz, L.E. Primary noncutaneous melanoma. Clin. Lab. Med. 2000, 20, 731–744. [Google Scholar] [PubMed]
- Xu, Y.; Zhang, J.; Shi, W.; Liu, Y. Anticancer effects of 3,3′-diindolylmethane are associated with G1 arrest and mitochondria-dependent apoptosis in human nasopharyngeal carcinoma cells. Oncol. Lett. 2013, 5, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.W.; Huang, S.M.; Han, F.; Wu, S.X.; Lu, L.X.; Lin, C.G.; Deng, X.W.; Lu, T.X.; Cui, N.J.; Zhao, C. Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: Long-term results of a phase 2 study. Cancer 2011, 117, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Zhao, M.; Qiu, H.; Shi, D.; Wang, J.; Tian, Y.; Lin, L.; Deng, W. Effusanin E suppresses nasopharyngeal carcinoma cell growth by inhibiting Nf-κB and COX-2 signaling. PLoS ONE 2014, 9, e109951. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liang, Z.D.; Wu, T.T.; Hoque, A.; Chen, H.; Jiang, Y.; Zhang, H.; Xu, X.C. Tumor-suppressive effect of retinoid receptor-induced gene-1 (RRIG1) in esophageal cancer. Cancer Res. 2007, 67, 1589–1593. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, Z.; Xiao, J.; Zhang, Y.; Lin, S.; Duan, W.; Yao, J.; Liu, C.; Huang, X.; Wang, T.; et al. Effects of triptolide from Tripterygium wilfordii on ERα and p53 expression in two human breast cancer cell lines. Phytomedicine 2009, 16, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Jiang, Z.Z.; Zhang, L.Y. Triptolide: Progress on research in pharmacodynamics and toxicology. J. Ethnopharmacol. 2014, 155, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, R.; Yang, Y.; Huang, Y.; Li, X.; Shen, X. Triptolide-induced in vitro and in vivo cytotoxicity in human breast cancer stem cells and primary breast cancer cells. Oncol. Rep. 2014, 31, 2181–2186. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, W.; Leng, T.; Shu, M.; Huang, Y.; Xu, D.; Qiu, P.; Su, X.; Yan, G. Triptolide-induced cell cycle arrest and apoptosis in human renal cell carcinoma cells. Oncol. Rep. 2011, 25, 979–987. [Google Scholar] [PubMed]
- Zhu, W.; Hu, H.; Qiu, P.; Yan, G. Triptolide induces apoptosis in human anaplastic thyroid carcinoma cells by a p53-independent but NF-κB-related mechanism. Oncol. Rep. 2009, 22, 1397–1401. [Google Scholar] [PubMed]
- Yang, S.; Chen, J.; Guo, Z.; Xu, X.M.; Wang, L.; Pei, X.F.; Yang, J.; Underhill, C.B.; Zhang, L. Triptolide inhibits the growth and metastasis of solid tumors. Mol. Cancer Ther. 2003, 2, 65–72. [Google Scholar] [PubMed]
- Tang, X.Y.; Zhu, Y.Q.; Tao, W.H.; Wei, B.; Lin, X.L. Synergistic effect of triptolide combined with 5-fluorouracil on colon carcinoma. Postgrad. Med. J. 2007, 83, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Pigneux, A.; Mahon, F.X.; Uhalde, M.; Jeanneteau, M.; Lacombe, F.; Milpied, N.; Reiffers, J.; Belloc, F. Triptolide cooperates with chemotherapy to induce apoptosis in acute myeloid leukemia cells. Exp. Hematol. 2008, 36, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Sangwan, V.; Banerjee, S.; Chugh, R.; Dudeja, V.; Vickers, S.M.; Saluja, A.K. Triptolide sensitizes pancreatic cancer cells to trail-induced activation of the death receptor pathway. Cancer Lett. 2014, 348, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, S.; Su, Y.; Xiao, Z.; Wang, C.; Li, X.; Lin, L.; Fenton, B.M.; Paoni, S.F.; Ding, I.; et al. Enhanced antitumor effect of combined triptolide and ionizing radiation. Clin. Cancer Res. 2007, 13, 4891–4899. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Takada, Y.; Shishodia, S.; Gutierrez, A.M.; Oommen, O.V.; Ichikawa, H.; Baba, Y.; Kumar, A. Nuclear transcription factor NF-κB: Role in biology and medicine. Indian J. Exp. Biol. 2004, 42, 341–353. [Google Scholar] [PubMed]
- Guo, L.; Guo, Y.; Xiao, S. Expression of tyrosine kinase Etk/Bmx and its relationship with AP-1- and NF-κB-associated proteins in hepatocellular carcinoma. Oncology 2007, 72, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Sudoyo, A.W.; Pranowo, B.S.; Rini, D.; Sutrisna, B.; Rani, A.A. Expression of NF-κB and COX-2 in young versus older patients with sporadic colorectal cancer. Acta Med. Indones. 2009, 41, 70–74. [Google Scholar] [PubMed]
- Annunziata, C.M.; Stavnes, H.T.; Kleinberg, L.; Berner, A.; Hernandez, L.F.; Birrer, M.J.; Steinberg, S.M.; Davidson, B.; Kohn, E.C. Nuclear factor κB transcription factors are coexpressed and convey a poor outcome in ovarian cancer. Cancer 2010, 116, 3276–3284. [Google Scholar] [CrossRef] [PubMed]
- Keats, J.J.; Fonseca, R.; Chesi, M.; Schop, R.; Baker, A.; Chng, W.J.; van Wier, S.; Tiedemann, R.; Shi, C.X.; Sebag, M.; et al. Promiscuous mutations activate the noncanonical NF-κB pathway in multiple myeloma. Cancer Cell 2007, 12, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Lerebours, F.; Vacher, S.; Andrieu, C.; Espie, M.; Marty, M.; Lidereau, R.; Bieche, I. NF-κB genes have a major role in inflammatory breast cancer. BMC Cancer 2008, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Levidou, G.; Korkolopoulou, P.; Nikiteas, N.; Tzanakis, N.; Thymara, I.; Saetta, A.A.; Tsigris, C.; Rallis, G.; Vlasis, K.; Patsouris, E. Expression of nuclear factor κB in human gastric carcinoma: Relationship with IκB a and prognostic significance. Virchows Arch. 2007, 450, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Sato, H.; Murono, S.; Furukawa, M.; Yoshizaki, T. Epstein-Barr virus (EBV) latent membrane protein 1 induces interleukin-8 through the nuclear factor-κB signaling pathway in EBV-infected nasopharyngeal carcinoma cell line. Laryngoscope 2004, 114, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.K.; Lo, K.W.; Tsao, S.W.; Wong, H.L.; Hui, J.W.; To, K.F.; Hayward, D.S.; Chui, Y.L.; Lau, Y.L.; Takada, K.; et al. Epstein-Barr virus infection alters cellular signal cascades in human nasopharyngeal epithelial cells. Neoplasia 2006, 8, 173–180. [Google Scholar] [PubMed]
- Cheung, A.K.; Ko, J.M.; Lung, H.L.; Chan, K.W.; Stanbridge, E.J.; Zabarovsky, E.; Tokino, T.; Kashima, L.; Suzuki, T.; Kwong, D.L.; et al. Cysteine-rich intestinal protein 2 (CRIP2) acts as a repressor of NF-κB-mediated proangiogenic cytokine transcription to suppress tumorigenesis and angiogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 8390–8395. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Guo, M.M.; Han, P.; Lin, J.Z.; Liang, F.Y.; Tan, G.M.; Li, H.B.; Zeng, M.; Huang, X.M. Id-1 and the p65 subunit of NF-κB promote migration of nasopharyngeal carcinoma cells and are correlated with poor prognosis. Carcinogenesis 2012, 33, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Mareel, M.; Leroy, A. Clinical, cellular, and molecular aspects of cancer invasion. Physiol. Rev. 2003, 83, 337–376. [Google Scholar] [CrossRef] [PubMed]
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Horbach, A.; Kubitz, R.; Frilling, A.; Haussinger, D. Disruption of hepatocellular tight junctions by vascular endothelial growth factor (VEGF): A novel mechanism for tumor invasion. J. Hepatol. 2004, 41, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.; Cui, J.; Barnes, L.; Cheresh, D. Endothelial barrier disruption by VEGF-mediated SRC activity potentiates tumor cell extravasation and metastasis. J. Cell Biol. 2004, 167, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Chilov, D.; Kukk, E.; Taira, S.; Jeltsch, M.; Kaukonen, J.; Palotie, A.; Joukov, V.; Alitalo, K. Genomic organization of human and mouse genes for vascular endothelial growth factor C. J. Biol. Chem. 1997, 272, 25176–25183. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ou, Y.; Li, Y.; Xiao, R.; Shu, M.; Zhou, Y.; Xie, J.; He, S.; Qiu, P.; Yan, G. A small-molecule triptolide suppresses angiogenesis and invasion of human anaplastic thyroid carcinoma cells via down-regulation of the nuclear factor-κB pathway. Mol. Pharmacol. 2009, 75, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Salnikov, A.V.; Bauer, N.; Aleksandrowicz, E.; Labsch, S.; Nwaeburu, C.; Mattern, J.; Gladkich, J.; Schemmer, P.; Werner, J.; et al. Triptolide reverses hypoxia-induced epithelial-mesenchymal transition and stem-like features in pancreatic cancer by NF-κB downregulation. Int. J. Cancer 2014, 134, 2489–2503. [Google Scholar] [CrossRef] [PubMed]
- Brinker, A.M.; Ma, J.; Lipsky, P.E.; Raskin, I. Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae). Phytochemistry 2007, 68, 732–766. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Kang, J.J.; Lee, K.Y.; Wei, K.; Anderson, E.; Gotmare, S.; Ross, J.A.; Rosen, G.D. Triptolide and chemotherapy cooperate in tumor cell apoptosis. A role for the p53 pathway. J. Biol. Chem. 2001, 276, 2221–2227. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.J.; Sun, Y.M.; Zhang, J.; Wang, L.R.; Li, J.C.; Liu, H. Triptolide induces apoptosis and synergizes with cisplatin in cisplatin-resistant HNE1/DDP nasopharyngeal cancer cells. Folia Biol. (Praha) 2015, 61, 195–202. [Google Scholar] [PubMed]
- Chen, Y.W.; Lin, G.J.; Hueng, D.Y.; Huang, S.H.; Chia, W.T.; Shieh, Y.S.; Ma, K.H.; Sytwu, H.K. Enhanced anti-tumor activity of triptolide in combination with irradiation for the treatment of oral cancer. Planta Med. 2014, 80, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, X.; Bhalla, K.; Kim, C.N.; Ibrado, A.M.; Cai, J.; Peng, T.I.; Jones, D.P.; Wang, X. Prevention of apoptosis by BCL-2: Release of cytochrome c from mitochondria blocked. Science 1997, 275, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Gerber, H.P. Anti-angiogenesis: Biology is the foundation for therapy. Drug Discov. Today 2003, 8, 344–346. [Google Scholar] [CrossRef]
- Ni, J.; Wu, Q.; Sun, Z.H.; Zhong, J.; Cai, Y.; Huang, X.E. The inhibition effect of triptolide on human endometrial carcinoma cell line HEC-1B: A in vitro and in vivo studies. Asian Pac. J. Cancer Prev. 2015, 16, 4571–4576. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Pettaway, C.A.; Uehara, H.; Bucana, C.D.; Fidler, I.J. Blockade of NF-κB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene 2001, 20, 4188–4197. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Li, Y.; Wang, Z.; Banerjee, S.; Sarkar, F.H. Inhibition of angiogenesis and invasion by 3,3′-diindolylmethane is mediated by the nuclear factor-κB downstream target genes MMP-9 and UPA that regulated bioavailability of vascular endothelial growth factor in prostate cancer. Cancer Res. 2007, 67, 3310–3319. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Chiba, H.; Miyoshi, H.; Sugita, T.; Toriumi, W. IκB kinases phosphorylate Nf-κB p65 subunit on serine 536 in the transactivation domain. J. Biol. Chem. 1999, 274, 30353–30356. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, A.M.; Montano, M.; van Beneden, K.; Chen, L.F.; Greene, W.C. Human T-cell lymphotropic virus type 1 tax induction of biologically active NF-κB requires IκB kinase-1-mediated phosphorylation of RelA/p65. J. Biol. Chem. 2004, 279, 18137–18145. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Takahashi, N.; Matsui, N.; Tetsuka, T.; Okamoto, T. The NF-κB activation in lymphotoxin β receptor signaling depends on the phosphorylation of p65 at serine 536. J. Biol. Chem. 2003, 278, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Buss, H.; Dorrie, A.; Schmitz, M.L.; Hoffmann, E.; Resch, K.; Kracht, M. Constitutive and interleukin-1-inducible phosphorylation of p65 NFκB at serine 536 is mediated by multiple protein kinases including IκB kinase (IKK)-α, IKKβ, IKKε, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. J. Biol. Chem. 2004, 279, 55633–55643. [Google Scholar] [PubMed]
- Fujita, F.; Taniguchi, Y.; Kato, T.; Narita, Y.; Furuya, A.; Ogawa, T.; Sakurai, H.; Joh, T.; Itoh, M.; Delhase, M.; et al. Identification of NAP1, a regulatory subunit of IκB kinase-related kinases that potentiates NF-κB signaling. Mol. Cell. Biol. 2003, 23, 7780–7793. [Google Scholar] [CrossRef] [PubMed]
- Bohuslav, J.; Chen, L.F.; Kwon, H.; Mu, Y.; Greene, W.C. p53 Induces NF-κB activation by an IκB kinase-independent mechanism involving phosphorylation of p65 by ribosomal S6 kinase 1. J. Biol. Chem. 2004, 279, 26115–26125. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.C.; Dickson, K.M.; Barker, P.A. Nuclear factor-κB induced by doxorubicin is deficient in phosphorylation and acetylation and represses nuclear factor-κB-dependent transcription in cancer cells. Cancer Res. 2005, 65, 4273–4281. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.S., Jr. The NF-κB and IκB proteins: New discoveries and insights. Annu. Rev. Immunol. 1996, 14, 649–683. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Kang, M.; Zhang, T.; Li, B.; Liao, X.; Wang, R. Triptolide Combined with Radiotherapy for the Treatment of Nasopharyngeal Carcinoma via NF-κB-Related Mechanism. Int. J. Mol. Sci. 2016, 17, 2139. https://doi.org/10.3390/ijms17122139
Zhang W, Kang M, Zhang T, Li B, Liao X, Wang R. Triptolide Combined with Radiotherapy for the Treatment of Nasopharyngeal Carcinoma via NF-κB-Related Mechanism. International Journal of Molecular Sciences. 2016; 17(12):2139. https://doi.org/10.3390/ijms17122139
Chicago/Turabian StyleZhang, Weiying, Min Kang, Tingting Zhang, Bo Li, Xueyin Liao, and Rensheng Wang. 2016. "Triptolide Combined with Radiotherapy for the Treatment of Nasopharyngeal Carcinoma via NF-κB-Related Mechanism" International Journal of Molecular Sciences 17, no. 12: 2139. https://doi.org/10.3390/ijms17122139
APA StyleZhang, W., Kang, M., Zhang, T., Li, B., Liao, X., & Wang, R. (2016). Triptolide Combined with Radiotherapy for the Treatment of Nasopharyngeal Carcinoma via NF-κB-Related Mechanism. International Journal of Molecular Sciences, 17(12), 2139. https://doi.org/10.3390/ijms17122139




