Optimizing a Male Reproductive Aging Mouse Model by d-Galactose Injection
Abstract
:1. Introduction
1.1. Aging and Male Infertility
1.2. Reactive Oxygen Species (ROS) Generation in Testes
1.3. Effects of Oral Antioxidants on Male Infertility
1.4. Model of Male Reproductive Aging
2. Results and Discussion
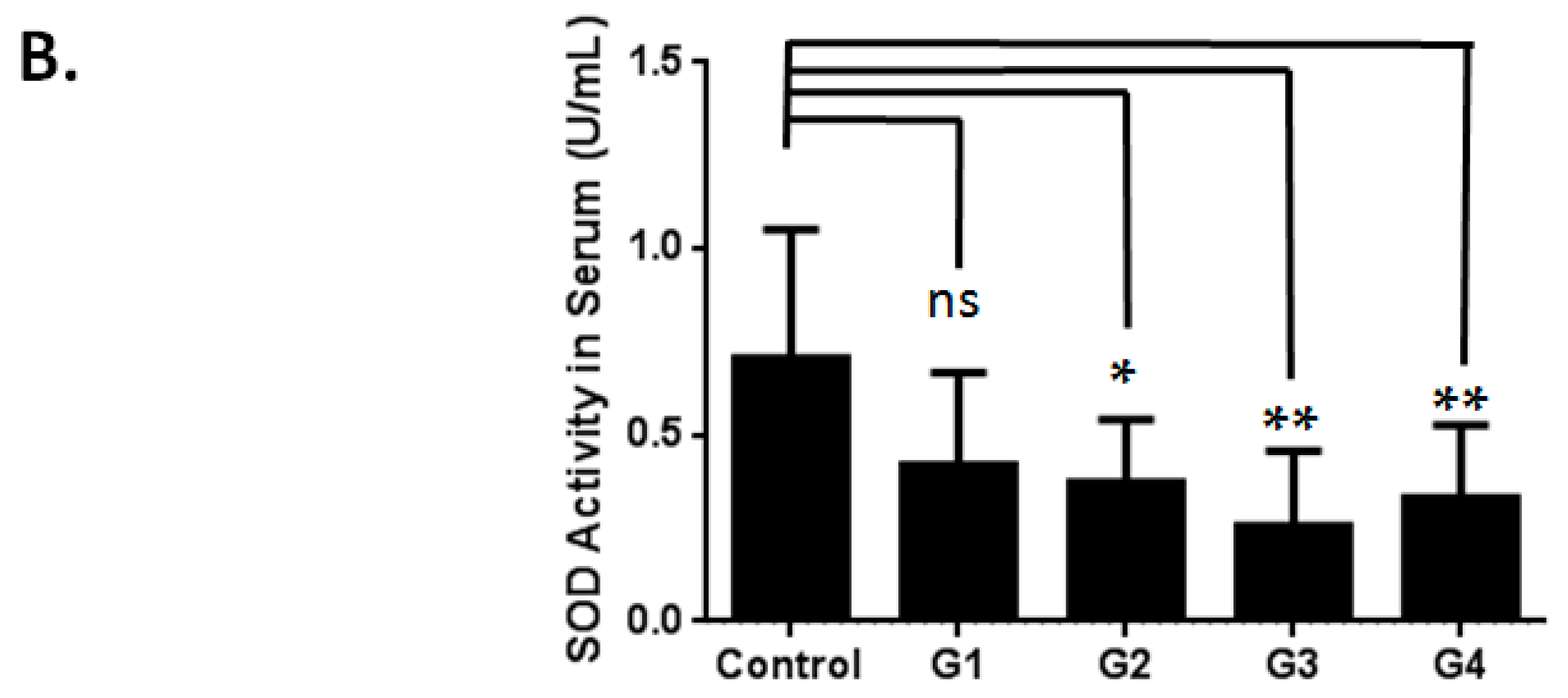
2.1. Optimizing a Model of Male Reproductive Aging through d-Galactose (d-Gal) Injection
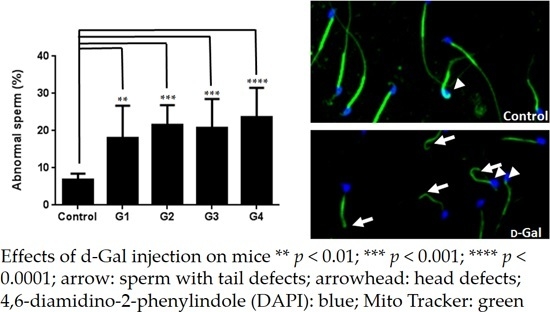
2.2. Sperm Quality Decreased in d-Gal-Injected Mice
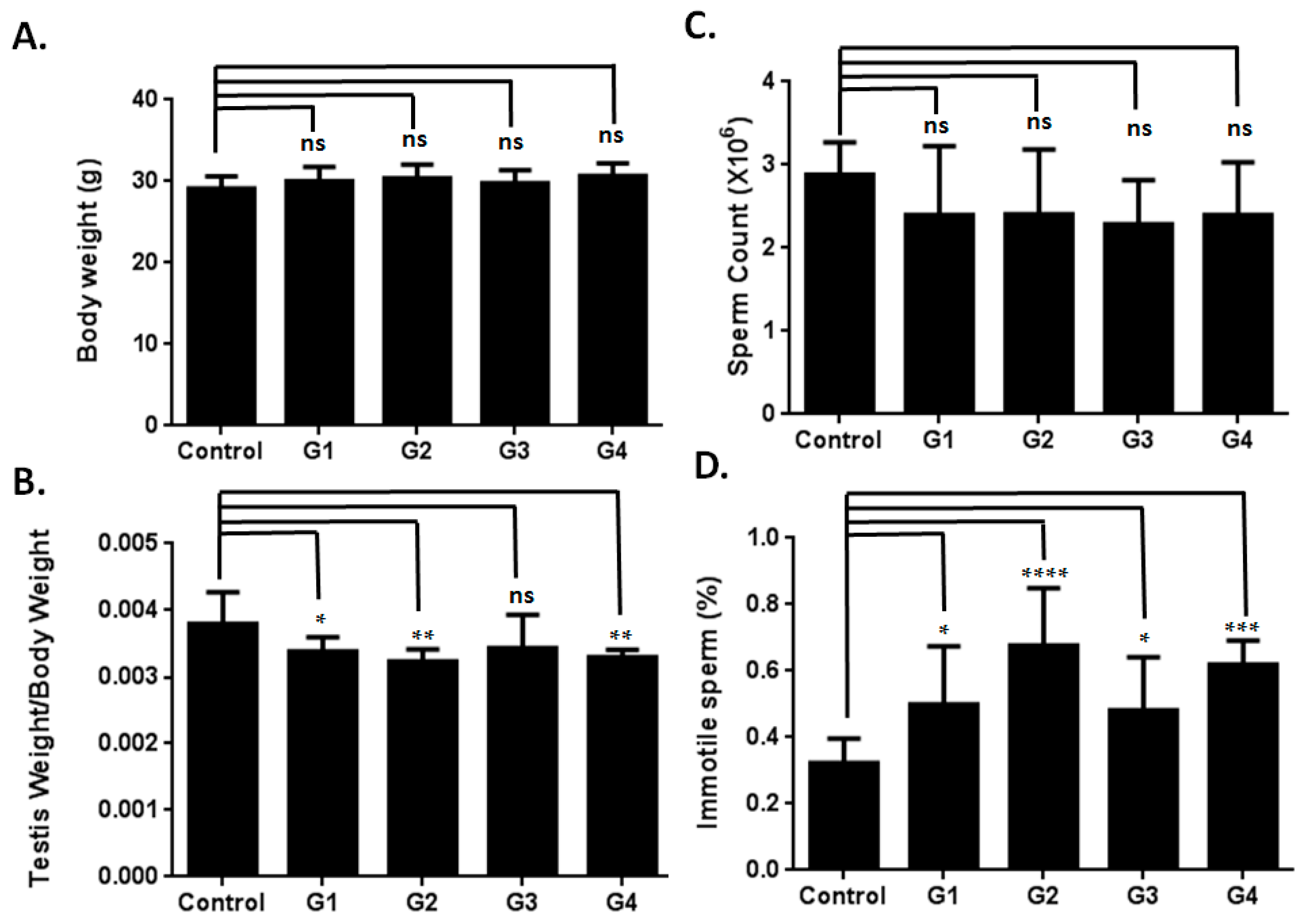
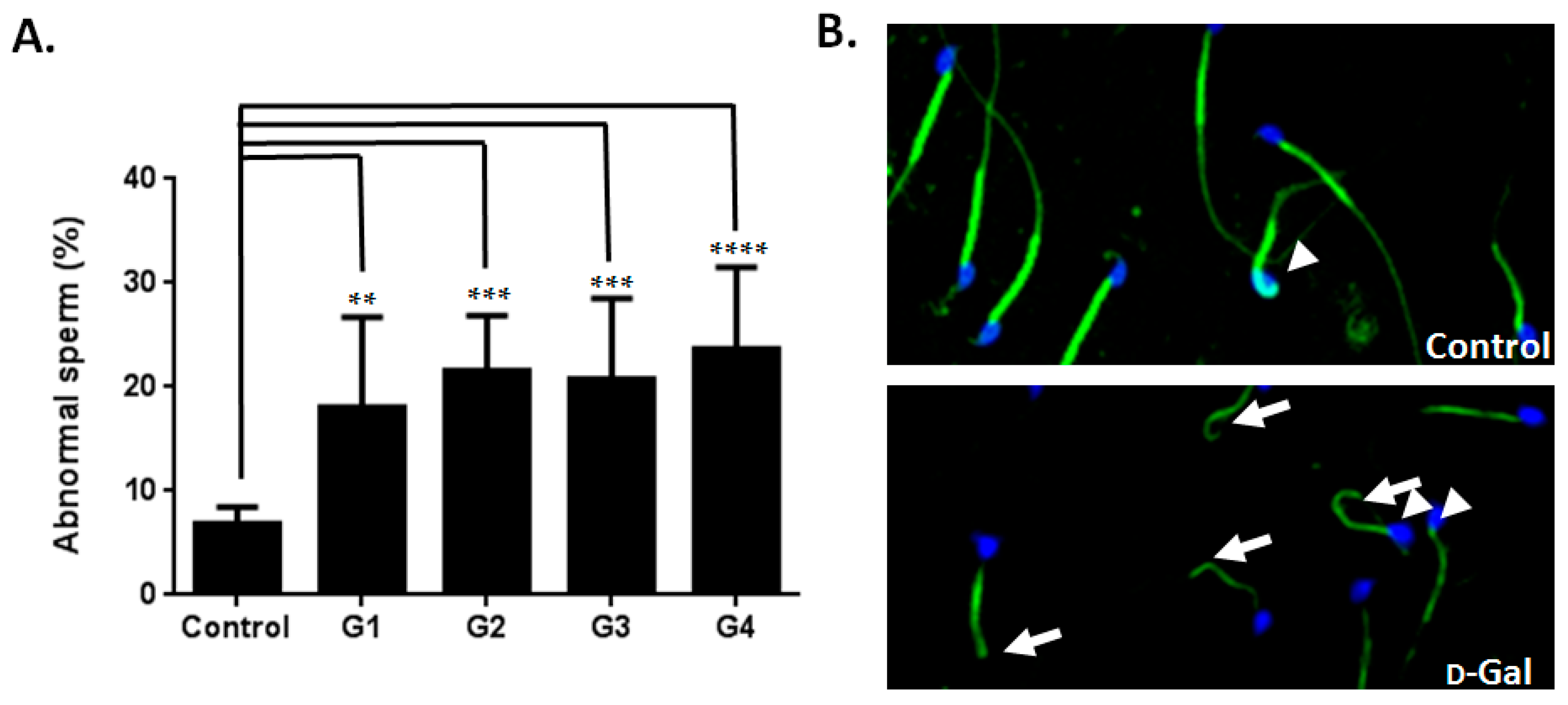
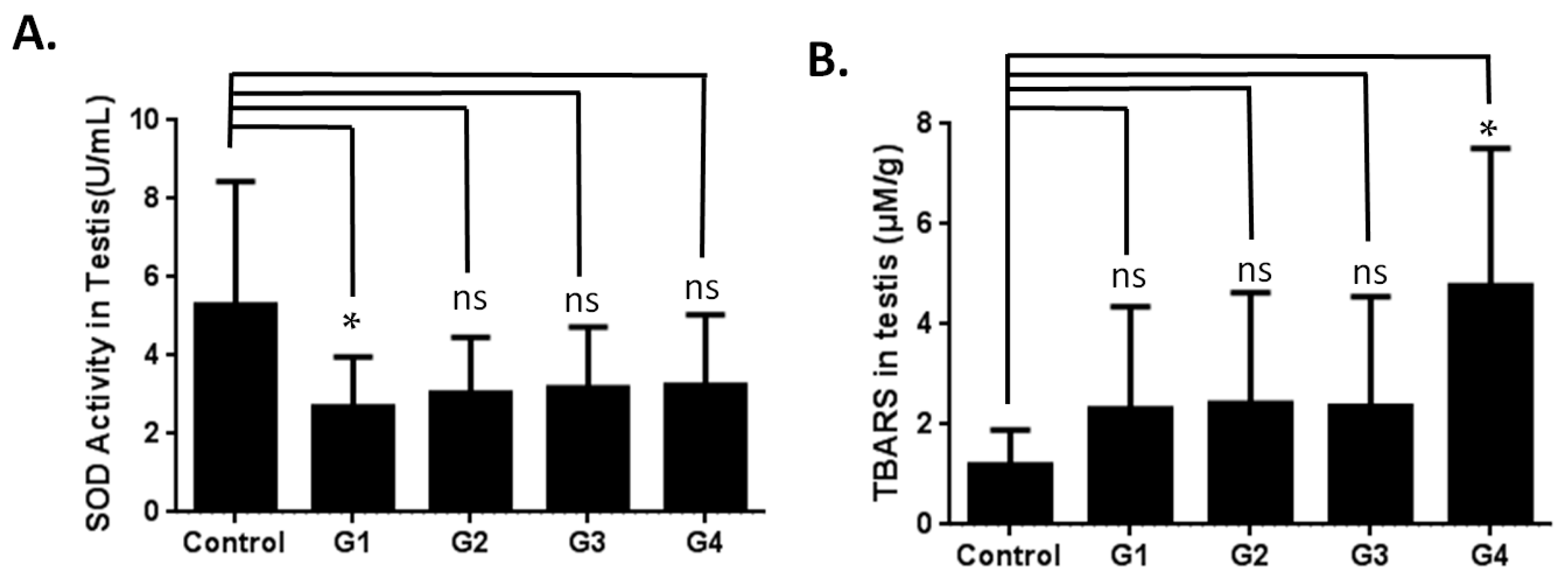
2.3. Investigating the Possible Mechanism of Reproductive Aging in d-Gal-Injected Mice
| Gene | Full Name | Fold |
|---|---|---|
| Cylc2 | Cylicin, basic protein of sperm head cytoskeleton 2 | 5.62 |
| Hk1 | Hexokinase 1 | 2.40 |
| Pltp | Phospholipid transfer protein | 2.25 |
| Utp3 | UTP3, small subunit (SSU) processome component | 2.20 |
| Cabyr | Calcium-binding tyrosine-(Y)-phosphorylation regulated | 2.19 |
| Zpbp2 | Zona pellucida binding protein 2 | 2.13 |
| Speer2 | Spermatogenesis associated glutamate (E)-rich protein 2 | 2.03 |
| Csnka2ip | Casein kinase 2, alpha prime interacting protein | 2.06 |
| Katnb1 | Katanin p80 (WD40-containing) subunit B1 | 0.48 |
3. Materials and Methods
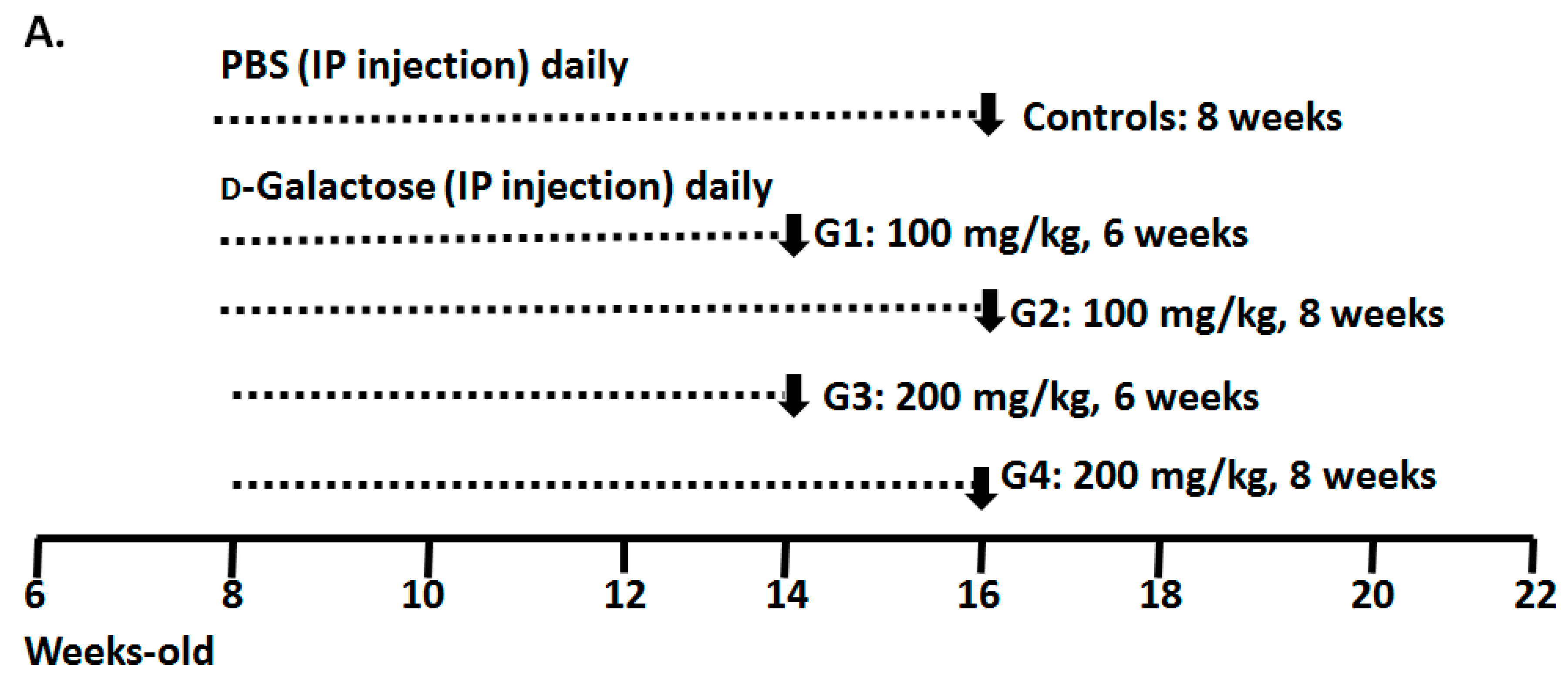
3.1. Animal Preparation and Experimental Design
3.2. Evaluation of Superoxide Dismutase Activity
3.3. Thiobarbituric Acid Reactive Substances (TBARS) Analysis
3.4. Semen Parameter
3.5. Microarray Analysis
4. Conclusions
4.1. Effect of d-Gal Injection on the Expression Levels of Spermatogenesis-Related Genes in Mice
4.2. Model of Male Reproductive Aging
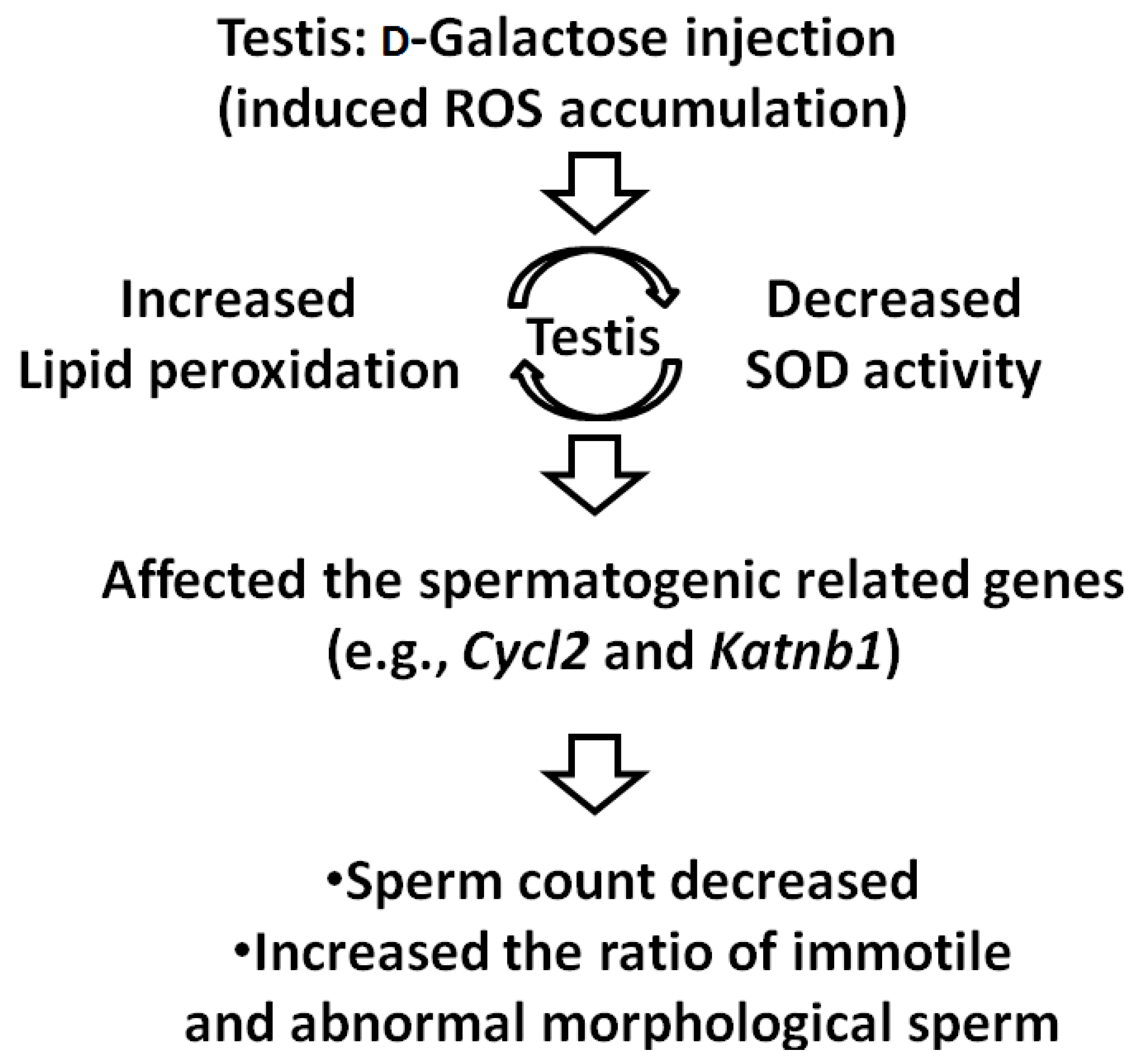
4.3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Charlton, B.G. Psychological neoteny and higher education: Associations with delayed parenthood. Med. Hypotheses 2007, 69, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Kovac, J.R.; Addai, J.; Smith, R.P.; Coward, R.M.; Lamb, D.J.; Lipshultz, L.I. The effects of advanced paternal age on fertility. Asian J. Androl. 2013, 15, 723–728. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Mukhopadhyay, D.; Varghese, A.C.; Pal, M.; Banerjee, S.K.; Bhattacharyya, A.K.; Sharma, R.K.; Agarwal, A. Semen quality and age-specific changes: A study between two decades on 3729 male partners of couples with normal sperm count and attending an andrology laboratory for infertility-related problems in an Indian city. Fertil. Steril. 2010, 93, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Wyrobek, A.J.; Eskenazi, B.; Young, S.; Arnheim, N.; Tiemann-Boege, I.; Jabs, E.W.; Glaser, R.L.; Pearson, F.S.; Evenson, D. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc. Natl. Acad. Sci. USA 2006, 103, 9601–9606. [Google Scholar] [CrossRef] [PubMed]
- Cocuzza, M.; Athayde, K.S.; Agarwal, A.; Sharma, R.; Pagani, R.; Lucon, A.M.; Srougi, M.; Hallak, J. Age-related increase of reactive oxygen species in neat semen in healthy fertile men. Urology 2008, 71, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Sabanegh, E., Jr.; Kim, T.; Agarwal, A. Free radical theory of aging: Implications in male infertility. Urology 2010, 75, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Raha, S.; Robinson, B.H. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem. Sci. 2000, 25, 502–508. [Google Scholar] [CrossRef]
- Muller, F.L.; Lustgarten, M.S.; Jang, Y.; Richardson, A.; van Remmen, H. Trends in oxidative aging theories. Free Radic. Biol. Med. 2007, 43, 477–503. [Google Scholar] [CrossRef] [PubMed]
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [PubMed]
- D’Agata, R.; Vicari, E.; Moncada, M.L.; Sidoti, G.; Calogero, A.E.; Fornito, M.C.; Minacapilli, G.; Mongioi, A.; Polosa, P. Generation of reactive oxygen species in subgroups of infertile men. Int. J. Androl. 1990, 13, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Agarwal, A. Role of reactive oxygen species in male infertility. Urology 1996, 48, 835–850. [Google Scholar] [CrossRef]
- Ross, C.; Morriss, A.; Khairy, M.; Khalaf, Y.; Braude, P.; Coomarasamy, A.; El-Toukhy, T. A systematic review of the effect of oral antioxidants on male infertility. Reprod. Biomed. Online 2010, 20, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Blahak, P. Vitamin E in the treatment of male sterility. Ceskoslovenska Gynekol. 1947, 12, 80–85. [Google Scholar]
- Abel, B.J.; Carswell, G.; Elton, R.; Hargreave, T.B.; Kyle, K.; Orr, S.; Rogers, A.; Baxby, K.; Yates, A. Randomised trial of clomiphene citrate treatment and vitamin C for male infertility. Br. J. Urol. 1982, 54, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Hargreave, T.B.; Kyle, K.F.; Baxby, K.; Rogers, A.C.; Scott, R.; Tolley, D.A.; Abel, B.J.; Orr, P.S.; Elton, R.A. Randomised trial of mesterolone versus vitamin C for male infertility. Scottish Infertility Group. Br. J. Urol. 1984, 56, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Bruni, J.F.; Huang, H.H.; Marshall, S.; Meites, J. Effects of single and multiple injections of synthetic GnRH on serum LH, FSH and testosterone in young and old male rats. Biol. Reprod. 1977, 17, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Bethea, C.L.; Walker, R.F. Age-related changes in reproductive hormones and in Leydig cell responsivity in the male Fischer 344 rat. J. Gerontol. 1979, 34, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Oroojan, A.A.; Heidari, H. Effects of exendin-4 on male reproductive parameters of d-galactose induced aging mouse model. World J. Mens Health 2014, 32, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Bao, M.; Li, D.; Li, Y.M. Advanced glycation in d-galactose induced mouse aging model. Mech. Ageing Dev. 1999, 108, 239–251. [Google Scholar] [CrossRef]
- Pirke, K.M.; Vogt, H.J.; Geiss, M. In vitro and in vivo studies on Leydig cell function in old rats. Acta Endocrinol. 1978, 89, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Nagano, M.; Robaire, B. Aging results in differential regulation of DNA repair pathways in pachytene spermatocytes in the Brown Norway rat. Biol. Reprod. 2011, 85, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Liu, J.H.; Wu, R.Y. Establishment of the mimetic aging effect in mice caused by d-galactose. Biogerontology 2003, 4, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Bucala, R.; Cerami, A. Advanced glycosylation: Chemistry, biology, and implications for diabetes and aging. Adv. Pharmacol. 1992, 23, 1–34. [Google Scholar] [PubMed]
- Lu, J.; Zheng, Y.L.; Wu, D.M.; Luo, L.; Sun, D.X.; Shan, Q. Ursolic acid ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by d-galactose. Biochem. Pharmacol. 2007, 74, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Novak, R.; Bosze, Z.; Matkovics, B.; Fachet, J. Gene affecting superoxide dismutase activity linked to the histocompatibility complex in H-2 congenic mice. Science 1980, 207, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.; Browne, R. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Adv. Exp. Med. Biol. 1994, 366, 43–58. [Google Scholar] [PubMed]
- Von Bulow, M.; Heid, H.; Hess, H.; Franke, W.W. Molecular nature of calicin, a major basic protein of the mammalian sperm head cytoskeleton. Exp. Cell Res. 1995, 219, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Hess, H.; Heid, H.; Zimbelmann, R.; Franke, W.W. The protein complexity of the cytoskeleton of bovine and human sperm heads: the identification and characterization of cylicin II. Exp. Cell Res. 1995, 218, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.J.; Mahr, J.; McNally, K.; Okawa, K.; Iwamatsu, A.; Thomas, S.; Cheesman, S.; Heuser, J.; Vale, R.D.; McNally, F.J. Katanin, a microtubule-severing protein, is a novel AAA ATPase that targets to the centrosome using a WD40-containing subunit. Cell 1998, 93, 277–287. [Google Scholar] [CrossRef]
- O’Donnell, L.; Rhodes, D.; Smith, S.J.; Merriner, D.J.; Clark, B.J.; Borg, C.; Whittle, B.; O’Connor, A.E.; Smith, L.B.; McNally, F.J.; et al. An essential role for katanin p80 and microtubule severing in male gamete production. PLoS Genet. 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.; McLachlan, R.I.; Merriner, D.J.; O’Bryan, M.K.; Jamsai, D. KATNB1 in the human testis and its genetic variants in fertile and oligoasthenoteratozoospermic infertile men. Andrology 2014, 2, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.J.; Yin, M.C. Anti-oxidative, anti-glycative and anti-apoptotic effects of oleanolic acid in brain of mice treated by d-galactose. Eur. J. Pharmacol. 2012, 689, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Chen, H.; Trush, M.A.; Show, M.D.; Anway, M.D.; Zirkin, B.R. Aging and the brown Norway rat leydig cell antioxidant defense system. J. Androl. 2006, 27, 240–247. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.-H.; Chen, B.-H.; Chiang, H.-S.; Chen, C.-W.; Chen, M.-F.; Ke, C.-C.; Wang, Y.-Y.; Lin, W.-N.; Wang, C.-C.; Lin, Y.-H. Optimizing a Male Reproductive Aging Mouse Model by d-Galactose Injection. Int. J. Mol. Sci. 2016, 17, 98. https://doi.org/10.3390/ijms17010098
Liao C-H, Chen B-H, Chiang H-S, Chen C-W, Chen M-F, Ke C-C, Wang Y-Y, Lin W-N, Wang C-C, Lin Y-H. Optimizing a Male Reproductive Aging Mouse Model by d-Galactose Injection. International Journal of Molecular Sciences. 2016; 17(1):98. https://doi.org/10.3390/ijms17010098
Chicago/Turabian StyleLiao, Chun-Hou, Bing-Huei Chen, Han-Sun Chiang, Chiu-Wei Chen, Mei-Feng Chen, Chih-Chun Ke, Ya-Yun Wang, Wei-Ning Lin, Chi-Chung Wang, and Ying-Hung Lin. 2016. "Optimizing a Male Reproductive Aging Mouse Model by d-Galactose Injection" International Journal of Molecular Sciences 17, no. 1: 98. https://doi.org/10.3390/ijms17010098
APA StyleLiao, C.-H., Chen, B.-H., Chiang, H.-S., Chen, C.-W., Chen, M.-F., Ke, C.-C., Wang, Y.-Y., Lin, W.-N., Wang, C.-C., & Lin, Y.-H. (2016). Optimizing a Male Reproductive Aging Mouse Model by d-Galactose Injection. International Journal of Molecular Sciences, 17(1), 98. https://doi.org/10.3390/ijms17010098