Neuropeptide Substance-P-Conjugated Chitosan Nanofibers as an Active Modulator of Stem Cell Recruiting
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological Analysis of Nanofibers
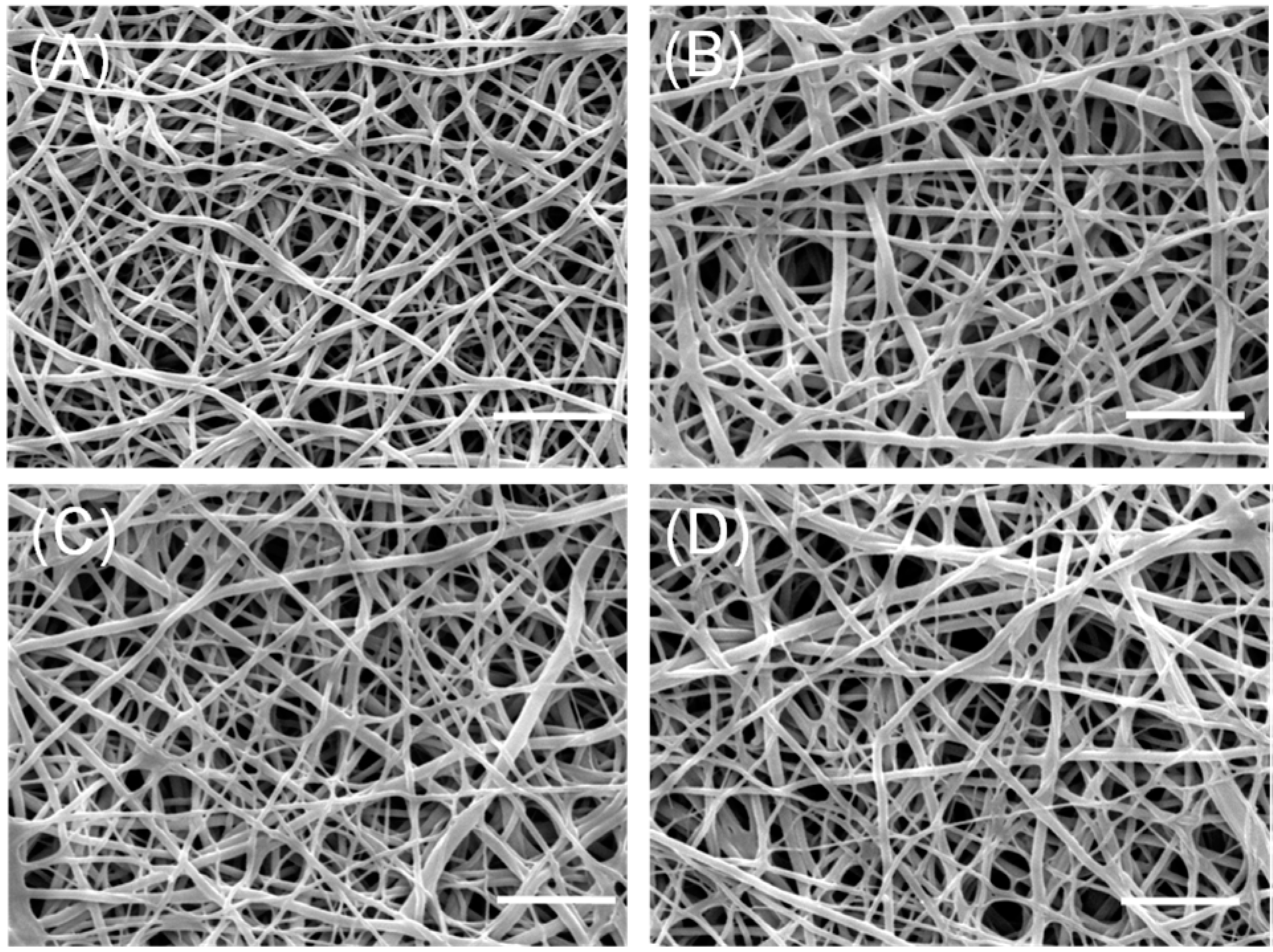
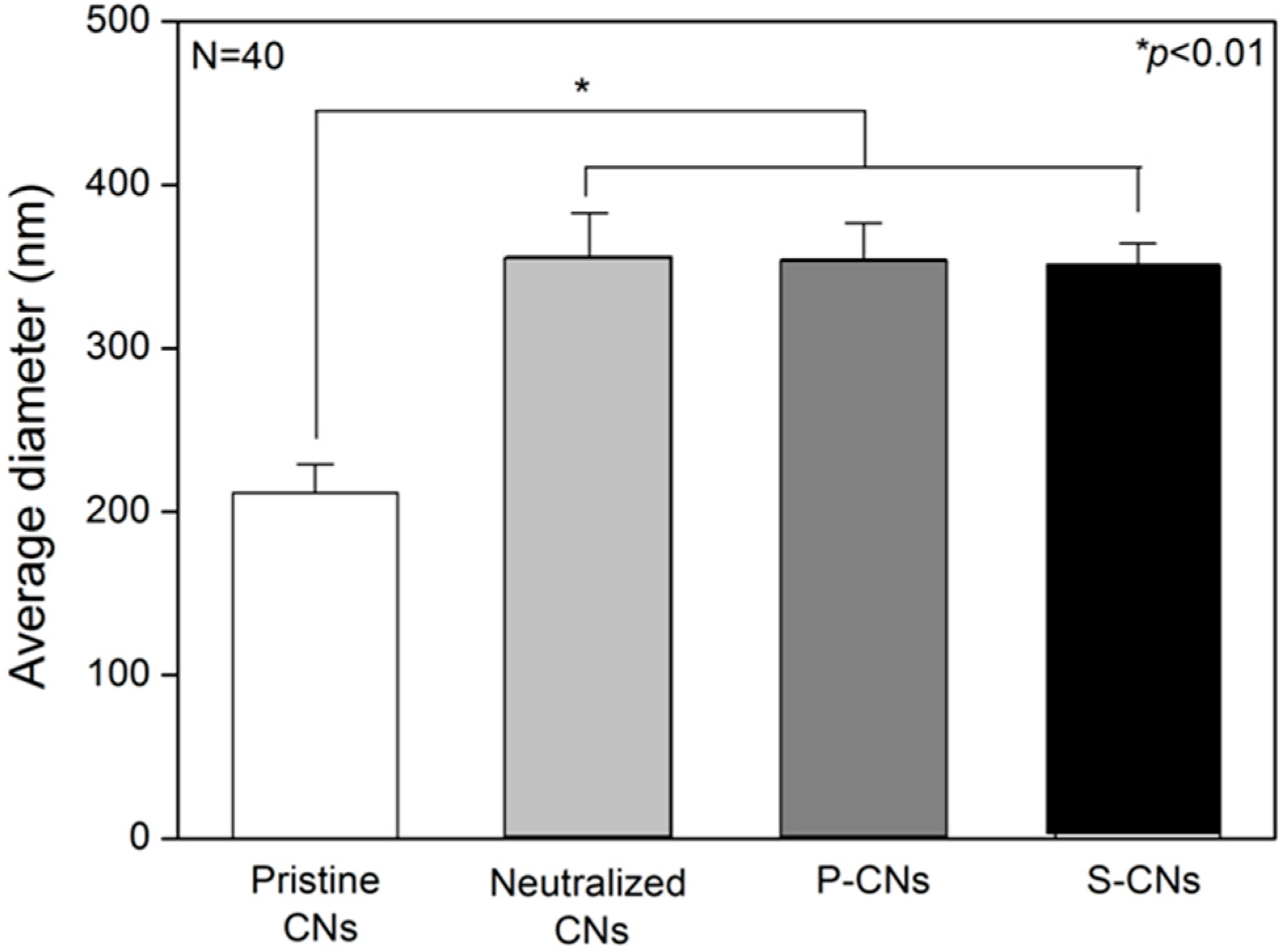
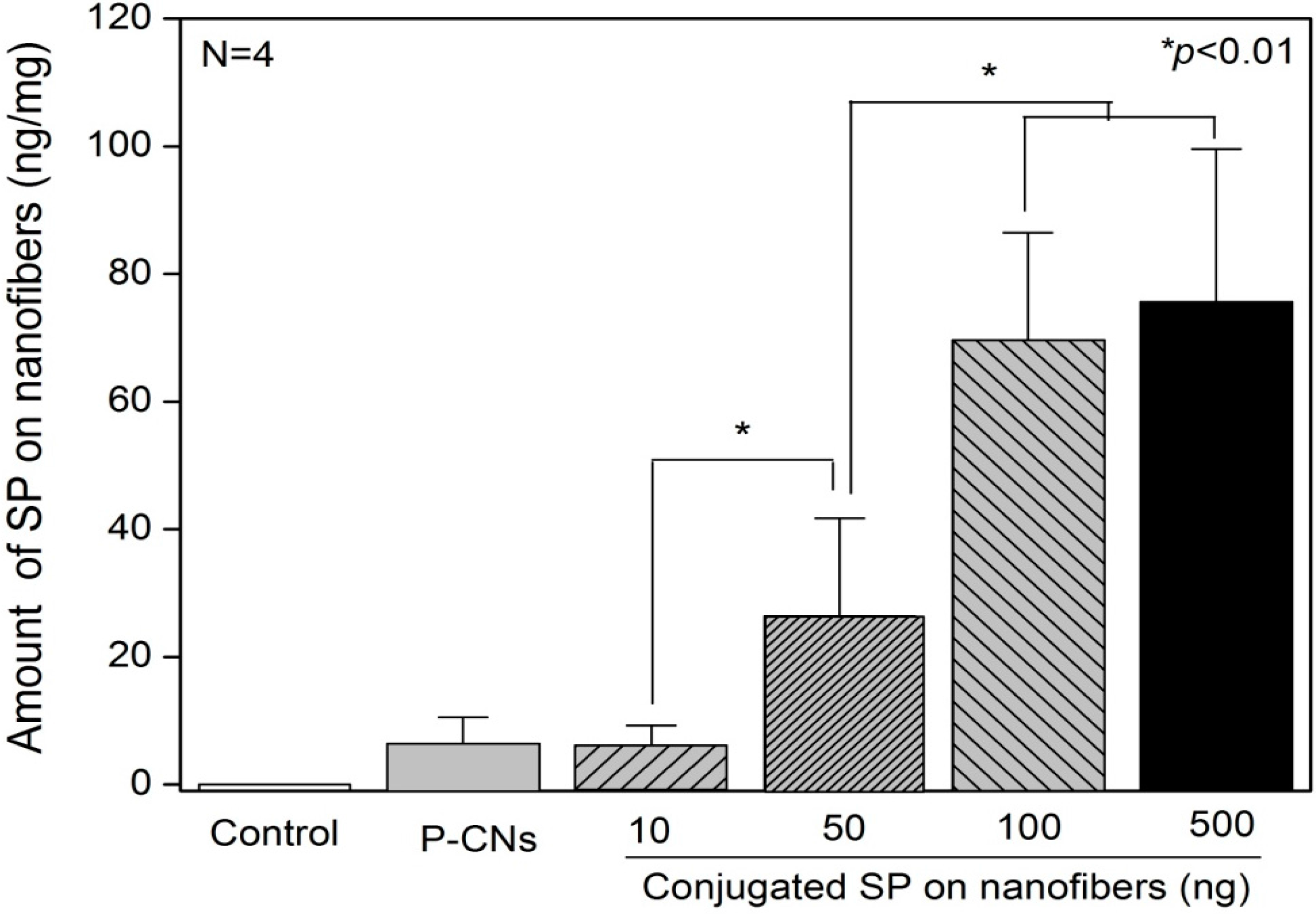
2.2. Quantification of SP on Nanofibers
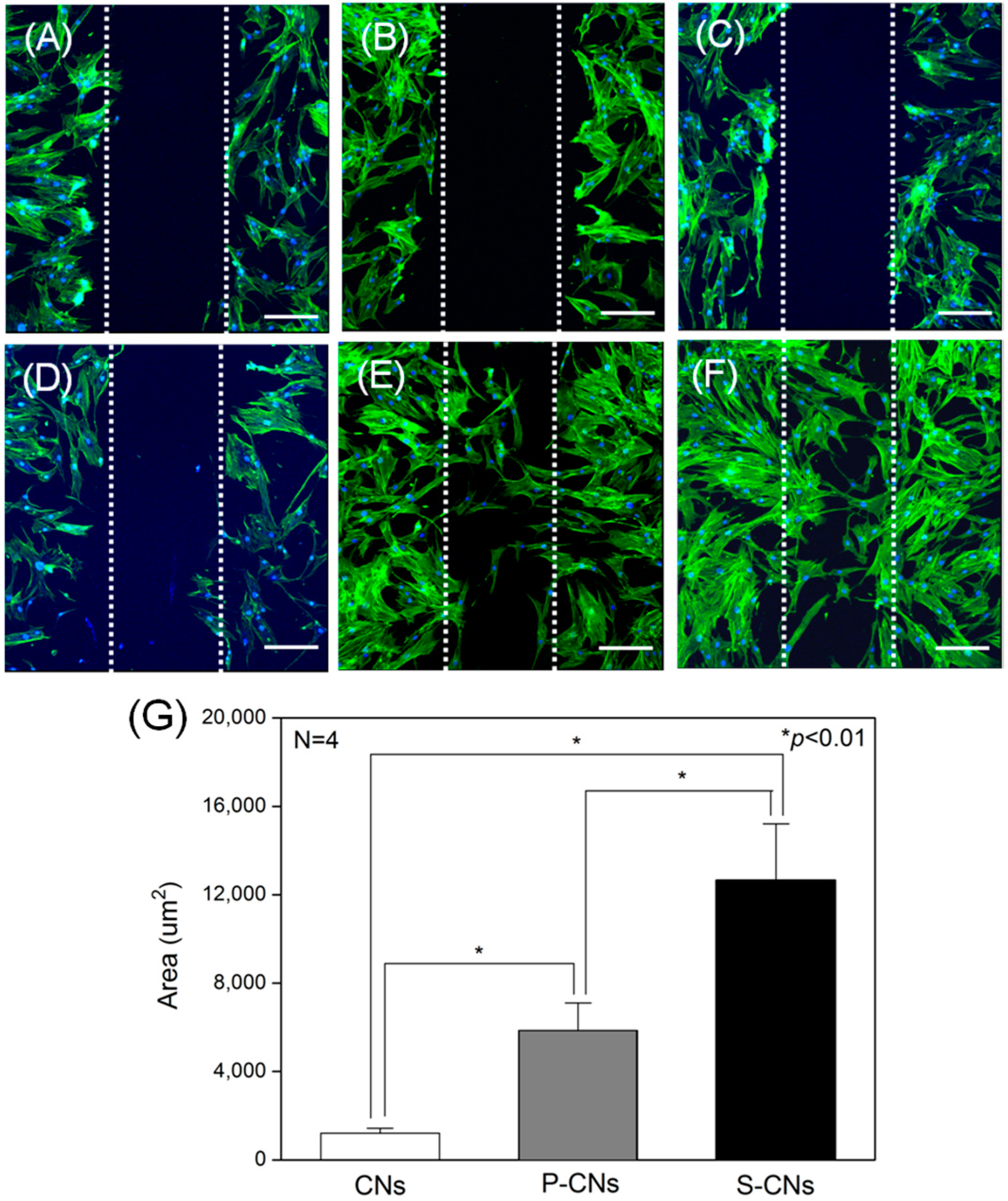
2.3. In Vitro Analysis of Nanofibers Using Human Mesenchymal Stem Cells


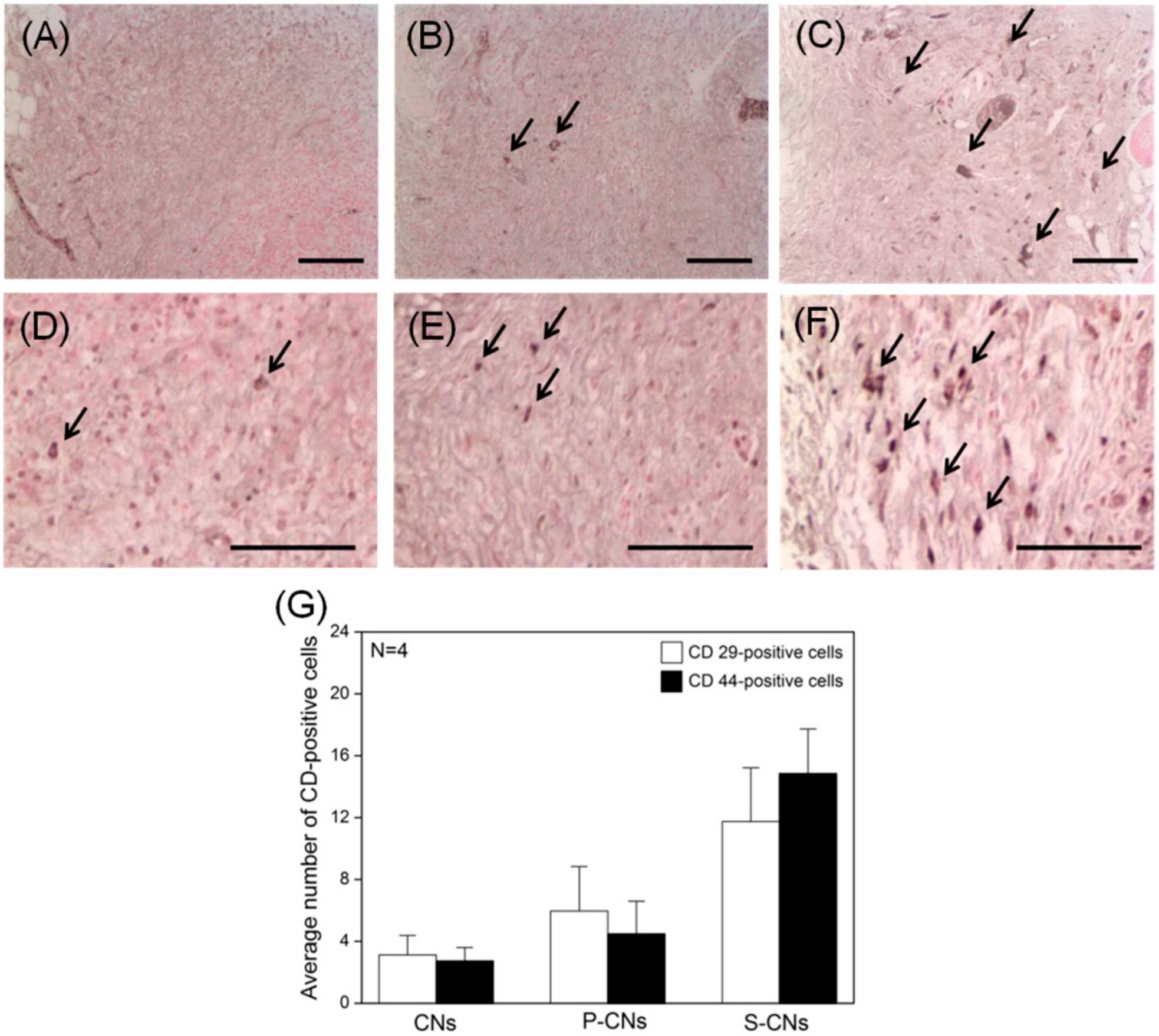
2.4. In Vivo Evaluation of Foreign Body Reaction and Recruited Stem Cells

3. Experimental Section
3.1. Materials
3.2. Fabrication of Chitosan Nanofibers
3.3. SP Coupling Process on Chitosan Nanofibers
3.4. Morphological Analysis
3.5. Quantification of SP on Nanofibers
3.6. Metabolic Activities of hMSCs on Nanofibers
3.7. Migration of hMSCs on Nanofibers
3.8. In Vivo Evaluation of Implanted Nanofibers
3.9. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G., II; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cell 2007, 25, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.E.; Wu, P.; Wu, Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [PubMed]
- Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Concise review: Clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cell Transl. Med. 2012, 1, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.V.; McManus, A.T.; Chambers, J.P. Exogenous administration of substance p enhances wound healing in a novel skin-injury model. Exp. Biol. Med. 2005, 230, 271–280. [Google Scholar]
- Muangman, P.; Tamura, R.N.; Muffley, L.A.; Isik, F.F.; Scott, J.R.; Xie, C.; Kegel, G.; Sullivan, S.R.; Liang, Z.; Gibran, N.S. Substance p enhances wound closure in nitric oxide synthase knockout mice. J. Surg. Res. 2009, 153, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.S.; Lee, J.; Lee, E.; Kwon, Y.S.; Lee, E.; Ahn, W.; Jiang, M.H.; Kim, J.C.; Son, Y. A new role of substance P as an injury-inducible messenger for mobilization of CD29+ stromal-like cells. Nat. Med. 2009, 15, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.-S.; Yoon, K.J.; Son, Y. A new paradigm for stem cell therapy: Substance-P as a stem cell-stimulating agent. Arch. Pharm. Res. 2011, 34, 2003–2006. [Google Scholar] [CrossRef] [PubMed]
- Kohara, H.; Tajima, S.; Yamamoto, M.; Tabata, Y. Angiogenesis induced by controlled release of neuropeptide substance P. Biomaterials 2010, 31, 8617–8625. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-X.; Mo, X.-M.; Zhang, K.-H.; Fan, L.-P.; Yin, A.-L.; He, C.-L.; Wang, H.-S. Fabrication of chitosan/silk fibroin composite nanofibers for wound-dressing applications. Int. J. Mol. Sci. 2010, 11, 3529–3539. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Lee, B.N.; Ko, J.H.; Kim, G.H.; Kang, K.N.; Kim, D.Y.; Kim, J.H.; Park, Y.H.; Chun, H.J.; Kim, C.H. In vivo biocompatibility study of electrospun chitosan microfiber for tissue engineering. Int. J. Mol. Sci. 2010, 11, 4140–4148. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Park, S.J.; Gu, B.K.; Kim, C.-H. Inter-connecting pores of chitosan scaffold with basic fibroblast growth factor modulate biological activity on human mesenchymal stem cells. Carbohydr. Polym. 2012, 87, 2683–2689. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Cationic antimicrobial polymers and their assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Krishnan, U.M.; Sethuraman, S. Fabrication and characterization of chitosan-gelatin blend nanofibers for skin tissue engineering. J. Biomed. Mater. Res. B 2010, 94, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Park, S.J.; Gu, B.K.; Kang, C.-M.; Kim, C.-H. Substance-p immobilized chitosan nanofibers. Mol. Cryst. Liq. Cryst. 2014, 603, 146–156. [Google Scholar] [CrossRef]
- Gu, B.K.; Park, S.J.; Kim, M.S.; Kang, C.M.; Kim, J.-I.; Kim, C.-H. Fabrication of sonicated chitosan nanofiber mat with enlarged porosity for use as hemostatic materials. Carbohydr. Polym. 2013, 97, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Amadesi, S.; Reni, C.; Katare, R.; Meloni, M.; Oikawa, A.; Beltrami, A.P.; Avolio, E.; Cesselli, D.; Fortunato, O.; Spinetti, G. Role for substance p-based nociceptive signaling in progenitor cell activation and angiogenesis during ischemia in mice and in human subjects. Circulation 2012, 125, 1774–1786. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hur, W.; Kim, J.E.; Min, H.J.; Kim, S.; Min, H.S.; Kim, B.K.; Kim, S.H.; Choi, T.H.; Jung, Y. Self-assembling peptide nanofibers coupled with neuropeptide substance P for bone tissue engineering. Tissue Eng. A 2015, 21, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Thevenot, P.T.; Nair, A.M.; Shen, J.; Lotfi, P.; Ko, C.-Y.; Tang, L. The effect of incorporation of SDF-1α into plga scaffolds on stem cell recruitment and the inflammatory response. Biomaterials 2010, 31, 3997–4008. [Google Scholar] [CrossRef] [PubMed]
- Ko, I.K.; Ju, Y.M.; Chen, T.; Atala, A.; Yoo, J.J.; Lee, S.J. Combined systemic and local delivery of stem cell inducing/recruiting factors for in situ tissue regeneration. FASEB J. 2012, 26, 158–168. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.S.; Park, S.J.; Cho, W.; Gu, B.K.; Kim, C.-H. Neuropeptide Substance-P-Conjugated Chitosan Nanofibers as an Active Modulator of Stem Cell Recruiting. Int. J. Mol. Sci. 2016, 17, 68. https://doi.org/10.3390/ijms17010068
Kim MS, Park SJ, Cho W, Gu BK, Kim C-H. Neuropeptide Substance-P-Conjugated Chitosan Nanofibers as an Active Modulator of Stem Cell Recruiting. International Journal of Molecular Sciences. 2016; 17(1):68. https://doi.org/10.3390/ijms17010068
Chicago/Turabian StyleKim, Min Sup, Sang Jun Park, Wheemoon Cho, Bon Kang Gu, and Chun-Ho Kim. 2016. "Neuropeptide Substance-P-Conjugated Chitosan Nanofibers as an Active Modulator of Stem Cell Recruiting" International Journal of Molecular Sciences 17, no. 1: 68. https://doi.org/10.3390/ijms17010068
APA StyleKim, M. S., Park, S. J., Cho, W., Gu, B. K., & Kim, C.-H. (2016). Neuropeptide Substance-P-Conjugated Chitosan Nanofibers as an Active Modulator of Stem Cell Recruiting. International Journal of Molecular Sciences, 17(1), 68. https://doi.org/10.3390/ijms17010068






