Targeted Radionuclide Therapy of Human Tumors
Abstract
:1. Introduction
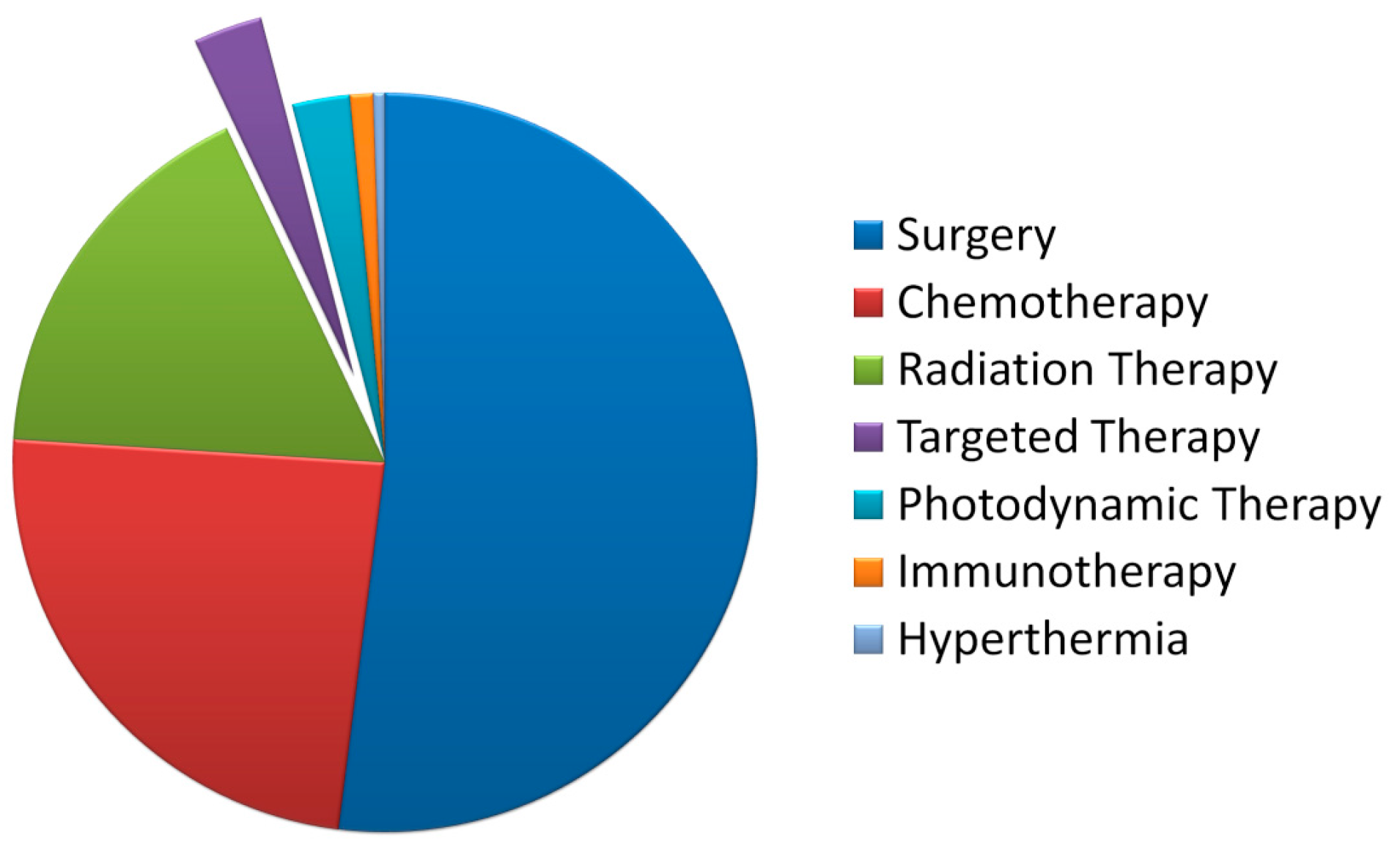
2. Features of Targeted Radionuclide Therapy
3. Radionuclide Selection

| Radionuclide | T1/2, h | Emax, MeV (*) | Method of Producing |
|---|---|---|---|
| 124I | 100.1 | β − 1.6 (~90%); 2.2 (~10%) | cyclotron |
| 131I | 192.0 | β − 0.7 (89%); γ − 0.4 (82%); β/γ ** = 1 | nuclear reactor |
| 86Y | 14.7 | β − 1.2 (~90%); 1.6 (~10%) | cyclotron |
| 90Y | 64.8 | β − 2.2 (100%) | generator 90Sr→90Y |
| 177Lu | 160.8 | β − 0.5 (100%) | nuclear reactor |
| 188Re | 17.0 | β − 2.0 (100%) | generator 188W→188Re |
| 64Cu | 12.7 | β − 0.65 (61.5%), β − 0.58 (38.5%) | Cyclotron |
| 67Cu | 61.9 | β − 0.4 (100%) | cyclotron |
| 89Zr | 78.0 | Β ± 0.9 (100%) | cyclotron |
| 212Pb | 10.6 | β − 0.6 (~80%); γ − 0.2 (44%); 0.08 (18%) | generator 228Th→220Rn→216Po→212Pb |
| 212Bi | 1.0 | α − 6.0 (100%); β − 2.0 (100%); α/β ** = 0.67 | generator 228Th→224Ra→212Bi |
| 213Bi | 0.7 | α − 5.8 (97%); β − 1.4 (100%); α/β ** = 0.02 | generator 229Th→225Ac→213Bi |
| 211At | 7.21 | α − 5.9 (42) | cyclotron |
| 225Ac | 240.2 | α − 5.7 (100%) | generator 229Th→225Ac |
| 223Ra | 273.6 | α − 5.7 (100%) | cyclotron |
| 149Tb | 4.1 | α − 4.0 (~80%) | cyclotron |
| 226Th | 0.5 | α − 6.3 (~50%) | generator 230U→226Th |
| 227Th | 448.8 | α − 6.0 (48%) | generator 227Ac→227Th |
| 89Sr | 1212 | β − 1.5 (100%) | nuclear reactor |
| 153Sm | 46.3 | β − 0.81 (100%) | cyclotron |
4. Choice of Carrier Molecule
- (1)
- The carrier molecule must possess high affinity and specificity for the target.
- (2)
- The carrier molecule should not be toxic or immunogenic, with a preferable LD50 value of greater than 1.5 g per kg of body weight.
- (3)
- The carrier molecule has no resistance to self-radiolysis and well-preserved both under storage conditions and upon contact with biological liquids.
- (4)
- Production of the carrier molecules with sufficient chemical purity ought to be simple and cost effective.
- (5)
- The carrier molecule has binding affinity to a variety of radionuclides. Specific chemical modifications are preferred to involve a minimum number of reactions.
5. Selection of a Target Antigen of Tumor Cells
- (1)
- Even expression and distribution over the entire cells surface of malignant tumors;
- (2)
- Low or negligible expression in normal cells to minimize the side effects;
- (3)
- Detainment in the cancer site, with no leakage to the blood flow.
6. Targeted Radionuclide Therapy of Hematological Malignancies
7. Targeted Radionuclide Therapy of Solid Tumors
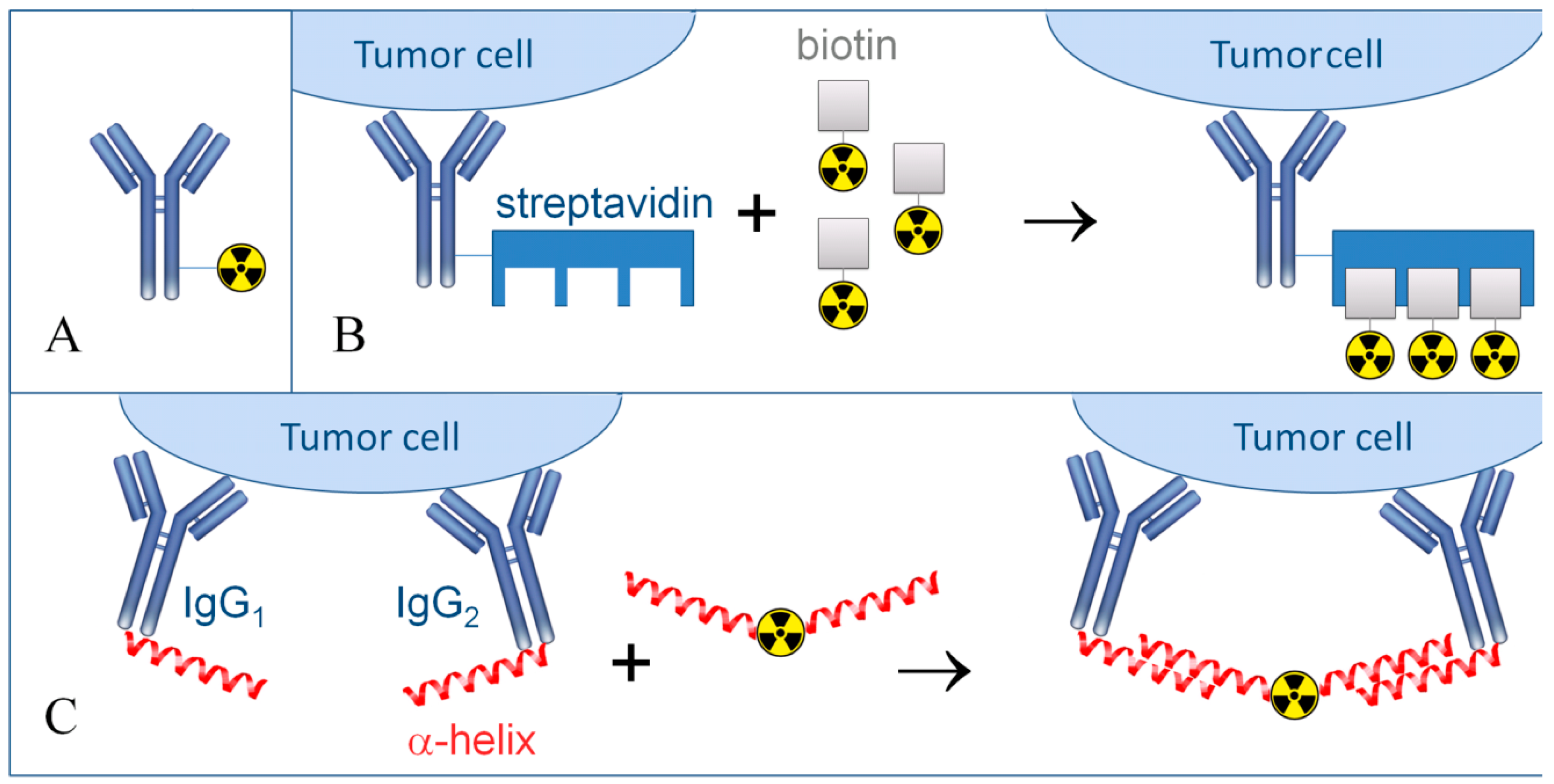
8. Determination of the Dose Load in TRNT
9. Available Commercial Pharmaceuticals for Targeted Radionuclide Therapy
| Commercial Name (Other Names) | Antigen/Radionuclide | Disease | Clinical Trial Status |
|---|---|---|---|
| Zevalin (90Y–ibritumomab tiuxetan) | CD20/90Y | non-Hodgkin‘s lymphoma | Approved by FDA |
| Bexxar ( 131I–tositumomab) | CD20/131I | non-Hodgkin‘s lymphoma | Approved by FDA |
| Oncolym (131I–Lym 1) | HLA-DR10/131I | non-Hodgkin‘s lymphoma, chronic lymphocytic leukaemia | Phase III |
| Lymphocide (Epratuzumab) | CD22/90Y | non-Hodgkin‘s lymphoma, chronic lymphocytic leukaemia, immune diseases | Phase III |
| Cotara (131I–chTNT–1/B) | DNA/131I | glioblastoma, anaplastic astrocytoma | Phase III |
| Labetuzumab (CEA–Cide) | CEA/90Y or 131I | breast, lung, pancreatic, stomach, colorectal carcinoma | Phase III |
| Theragin (Pemtumomab) | PEM/90Y | ovarian, gastric carcinoma | Phase III |
| Licartin (131I–metuximab) | (Hab18G/CD147)/131I | hepatocellular carcinoma | Phase II |
| Radretumab (131I–L19) | Fibronectin/131I | hepatological malignancy, refractory Hodgkin‘s lymphoma, non-small cell lung cancer, melanoma, head and neck carcinoma | Phase II |
| PAM4 (90Y–clivatuzumab tetraxetan) | MUC1/90Y | Pancreatic adenocarcinoma | Phase III |
| Xofigo (223Ra dichloride) | –/223Ra | metastatic castration-resistant prostate cancer | Approved by FDA |
| Lutathera (177Lu–DOTA–Tyr3–Octreotate) | SST/177Lu | metastatic GastroEnteroPancreatic NeuroEndocrine Tumors | Phase III |
| 131I–MIBG | norepinephrine (NE)/131I | neuroblastoma, Pheochromocytoma, Paraganglioma | Phase III |
10. Conclusions
Acknowledgments
Conflicts of Interest
References
- Curcio, C.G.; Vasile, C.; Gianciotta, A.; Casali, A.; Gionfra, T.; Rinaldi, M.; Guadagni, A.; Le Pera, V.; Sega, E. Short-term results of combined radioimmunotherapy in inoperable lung cancer. Tumori 1976, 62, 587–598. [Google Scholar] [PubMed]
- Sfakianakis, G.N.; DeLand, F.H. Radioimmunodiagnosis and radioimmunotherapy, 1982. J. Nucl. Med. 1982, 23, 840–850. [Google Scholar] [PubMed]
- Reilly, R.M. Radioimmunotherapy of malignancies. Clin. Pharm. 1991, 10, 359–375. [Google Scholar] [PubMed]
- Goldenberg, D.M.; Chang, C.H.; Rossi, E.A.; McBride, J.W.; Sharkey, R.M. Pretargeted molecular imaging and radioimmunotherapy. Theranostics 2012, 2, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Pouget, J.P.; Lozza, C.; Deshayes, E.; Boudousq, V.; Navarro-Teulon, I. Introduction to radiobiology of targeted radionuclide therapy. Front. Med. 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Apetoh, L. Radiotherapy and immunogenic cell death. Semin. Radiat. Oncol. 2015, 25, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.F. DNA damage produced by ionizing radiation in mammalian cells: Identities, mechanisms of formation, and reparability. Prog. Nucleic Acid Res. Mol. Biol. 1988, 35, 95–125. [Google Scholar] [PubMed]
- Jackson, M.R.; Falzone, N.; Vallis, K.A. Advances in anticancer radiopharmaceuticals. Clin. Oncol. 2013, 25, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Kassis, A.I.; Adelstein, S.J. Radiobiologic principles in radionuclide therapy. J. Nucl. Med. 2005, 46, 4S–12S. [Google Scholar] [PubMed]
- Barendsen, G.W.; Beusker, T.L.; Vergroesen, A.J.; Budke, L. Effects of different radiations on human cells in tissue culture. Ii. Biological experiments. Radiat. Res. 1960, 13, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Qaim, S.M. Therapeutic radionuclides and nuclear data. Radiochim. Acta 2001, 89, 297–302. [Google Scholar] [CrossRef]
- Kuroda, I. Effective use of strontium-89 in osseous metastases. Ann. Nucl. Med. 2012, 26, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Toohey, R.E.; Stabin, M.G.; Watson, E.E. The aapm/rsna physics tutorial for residents: Internal radiation dosimetry: Principles and applications. Radiographics 2000, 20, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Ocean, A.J.; Pennington, K.L.; Guarino, M.J.; Sheikh, A.; Bekaii-Saab, T.; Serafini, A.N.; Lee, D.; Sung, M.W.; Gulec, S.A.; Goldsmith, S.J.; et al. Fractionated radioimmunotherapy with (90) y-clivatuzumab tetraxetan and low-dose gemcitabine is active in advanced pancreatic cancer: A phase 1 trial. Cancer 2012, 118, 5497–5506. [Google Scholar] [CrossRef] [PubMed]
- Kraeber-Bodere, F.; Rousseau, C.; Bodet-Milin, C.; Frampas, E.; Faivre-Chauvet, A.; Rauscher, A.; Sharkey, R.M.; Goldenberg, D.M.; Chatal, J.F.; Barbet, J. A pretargeting system for tumor pet imaging and radioimmunotherapy. Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Pruszynski, M. Astatine-211: Production and availability. Curr. Radiopharm. 2011, 4, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Abmel, X.C.; Chinol, M.; Jin, X.-H.; Knapp, F.F., Jr.; Rostislav, K.; van So, L.; Renata, M.; Fabiola, M.; Osso, J.A., Jr.; Park, S.H.; et al. Therapeutic Radionuclide Generators: 90sr/90y and 188w/188re Generators; International Atomic Energy Agency: Vienna, Austria, 2009; p. 235. [Google Scholar]
- Pillai, M.R.; Dash, A.; Knapp, F.F., Jr. Rhenium-188: Availability from the (188)w/(188)re generator and status of current applications. Curr. Radiopharm. 2012, 5, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.M.; Carrasquillo, J.A.; Cheung, N.K.; Press, O.W. Radioimmunotherapy of human tumours. Nat. Rev. Cancer 2015, 15, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Press, O.W.; Shan, D.; Howell-Clark, J.; Eary, J.; Appelbaum, F.R.; Matthews, D.; King, D.J.; Haines, A.M.; Hamann, P.; Hinman, L.; et al. Comparative metabolism and retention of iodine-125, yttrium-90, and indium-111 radioimmunoconjugates by cancer cells. Cancer Res. 1996, 56, 2123–2129. [Google Scholar] [PubMed]
- Govindan, S.V.; Goldenberg, D.M. New antibody conjugates in cancer therapy. Sci. World J. 2010, 10, 2070–2089. [Google Scholar] [CrossRef] [PubMed]
- Seidl, C. Radioimmunotherapy with alpha-particle-emitting radionuclides. Immunotherapy 2014, 6, 431–458. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Aoki, M. Radiolabeled apoptosis imaging agents for early detection of response to therapy. Sci. World J. 2014, 2014, 732603. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.K.; Cheon, G.J. Radioiodine therapy in differentiated thyroid cancer: The first targeted therapy in oncology. Endocrinol. Metab. 2014, 29, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Seidlin, S.M.; Marinelli, L.D.; Oshry, E. Radioactive iodine therapy: Effect on functioning metastases of adenocarcinoma of the thyroid. J. Am. Med. Assoc. 1990, 40, 299–317. [Google Scholar] [CrossRef]
- Dai, G.; Levy, O.; Carrasco, N. Cloning and characterization of the thyroid iodide transporter. Nature 1996, 379, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zhang, R.; Pan, Y.; Xu, H.; Zhang, M.; Liang, S.; Wang, L.; Zhang, Y.; Li, B. Feasibility of a novel positive feedback effect of 131i-promoted bac-egr1-hnis expression in malignant glioma through baculovirus: A comparative study with bac-cmv-hnis. Nucl. Med. Commun. 2011, 32, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.P.; Ferreira, A.C. The importance of sodium/iodide symporter (nis) for thyroid cancer management. Arq. Bras. Endocrinol. Metab. 2007, 51, 672–682. [Google Scholar] [CrossRef]
- Puppin, C.; Arturi, F.; Ferretti, E.; Russo, D.; Sacco, R.; Tell, G.; Damante, G.; Filetti, S. Transcriptional regulation of human sodium/iodide symporter gene: A role for redox factor-1. Endocrinology 2004, 145, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Nicola, J.P.; Nazar, M.; Mascanfroni, I.D.; Pellizas, C.G.; Masini-Repiso, A.M. Nf-kappab p65 subunit mediates lipopolysaccharide-induced Na(+)/I(−) symporter gene expression by involving functional interaction with the paired domain transcription factor pax8. Mol. Endocrinol. 2010, 24, 1846–1862. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.-K.; Youn, H.W.; Kang, J.H.; Lee, H.Y.; Kang, K.W. Sodium iodide symporter and the radioiodine treatment of thyroid carcinoma. Nucl. Med. Mol. Imaging 2010, 44, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Pandit-Taskar, N.; Larson, S.M.; Carrasquillo, J.A. Bone-seeking radiopharmaceuticals for treatment of osseous metastases, part 1: Alpha therapy with 223ra-dichloride. J. Nucl. Med. 2014, 55, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Tomblyn, M. The role of bone-seeking radionuclides in the palliative treatment of patients with painful osteoblastic skeletal metastases. Cancer Control 2012, 19, 137–144. [Google Scholar] [PubMed]
- Maffioli, L.; Florimonte, L.; Costa, D.C.; Correia Castanheira, J.; Grana, C.; Luster, M.; Bodei, L.; Chinol, M. New radiopharmaceutical agents for the treatment of castration-resistant prostate cancer. Q. J. Nucl. Med. Mol. Imaging 2015, 59, 420–438. [Google Scholar] [PubMed]
- Allen, B. Systemic targeted alpha radiotherapy for cancer. J. Biomed. Phys. Eng. 2013, 3, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Dash, A.; Knapp, F.F.; Pillai, M.R. Targeted radionuclide therapy—An overview. Curr. Radiopharm. 2013, 6, 152–180. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Weiner, G.J. Building better monoclonal antibody-based therapeutics. Nat. Rev. Cancer 2015, 15, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Cremonesi, M.; Kidd, M.; Grana, C.M.; Severi, S.; Modlin, I.M.; Paganelli, G. Peptide receptor radionuclide therapy for advanced neuroendocrine tumors. Thorac. Surg. Clin. 2014, 24, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Shapira, S.; Fokra, A.; Arber, N.; Kraus, S. Peptides for diagnosis and treatment of colorectal cancer. Curr. Med. Chem. 2014, 21, 2410–2416. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.T.; Holland, N.B. Multifunctional nanoparticles for use in theranostic applications. Drug Deliv. Transl. Res. 2015, 5, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Toward a full understanding of the epr effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.M.; Subbiah, V.; Rohren, E. Bone-seeking radiopharmaceuticals as targeted agents of osteosarcoma: Samarium-153-edtmp and radium-223. Adv. Exp. Med. Biol. 2014, 804, 291–304. [Google Scholar] [PubMed]
- Hofmeister, V.; Schrama, D.; Becker, J.C. Anti-cancer therapies targeting the tumor stroma. Cancer Immunol. Immunother. 2008, 57, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; O’Brien, S.; Ravandi, F.; Kantarjian, H. Monoclonal antibodies in acute lymphoblastic leukemia. Blood 2015, 125, 4010–4016. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.P.; Bulkeley, W., 3rd; Zhang, L.; Menes, M.; Bui, M.M. A practical approach to diagnose soft tissue myeloid sarcoma preceding or coinciding with acute myeloid leukemia. Ann. Diagn. Pathol. 2014, 18, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Slovin, S. Biomarkers for immunotherapy in genitourinary malignancies. Urol. Oncol. 2015, 18, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Ronca, R.; Sozzani, S.; Presta, M.; Alessi, P. Delivering cytokines at tumor site: The immunocytokine-conjugated anti-edb-fibronectin antibody case. Immunobiology 2009, 214, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Garinchesa, P.; Sakamoto, J.; Welt, S.; Real, F.; Rettig, W.; Old, L. Organ-specific expression of the colon cancer antigen a33, a cell surface target for antibody-based therapy. Int. J. Oncol. 1996, 9, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Pourgholami, M.H.; Allen, B.J. Optimizing radioimmunoconjugate delivery in the treatment of solid tumor. Cancer Treat. Rev. 2012, 38, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Jurcic, J.G. Radioimmunotherapy for hematopoietic cell transplantation. Immunotherapy 2013, 5, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Tabata, R.; Iwama, H.; Tabata, C.; Yasumizu, R.; Kojima, M. Cd5- and cd23-positive splenic diffuse large b-cell lymphoma with very low cd20 expression. J. Clin. Exp. Hematop. 2014, 54, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Koon, H.B.; Junghans, R.P. Anti-cd30 antibody-based therapy. Curr. Opin. Oncol. 2000, 12, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yao, Z.; Zhang, Z.; Garmestani, K.; Talanov, V.S.; Plascjak, P.S.; Yu, S.; Kim, H.S.; Goldman, C.K.; Paik, C.H.; et al. The anti-cd25 monoclonal antibody 7g7/b6, armed with the alpha-emitter 211at, provides effective radioimmunotherapy for a murine model of leukemia. Cancer Res. 2006, 66, 8227–8232. [Google Scholar] [CrossRef] [PubMed]
- Liersch, T.; Meller, J.; Kulle, B.; Behr, T.M.; Markus, P.; Langer, C.; Ghadimi, B.M.; Wegener, W.A.; Kovacs, J.; Horak, I.D.; et al. Phase ii trial of carcinoembryonic antigen radioimmunotherapy with 131i-labetuzumab after salvage resection of colorectal metastases in the liver: Five-year safety and efficacy results. J. Clin. Oncol. 2005, 23, 6763–6770. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S.T.; Milowsky, M.I.; Morris, M.; Vallabhajosula, S.; Christos, P.; Akhtar, N.H.; Osborne, J.; Goldsmith, S.J.; Larson, S.; Taskar, N.P.; et al. Phase ii study of lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody j591 for metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2013, 19, 5182–5191. [Google Scholar] [CrossRef] [PubMed]
- Raja, C.; Graham, P.; Abbas Rizvi, S.M.; Song, E.; Goldsmith, H.; Thompson, J.; Bosserhoff, A.; Morgenstern, A.; Apostolidis, C.; Kearsley, J.; et al. Interim analysis of toxicity and response in phase 1 trial of systemic targeted alpha therapy for metastatic melanoma. Cancer Biol. Ther. 2007, 6, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.J.; Singla, A.A.; Rizvi, S.M.; Graham, P.; Bruchertseifer, F.; Apostolidis, C.; Morgenstern, A. Analysis of patient survival in a phase I trial of systemic targeted alpha-therapy for metastatic melanoma. Immunotherapy 2011, 3, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Sansovini, M.; Severi, S.; Ambrosetti, A.; Monti, M.; Nanni, O.; Sarnelli, A.; Bodei, L.; Garaboldi, L.; Bartolomei, M.; Paganelli, G. Treatment with the radiolabelled somatostatin analog lu-dotatate for advanced pancreatic neuroendocrine tumors. Neuroendocrinology 2013, 97, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Forrer, F.; Waldherr, C.; Maecke, H.R.; Mueller-Brand, J. Targeted radionuclide therapy with 90y-dotatoc in patients with neuroendocrine tumors. Anticancer Res. 2006, 26, 703–707. [Google Scholar] [PubMed]
- Waldherr, C.; Pless, M.; Maecke, H.R.; Schumacher, T.; Crazzolara, A.; Nitzsche, E.U.; Haldemann, A.; Mueller-Brand, J. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 gbq (90)y-dotatoc. J. Nucl. Med. 2002, 43, 610–616. [Google Scholar] [PubMed]
- Nisa, L.; Savelli, G.; Giubbini, R. Yttrium-90 dotatoc therapy in gep-net and other sst2 expressing tumors: A selected review. Ann. Nucl. Med. 2011, 25, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; da Silva, R.; Gravekamp, C.; Libutti, S.K.; Abraham, T.; Dadachova, E. Targeted radionuclide therapies for pancreatic cancer. Cancer Gene Ther. 2015, 22, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Kraeber-Bodere, F.; Bodet-Milin, C.; Rousseau, C.; Eugene, T.; Pallardy, A.; Frampas, E.; Carlier, T.; Ferrer, L.; Gaschet, J.; Davodeau, F.; et al. Radioimmunoconjugates for the treatment of cancer. Semin. Oncol. 2014, 41, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.; Held, K.D. Radiation Biology: A Handbook for Teachers and Students; International Atomic Energy Agency: Vienna, Austria, 2010; p. 166. [Google Scholar]
- Burnet, N.G.; Wurm, R.; Nyman, J.; Peacock, J.H. Normal tissue radiosensitivity--how important is it? Clin. Oncol. 1996, 8, 25–34. [Google Scholar] [CrossRef]
- Bruland, O.S.; Nilsson, S.; Fisher, D.R.; Larsen, R.H. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223ra: Adjuvant or alternative to conventional modalities? Clin. Cancer Res. 2006, 12, 6250s–6257s. [Google Scholar] [CrossRef] [PubMed]
- Trieu, M.; DuBois, S.G.; Pon, E.; Nardo, L.; Hawkins, R.A.; Marachelian, A.; Twist, C.J.; Park, J.R.; Matthay, K.K. Impact of whole-body radiation dose on response and toxicity in patients with neuroblastoma after therapy with i-metaiodobenzylguanidine (mibg). Pediatr. Blood Cancer 2016, 63. [Google Scholar] [CrossRef]
- Seo, Y.; Gustafson, W.C.; Dannoon, S.F.; Nekritz, E.A.; Lee, C.L.; Murphy, S.T.; VanBrocklin, H.F.; Hernandez-Pampaloni, M.; Haas-Kogan, D.A.; Weiss, W.A.; et al. Tumor dosimetry using [124i]m-iodobenzylguanidine micropet/ct for [131i]m-iodobenzylguanidine treatment of neuroblastoma in a murine xenograft model. Mol. Imaging Biol. 2012, 14, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Bolch, W.E.; Lee, C.; van Brocklin, H.F.; Pampaloni, M.H.; Hawkins, R.A.; Sznewajs, A.; DuBois, S.G.; Matthay, K.K.; Seo, Y. Patient-specific dosimetry using pretherapy [124I]m-iodobenzylguanidine ([124I]mibg) dynamic pet/ct imaging before [131I]mibg targeted radionuclide therapy for neuroblastoma. Mol. Imaging Biol. 2015, 17, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G. Yttrium-90 biodistribution by yttrium-87 imaging: A theoretical feasibility analysis. Med. Phys. 1998, 25, 1487–1490. [Google Scholar] [CrossRef] [PubMed]
- Gulec, S.A.; Cohen, S.J.; Pennington, K.L.; Zuckier, L.S.; Hauke, R.J.; Horne, H.; Wegener, W.A.; Teoh, N.; Gold, D.V.; Sharkey, R.M.; et al. Treatment of advanced pancreatic carcinoma with 90y-clivatuzumab tetraxetan: A phase I single-dose escalation trial. Clin. Cancer Res. 2011, 17, 4091–4100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yeong, C.H.; Cheng, M.H.; Ng, K.H. Therapeutic radionuclides in nuclear medicine: Current and future prospects. J. Zhejiang Univ. Sci. B 2014, 15, 845–863. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Ruiz, A.C.; de la Cruz-Merino, L.; Provencio Pulla, M. Role of consolidation with yttrium-90 ibritumomab tiuxetan in patients with advanced-stage follicular lymphoma. Ther. Adv. Hematol. 2014, 5, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Morschhauser, F.; Radford, J.; van Hoof, A.; Botto, B.; Rohatiner, A.Z.; Salles, G.; Soubeyran, P.; Tilly, H.; Bischof-Delaloye, A.; van Putten, W.L.; et al. 90yttrium-ibritumomab tiuxetan consolidation of first remission in advanced-stage follicular non-hodgkin lymphoma: Updated results after a median follow-up of 7. 3 years from the international, randomized, phase iii first-lineindolent trial. J. Clin. Oncol. 2013, 31, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Morschhauser, F.; Radford, J.; van Hoof, A.; Vitolo, U.; Soubeyran, P.; Tilly, H.; Huijgens, P.C.; Kolstad, A.; d’Amore, F.; Gonzalez Diaz, M.; et al. Phase iii trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J. Clin. Oncol. 2008, 26, 5156–5164. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.P.; Coleman, M.; Ketas, J.C.; Chadburn, A.; Furman, R.; Schuster, M.W.; Feldman, E.J.; Ashe, M.; Schuster, S.J.; Wegener, W.A.; et al. Epratuzumab, a humanized anti-cd22 antibody, in aggressive non-hodgkin's lymphoma: Phase i/ii clinical trial results. Clin. Cancer Res. 2004, 10, 5327–5334. [Google Scholar] [CrossRef] [PubMed]
- Beatson, R.E.; Taylor-Papadimitriou, J.; Burchell, J.M. Muc1 immunotherapy. Immunotherapy 2010, 2, 305–327. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Jin, G.; Wang, L.; Li, M.; He, C.; Guo, X.; Zhu, Q. The role of pam4 in the management of pancreatic cancer: Diagnosis, radioimmunodetection, and radioimmunotherapy. J. Immunol. Res. 2014, 2014, 268479. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, S.J. Radioimmunotherapy of lymphoma: Bexxar and zevalin. Semin. Nucl. Med. 2010, 40, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Press, O.W.; Unger, J.M.; Rimsza, L.M.; Friedberg, J.W.; LeBlanc, M.; Czuczman, M.S.; Kaminski, M.; Braziel, R.M.; Spier, C.; Gopal, A.K.; et al. Phase iii randomized intergroup trial of chop plus rituximab compared with chop chemotherapy plus (131)iodine-tositumomab for previously untreated follicular non-hodgkin lymphoma: Swog s0016. J. Clin. Oncol. 2013, 31, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Vose, J.M.; Carter, S.; Burns, L.J.; Ayala, E.; Press, O.W.; Moskowitz, C.H.; Stadtmauer, E.A.; Mineshi, S.; Ambinder, R.; Fenske, T.; et al. Phase iii randomized study of rituximab/carmustine, etoposide, cytarabine, and melphalan (beam) compared with iodine-131 tositumomab/beam with autologous hematopoietic cell transplantation for relapsed diffuse large b-cell lymphoma: Results from the bmt ctn 0401 trial. J. Clin. Oncol. 2013, 31, 1662–1668. [Google Scholar] [PubMed]
- Press, O.W.; Unger, J.M.; Rimsza, L.M.; Friedberg, J.W.; LeBlanc, M.; Czuczman, M.S.; Kaminski, M.; Braziel, R.M.; Spier, C.; Gopal, A.K.; et al. A comparative analysis of prognostic factor models for follicular lymphoma based on a phase iii trial of chop-rituximab versus chop + 131iodine—Tositumomab. Clin. Cancer Res. 2013, 19, 6624–6632. [Google Scholar] [CrossRef] [PubMed]
- Dechant, M.; Bruenke, J.; Valerius, T. Hla class ii antibodies in the treatment of hematologic malignancies. Semin. Oncol. 2003, 30, 465–475. [Google Scholar] [CrossRef]
- Hdeib, A.; Sloan, A. Targeted radioimmunotherapy: The role of 131I-chtnt-1/b mab (cotara) for treatment of high-grade gliomas. Future Oncol. 2012, 8, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, M.; Aucejo, F.; Kim, R. Adjuvant treatment of hepatocellular carcinoma after orthotopic liver transplantation: Do we really need this? Clin. Transplant. 2013, 27, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Erba, P.A.; Sollini, M.; Orciuolo, E.; Traino, C.; Petrini, M.; Paganelli, G.; Bombardieri, E.; Grana, C.; Giovannoni, L.; Neri, D.; et al. Radioimmunotherapy with radretumab in patients with relapsed hematologic malignancies. J. Nucl. Med. 2012, 53, 922–927. [Google Scholar] [CrossRef] [PubMed]
- FDA_USA. Xofigo (radium ra 223 dichloride) injection: Prescribing information. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/203971lbl.pdf (accessed on 31 October 2015).
- Humm, J.L.; Sartor, O.; Parker, C.; Bruland, O.S.; Macklis, R. Radium-223 in the treatment of osteoblastic metastases: A critical clinical review. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O'Sullivan, J.M.; Fossa, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Mittra, E.; Hobday, T.; Hendifar, A.; et al. 177-Lu-dotatate significantly improves progression-free survival in patients with midgut neuroendocrine tumours: Results of the Phase III NETTER-1 trial. In Proceedings of the 2015 European Cancer Congress, Vienna, Austria, 25–29 September 2015; p. 6LBA.
- Kayano, D.; Kinuya, S. Iodine-131 metaiodobenzylguanidine therapy for neuroblastoma: Reports so far and future perspective. Sci. World J. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Quach, A.; Ji, L.; Mishra, V.; Sznewajs, A.; Veatch, J.; Huberty, J.; Franc, B.; Sposto, R.; Groshen, S.; Wei, D.; et al. Thyroid and hepatic function after high dose (131)i-metaiodobenzylguanidine ((131)i-mibg) therapy for neuroblastoma. Pediatr. Blood Cancer 2011, 56, 191–201. [Google Scholar] [CrossRef] [PubMed]
- DuBois, S.G.; Groshen, S.; Park, J.R.; Haas-Kogan, D.A.; Yang, X.; Geier, E.; Chen, E.; Giacomini, K.; Weiss, B.; Cohn, S.L.; et al. Phase I study of vorinostat as a radiation sensitizer with 131i-metaiodobenzylguanidine (131i-mibg) for patients with relapsed or refractory neuroblastoma. Clin. Cancer Res. 2015, 21, 2715–2721. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.J.; Doral, M.Y.; DuBois, S.G.; Villablanca, J.G.; Yanik, G.A.; Matthay, K.K. Different outcomes for relapsed versus refractory neuroblastoma after therapy with (131)i-metaiodobenzylguanidine ((131)i-mibg). Eur. J. Cancer 2015, 51, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Popova, N.R.; Bruskov, V.I. Radioprotective substances: History, trends and prospects. Biophysics 2015, 60, 659–667. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Shtarkman, I.N.; Smirnova, V.S.; Chernikov, A.V.; Bruskov, V.I. Guanosine and inosine display antioxidant activity, protect DNA in vitro from oxidative damage induced by reactive oxygen species, and serve as radioprotectors in mice. Radiat. Res. 2006, 165, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Gudkova, O.Y.; Chernikov, A.V.; Bruskov, V.I. Protection of mice against X-ray injuries by the post-irradiation administration of guanosine and inosine. Int. J. Radiat. Biol. 2009, 85, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Asadullina, N.R.; Usacheva, A.M.; Smirnova, V.S.; Gudkov, S.V. Antioxidative and radiation modulating properties of guanosine-5′-monophosphate. Nucleosides Nucleotides Nucleic Acid 2010, 29, 786–799. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudkov, S.V.; Shilyagina, N.Y.; Vodeneev, V.A.; Zvyagin, A.V. Targeted Radionuclide Therapy of Human Tumors. Int. J. Mol. Sci. 2016, 17, 33. https://doi.org/10.3390/ijms17010033
Gudkov SV, Shilyagina NY, Vodeneev VA, Zvyagin AV. Targeted Radionuclide Therapy of Human Tumors. International Journal of Molecular Sciences. 2016; 17(1):33. https://doi.org/10.3390/ijms17010033
Chicago/Turabian StyleGudkov, Sergey V., Natalya Yu. Shilyagina, Vladimir A. Vodeneev, and Andrei V. Zvyagin. 2016. "Targeted Radionuclide Therapy of Human Tumors" International Journal of Molecular Sciences 17, no. 1: 33. https://doi.org/10.3390/ijms17010033
APA StyleGudkov, S. V., Shilyagina, N. Y., Vodeneev, V. A., & Zvyagin, A. V. (2016). Targeted Radionuclide Therapy of Human Tumors. International Journal of Molecular Sciences, 17(1), 33. https://doi.org/10.3390/ijms17010033










