Osteoclast Differentiation Is Impaired in a Subgroup of SLE Patients and Correlates Inversely with Mycophenolate Mofetil Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. In Vitro Monocyte-Derived Osteoclast Differentiation of SLE Patients and Controls

2.1.2. Osteoclast Differentiation and MMF, IFNα and Corticosteroids
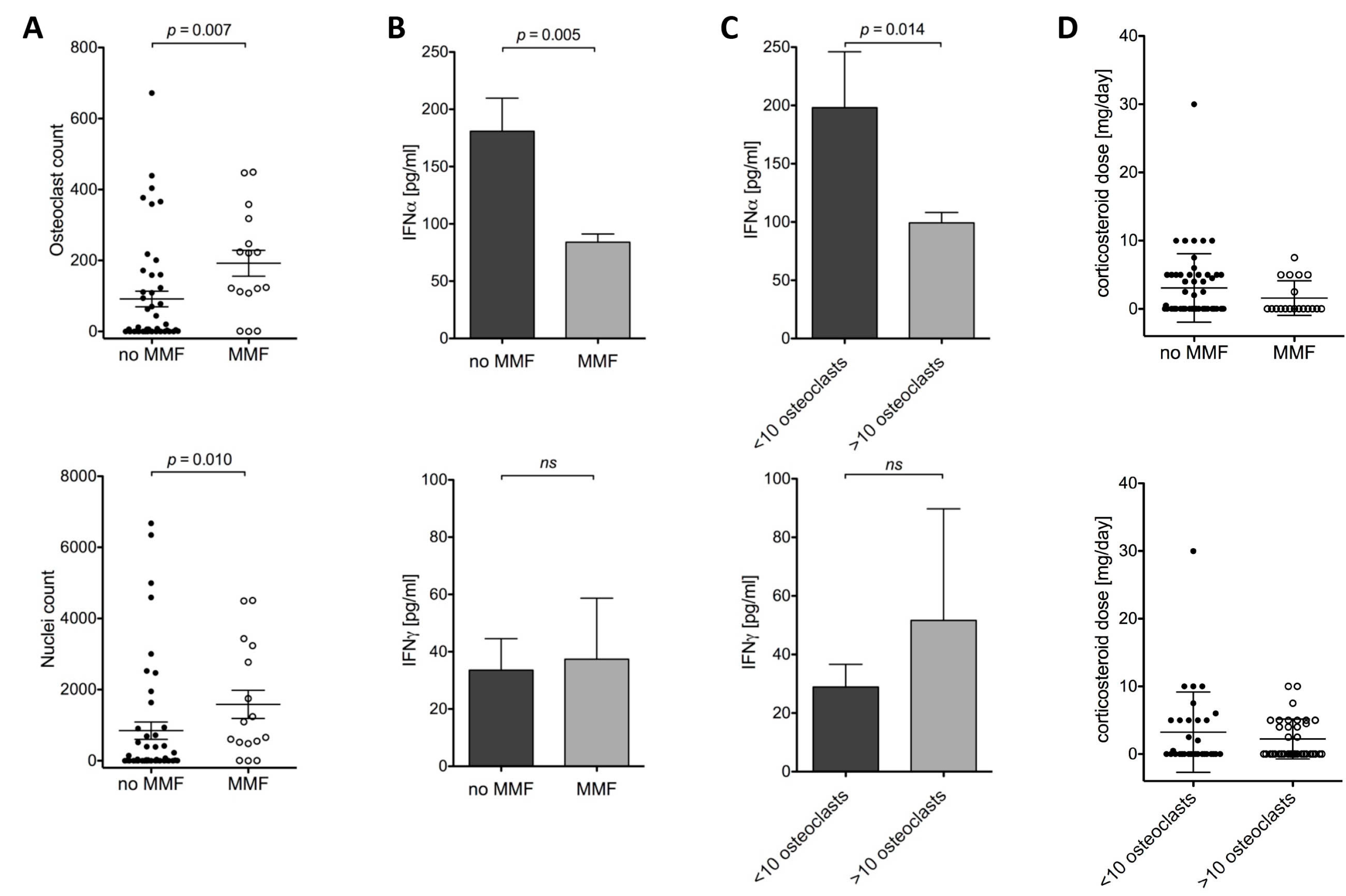
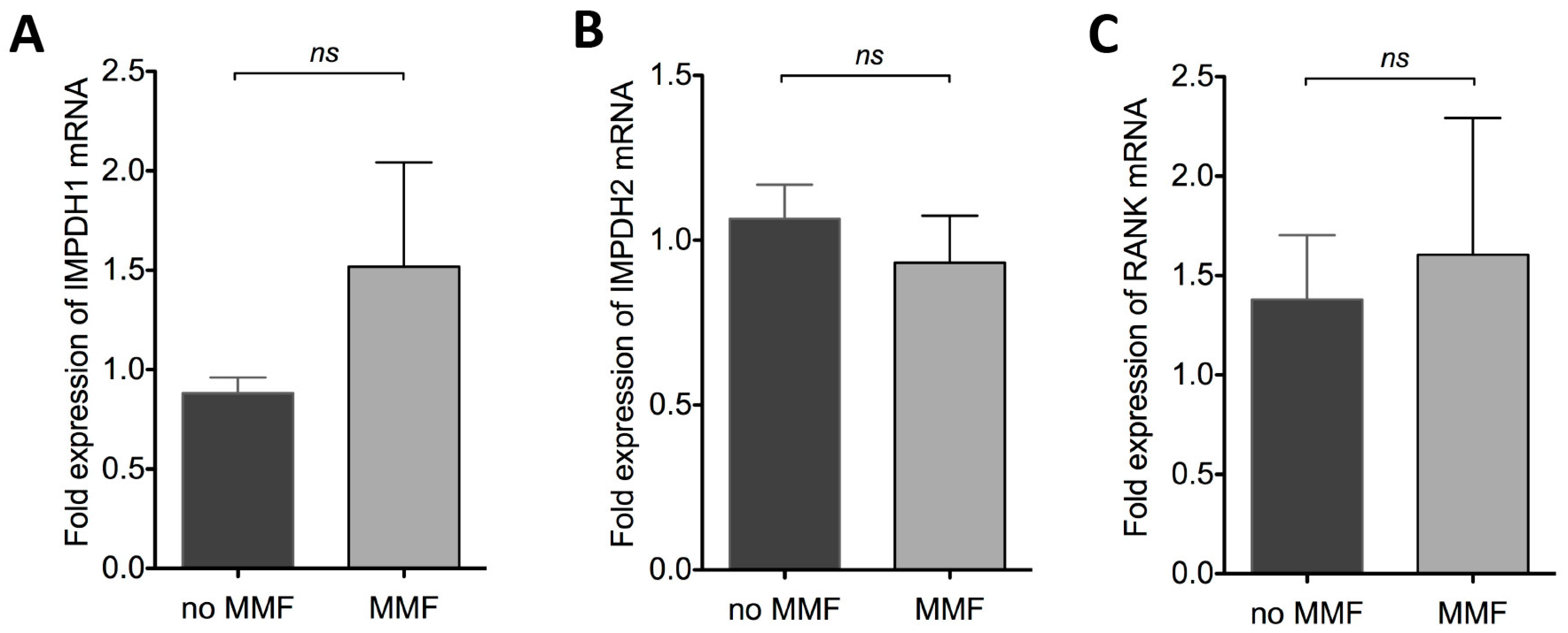
2.1.3. Effect of MMF on IMPDH1, IMPDH2 and RANK mRNA Expression
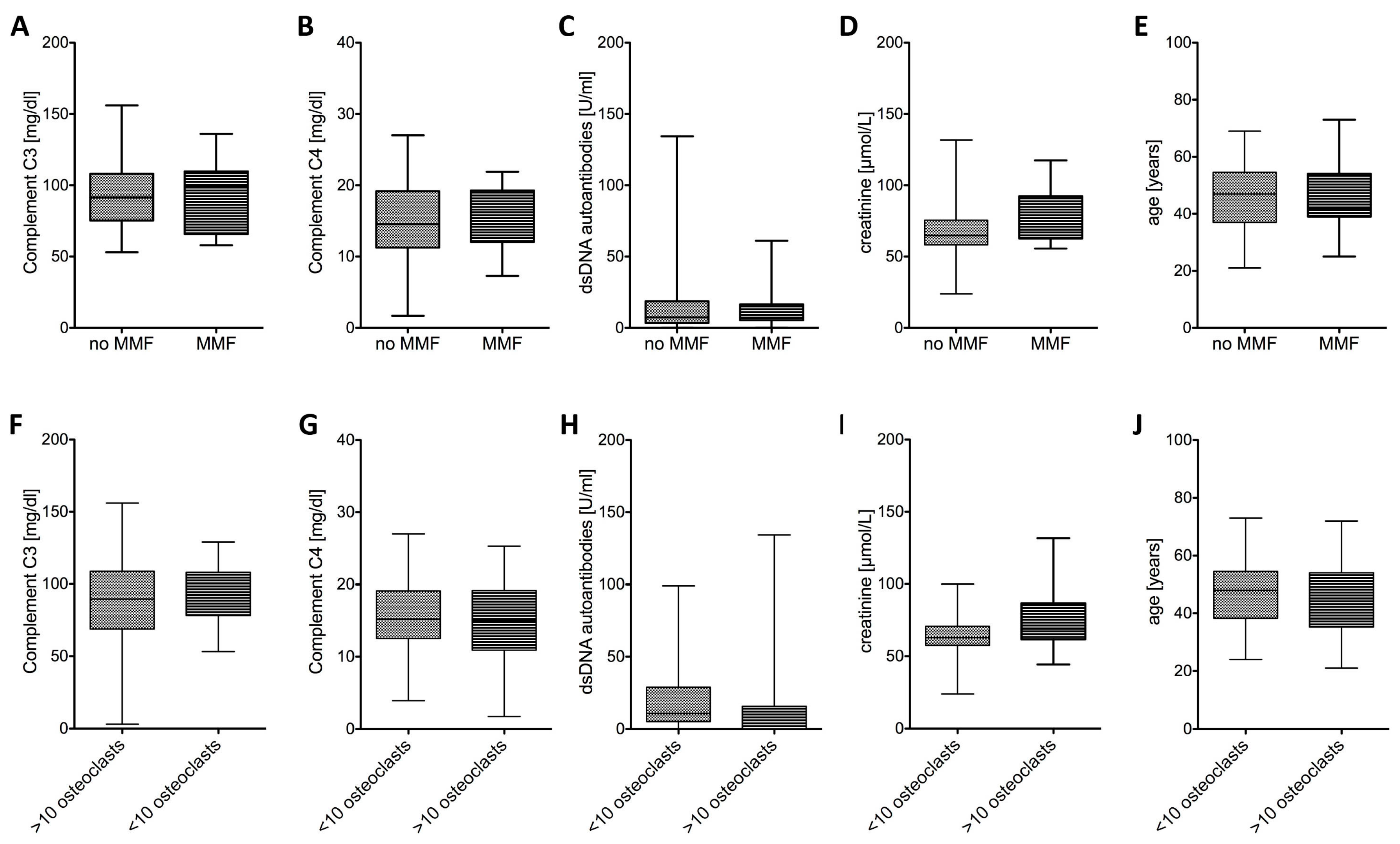
2.1.4. SLE Disease Activity Was Similar Irrespective of Osteoclast Differentiation or MMF Treatment
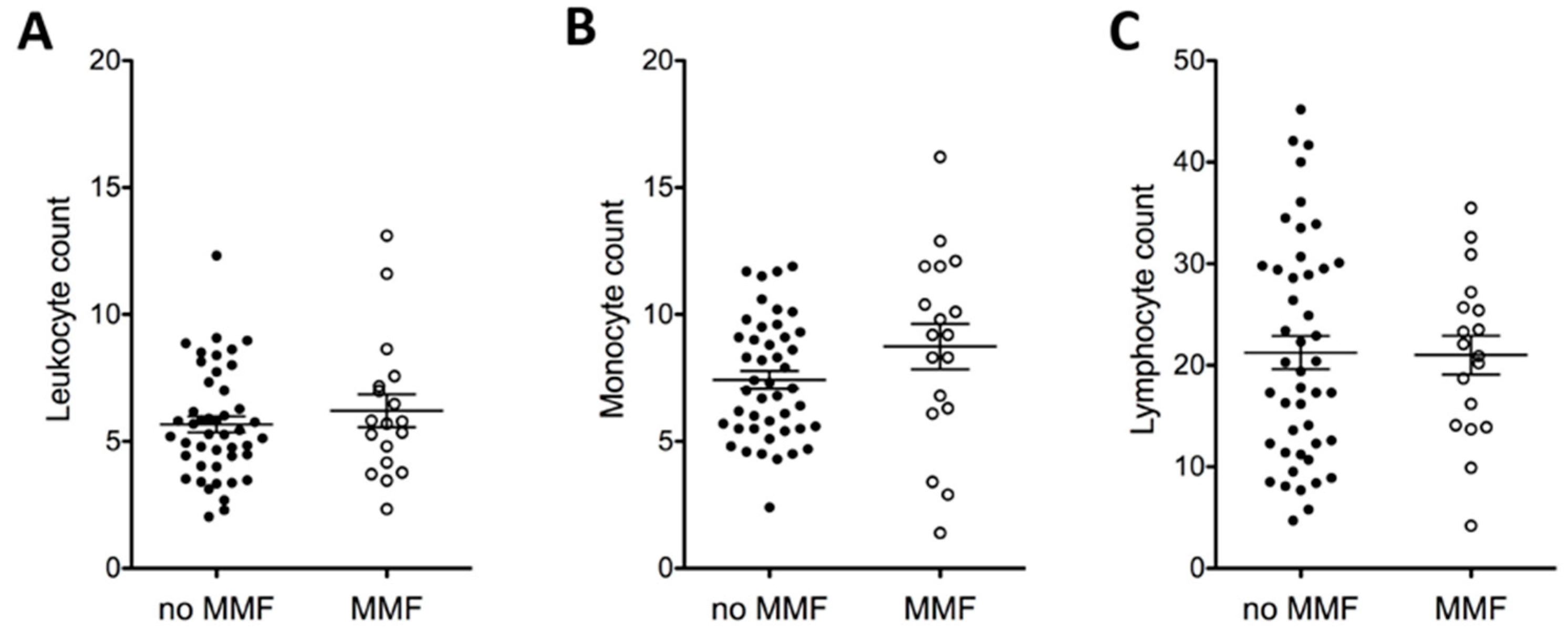
2.1.5. MMF and Full Blood Count
2.2. Discussion
3. Experimental Section
3.1. Patients and Controls
3.2. Isolation of Monocytes and Osteoclast Differentiation
3.3. Staining of Osteoclasts
3.4. RNA Isolation and Quantitative Real-Time PCR
- IMPDH1 5′-GAGCCCGAGAGCCCTAGATT-3′ & 5′-TCAGGTAGTCCGCCATGCTA-3′;
- IMPDH2 5′-TGGAGGCAATGTGGTCACTG-3′ & 5′-AGCAATGACCGGAACACCAA-3′;
- RANK 5′-ACCCTGGACCAACTGTACCT-3′ & 5′- CGGGCAAGTAAACATGGGGT-3′;
- GAPDH 5′-CATGAGAAGTATGACAACAGCCT-3′ & 5′-AGTCCTTCCACGATACCAAAGT-3′;
- RPL27 5′-ATCGCCAAGAGATCAAAGATAA-3′ & 5′-TCTGAAGACATCCTTATTGACG-3′.
3.5. ELISA for Interferon α and Interferon-γ
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Muñoz, L.E.; Frey, B.; Appelt, U.; Janko, C.; Sarter, K.; Voll, R.E.; Kern, P.; Herrmann, M.; Gaipl, U.S. 2010 Peripheral Blood Stem Cells of Patients with Systemic Lupus Erythematosus Show Altered Differentiation into Macrophages. Open Autoimmun. J. 2010, 2, 11–16. [Google Scholar]
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Westenfeld, R.; Schlieper, G.; Woltje, M.; Gawlik, A.; Brandenburg, V.; Rutkowski, P.; Floege, J.; Jahnen-Dechent, W.; Ketteler, M. Impact of sirolimus, tacrolimus and mycophenolate mofetil on osteoclastogenesis—Implications for post-transplantation bone disease. Nephrol. Dial. Transplant. 2011, 26, 4115–4123. [Google Scholar] [CrossRef] [PubMed]
- Eugui, E.M.; Almquist, S.J.; Muller, C.D.; Allison, A.C. Lymphocyte-selective cytostatic and immunosuppressive effects of mycophenolic acid in vitro: Role of deoxyguanosine nucleotide depletion. Scand. J. Immunol. 1991, 33, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Glomsda, B.A.; Blaheta, R.A.; Hailer, N.P. Inhibition of monocyte/endothelial cell interactions and monocyte adhesion molecule expression by the immunosuppressant mycophenolate mofetil. Spinal Cord 2003, 41, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.C.; Lee, Y.F.; Lan, J.L.; Wu, M.J.; Hsieh, T.Y.; Lin, N.N.; Wang, J.M.; Chiu, Y.T. Mycophenolate mofetil alleviates lupus nephritis through urokinase receptor signaling in a mice model. Lupus 2013, 22, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.F.; Papp, E.; Wu, J.C.; Natsumeda, Y. Characterization of human type I and type II IMP dehydrogenases. J. Biol. Chem. 1993, 268, 27286–27290. [Google Scholar] [PubMed]
- Natsumeda, Y.; Carr, S.F. Human type I and II IMP dehydrogenases as drug targets. Ann. N. Y. Acad. Sci. 1993, 696, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Adu, D.; Cross, J.; Jayne, D.R. Treatment of systemic lupus erythematosus with mycophenolate mofetil. Lupus 2001, 10, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Falcini, F.; Capannini, S.; Martini, G.; La Torre, F.; Vitale, A.; Mangiantini, F.; Nacci, F.; Cerinic, M.M.; Cimaz, R.; Zulian, F. Mycophenolate mofetil for the treatment of juvenile onset SLE: A multicenter study. Lupus 2009, 18, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Sanquer, S.; Maison, P.; Tomkiewicz, C.; Macquin-Mavier, I.; Legendre, C.; Barouki, R.; Lang, P. Expression of inosine monophosphate dehydrogenase type I and type II after mycophenolate mofetil treatment: A 2-year follow-up in kidney transplantation. Clin. Pharmacol. Ther. 2008, 83, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Avnet, S.; Cenni, E.; Perut, F.; Granchi, D.; Brandi, M.L.; Giunti, A.; Baldini, N. Interferon-α inhibits in vitro osteoclast differentiation and renal cell carcinoma-induced angiogenesis. Int. J. Oncol. 2007, 30, 469–476. [Google Scholar] [PubMed]
- Crow, M.K. Interferon-alpha: A therapeutic target in systemic lupus erythematosus. Rheum. Dis. Clin. N. Am. 2010, 36, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Harre, U.; Lang, S.C.; Pfeifle, R.; Rombouts, Y.; Fruhbeisser, S.; Amara, K.; Bang, H.; Lux, A.; Koeleman, C.A.; Baum, W.; et al. Glycosylation of immunoglobulin G determines osteoclast differentiation and bone loss. Nat. Commun. 2015, 6, 6651. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.M.; Itoh, K.; Udagawa, N.; Hausler, K.; Yasuda, H.; Shima, N.; Mizuno, A.; Higashio, K.; Takahashi, N.; Suda, T.; et al. Transforming growth factor beta affects osteoclast differentiation via direct and indirect actions. J. Bone Miner. Res.: J. Am. Soc. Bone Miner. Res. 2001, 16, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Sells Galvin, R.J.; Gatlin, C.L.; Horn, J.W.; Fuson, T.R. TGF-β enhances osteoclast differentiation in hematopoietic cell cultures stimulated with RANKL and M-CSF. Biochem. Biophys. Res. Commun. 1999, 265, 233–239. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fürnrohr, B.G.; Rhodes, B.; Munoz, L.E.; Weiß, K.; Vyse, T.J.; Schett, G. Osteoclast Differentiation Is Impaired in a Subgroup of SLE Patients and Correlates Inversely with Mycophenolate Mofetil Treatment. Int. J. Mol. Sci. 2015, 16, 18825-18835. https://doi.org/10.3390/ijms160818825
Fürnrohr BG, Rhodes B, Munoz LE, Weiß K, Vyse TJ, Schett G. Osteoclast Differentiation Is Impaired in a Subgroup of SLE Patients and Correlates Inversely with Mycophenolate Mofetil Treatment. International Journal of Molecular Sciences. 2015; 16(8):18825-18835. https://doi.org/10.3390/ijms160818825
Chicago/Turabian StyleFürnrohr, Barbara G., Benjamin Rhodes, Luis E. Munoz, Katrin Weiß, Tim J. Vyse, and Georg Schett. 2015. "Osteoclast Differentiation Is Impaired in a Subgroup of SLE Patients and Correlates Inversely with Mycophenolate Mofetil Treatment" International Journal of Molecular Sciences 16, no. 8: 18825-18835. https://doi.org/10.3390/ijms160818825
APA StyleFürnrohr, B. G., Rhodes, B., Munoz, L. E., Weiß, K., Vyse, T. J., & Schett, G. (2015). Osteoclast Differentiation Is Impaired in a Subgroup of SLE Patients and Correlates Inversely with Mycophenolate Mofetil Treatment. International Journal of Molecular Sciences, 16(8), 18825-18835. https://doi.org/10.3390/ijms160818825





