Combined Spectroscopic and Calorimetric Studies to Reveal Absorption Mechanisms and Conformational Changes of Protein on Nanoporous Biomaterials
Abstract
:1. Introduction
2. Results and Discussion
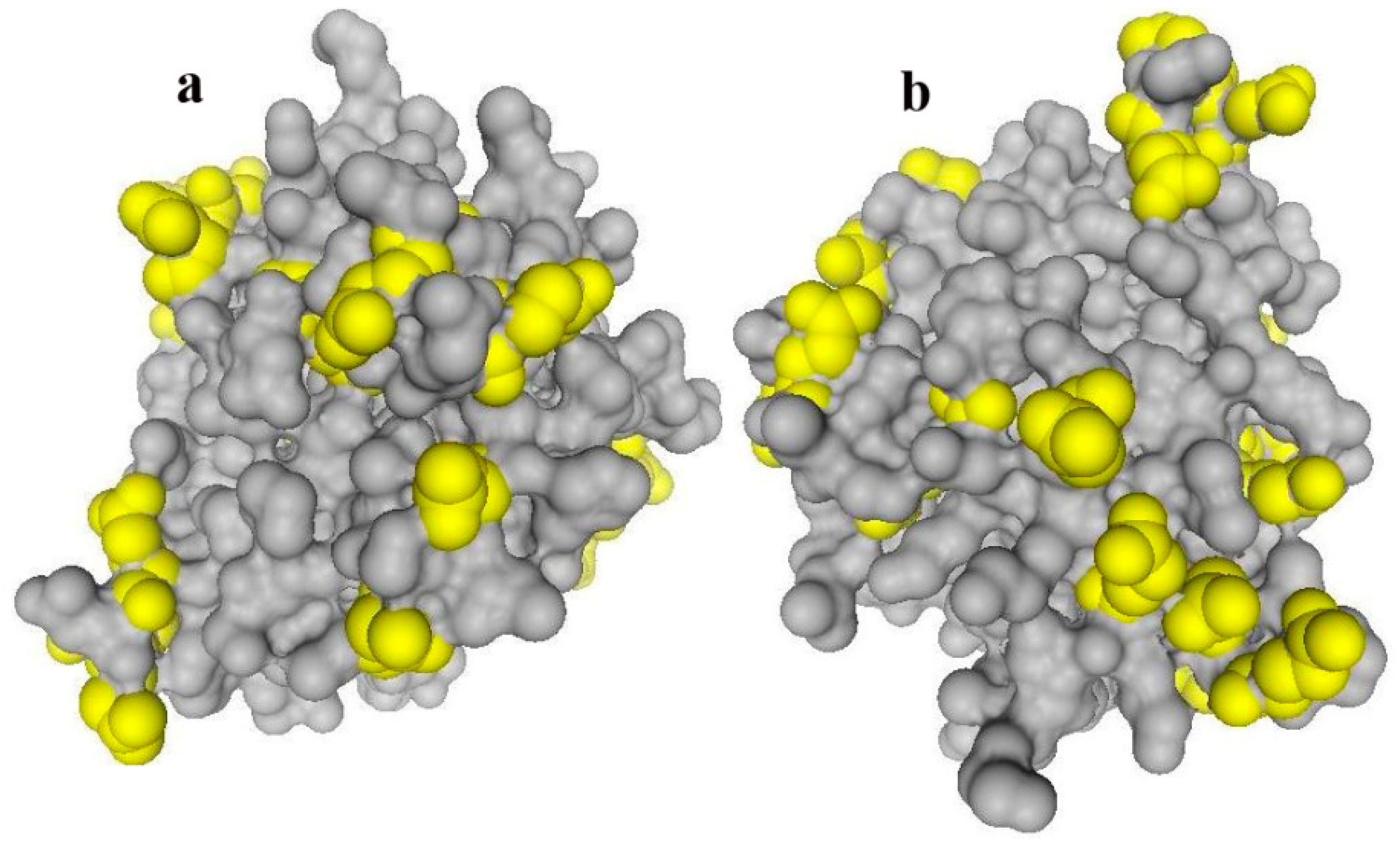
2.1. Effect of Aminopropyl-Functionalization of Mesoporous Nanoparticles on the Amount of Adsorbed BLG
| Supports | Pore Size (nm) | BLG Adsorbed (%) | BLG Leached (%) |
|---|---|---|---|
| KIT-6 | 7.2 | 28.1 | 21.5 |
| [n-PrNH2-KIT-6] [46] | 6.5 | 63.8 | 0 |
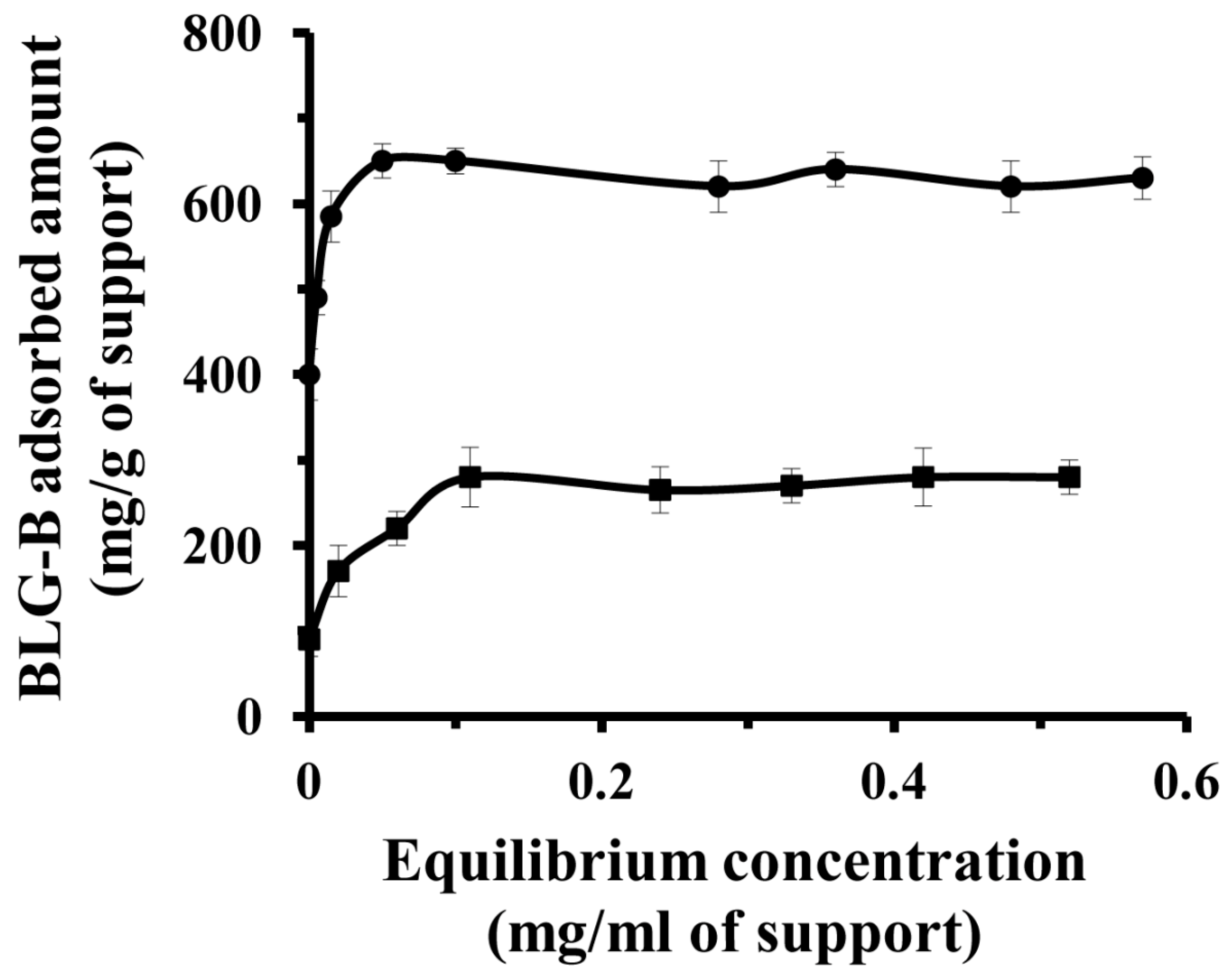
2.2. Effect of Initial Concentration of BLG Solution on the Amount of Protein Immobilization on the KIT-6 and [n-PrNH2-KIT-6]
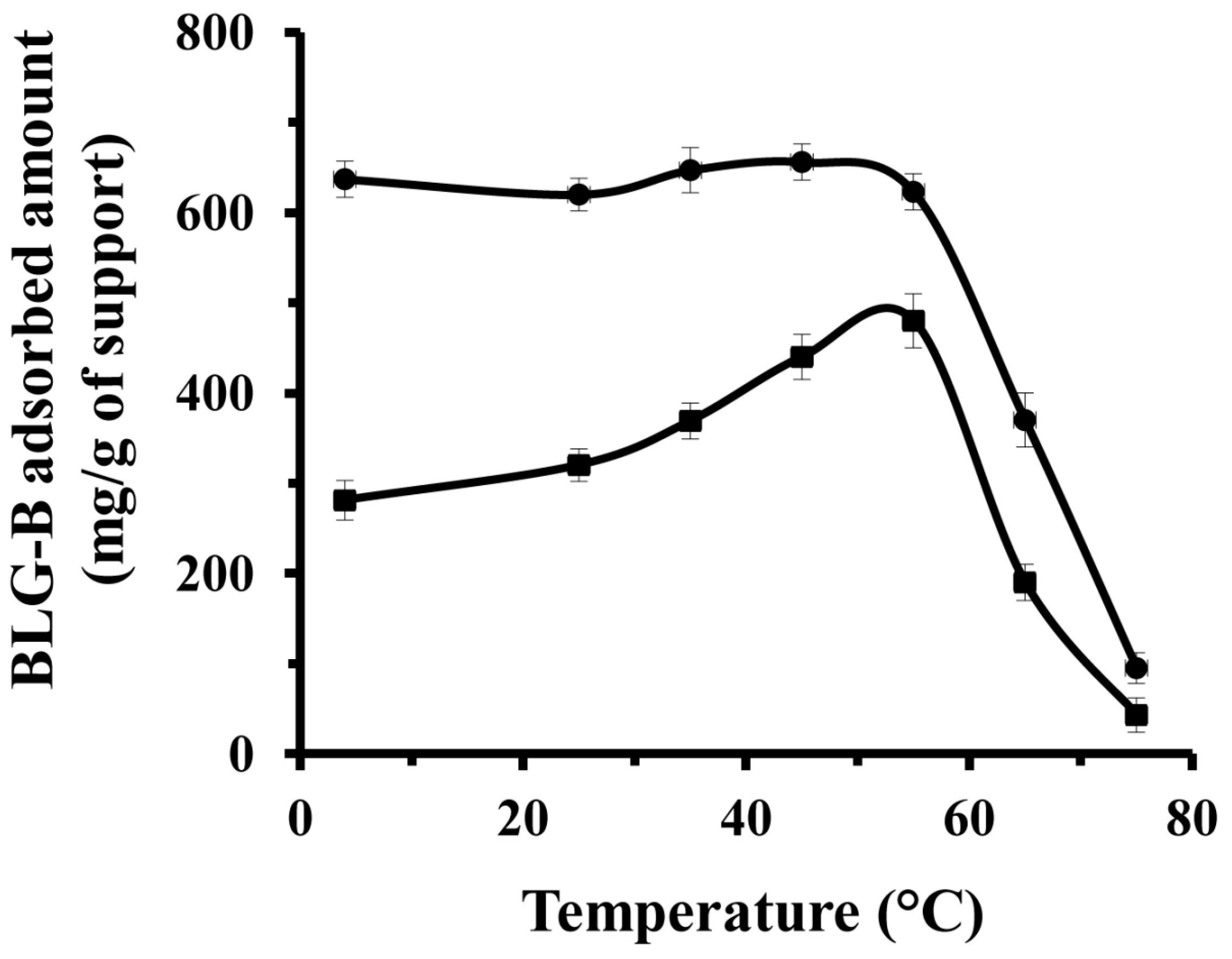
2.3. Effect of Temperature on the Amount of BLG-B Adsorption on the KIT-6 and [n-PrNH2-KIT-6]
2.4. DSC Measurements of Free and Immobilized BLG-B
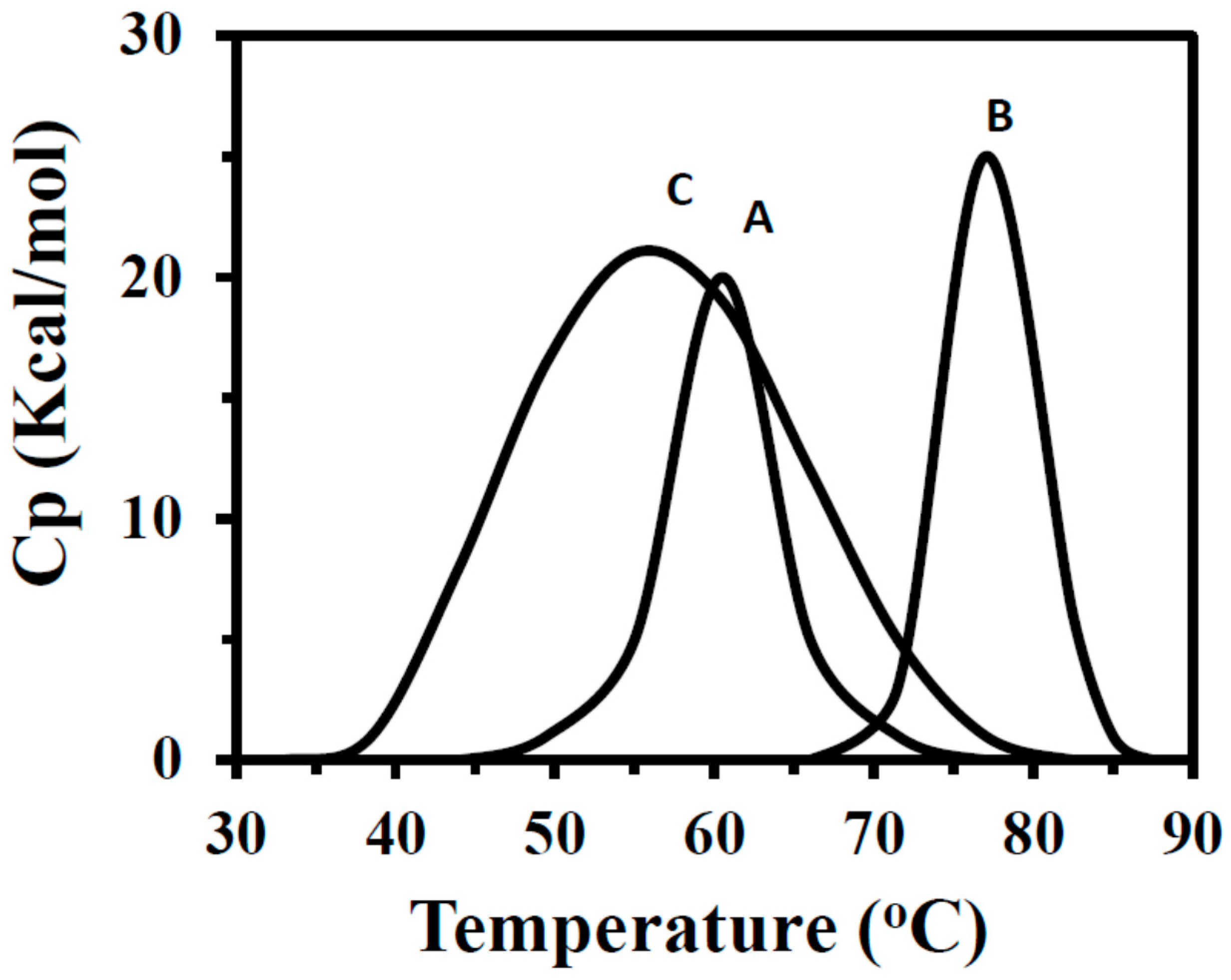
| Supports | Tm (°C) | ∆H° (Kcal/mol) |
|---|---|---|
| Free BLG | 60.5 | 37.5 |
| BLG-[n-PrNH2-KIT-6] | 77 | 54.7 |
| BLG-KIT-6 | 55 | 173.5 |
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Synthesis and Characterization of Non-Functionalized (KIT-6) and Aminopropyl-Functionalized Nanoparticles ([n-PrNH2-KIT-6])
3.2.2. Immobilization of BLG-B on Non-Functionalized (KIT-6) and Aminopropyl-Functionalized Mesoporous Silica Nanoparticles ([n-PrNH2-KIT-6])
3.2.3. Assessment of Leaching of BLG-B from Non-Functionalized (KIT-6) and Aminopropyl-Functionalized Nanoparticles ([n-PrNH2-KIT-6])
3.2.4. Effect of Initial BLG Concentration on the Amount of Protein Adsorption on Non-Functionalized (KIT-6) and Aminopropyl-Functionalized Nanoparticles ([n-PrNH2-KIT-6])
3.2.5. Effect of Temperature on the Amount of BLG-B Adsorption on Non-Functionalized (KIT-6) and Aminopropyl-Functionalized Nanoparticles ([n-PrNH2-KIT-6])
3.2.6. Calorimetric Study of Free and Immobilized BLG-B onto Mesoporous Silica Supports
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hartmann, M. Ordered mesoporous materials for bioadsorption and biocatalysis. Chem. Mater. 2005, 17, 4577–4593. [Google Scholar] [CrossRef]
- Lee, C.H.; Lang, J.; Yen, C.W.; Shih, P.C.; Lin, T.S.; Mou, C.Y. Enhancing stability and oxidation activity of cytochrome c by immobilization in the nanochannels of mesoporous alumino silicates. J. Phys. Chem. B 2005, 109, 12277–12286. [Google Scholar] [CrossRef] [PubMed]
- Kresge, C.T.; Leonowicz, M.; Roth, W.J.; Vartuli, J.C.; Beck, J.C. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Beck, J.C.; Vartuli, J.S.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Diaz, J.F.; Balkus, K.J. Enzyme immobilization in MCM-4 1 molecular sieve. J. Mol. Catal. B 1996, 2, 115–126. [Google Scholar] [CrossRef]
- Serra, E.; Mayoral, A.; Sakamoto, Y.; Blanco, R.M.; Diaz, I. Immobilization of lipase in ordered mesoporous materials, Effect of textural and structural parameters. Microporous Mesoporous Mater. 2008, 114, 201–213. [Google Scholar] [CrossRef]
- Khatibi, A.; Ma’mani, L.; Khodarahmi, R.; Shafiee, A.; Maghami, P.; Ahmad, F.; Sheibani, N.; Moosavi-Movahedi, A.A. Enhancement of thermal reversibility and stability of human carbonic anhydrase II by mesoporous nanoparticles. Int. J. Biol. Macromol. 2015, 75, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Dong, S.; Mei, F.; Ping, S.; Chun, H. Immobilization of Candida rugosa lipase on MSU-H type mesoporous silica for selective esterification of conjugated linoleic acid isomers with ethanol. J. Mol. Catal. B Enzym. 2015, 111, 43–50. [Google Scholar]
- Zhou, Z.; Piepenbreier, F.; Reddy Marthala, V.R.; Karbacher, K.; Hartmann, M. Immobilizationof lipase in cage-type mesoporous organosilicas via covalent bonding and crosslinking. Catal. Today 2015, 243, 173–183. [Google Scholar] [CrossRef]
- Muñoz-Guerrero, F.A.; Águila, S.; Vazquez-Duhalt, R.; Alderete, J.B. Enhancement of operational stability of chloroperoxidase from Caldariomycesfumago by immobilization onto mesoporous supports and the use of co-solvents. J. Mol. Catal. B Enzym. 2015, 116, 1–8. [Google Scholar] [CrossRef]
- NabaviZadeh, P.S.; Abdel Mallak, K.; Carlsson, N.; Akerman, B. A fluorescence spectroscopy assay for real-time monitoring of enzyme immobilization into mesoporous silica particles. Anal. Biochem. 2015, 476, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Radu, D.R.; Lai, C.Y.; Jeftinija, K.; Rowe, E.W.; Jeftinija, S.; Lin, V.S.Y. A poly amido aminopropyl dendrimer-capped mesoporous silica nanosphere-based gene transfection reagent. J. Am. Chem. Soc. 2004, 126, 13216–13217. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.M.; Hung, Y.; Ko, B.S.; Hsu, S.C.; Chen, W.H.; Chien, C.L.; Tsai, C.P.; Chen, Y.C. Highly efficient cellular labeling of mesoporous nanoparticles in human mesenchymal stem cells, Implication for stem cell tracking. FASEB J. 2005, 19, 2014–2016. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Tsai, C.P.; Huang, H.Y.; Kuo, C.T.; Hung, Y.; Huang, D.M.; Chen, Y.C.; Mou, C.Y. Well-ordered mesoporous silica nanoparticles as cell markers. Chem. Mater. 2005, 17, 4570–4573. [Google Scholar] [CrossRef]
- Slowing, I.; Trewyn, B.G.; Lin, V.S.Y. Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef] [PubMed]
- Radin, S.; El-Bassyouni, G.; Vresilovic, E.J.; Schepers, E.; Ducheyne, P. In vivo tissue response to resorbable silica xerogels as controlled-release material. Biomaterial 2005, 26, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.H.; Wu, S.H.; Yao, M.; Lu, C.W.; Lin, Y.S.; Hung, Y. The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles 3T3-L1 cells and human mesenchymal stem cells. Biomaterial 2007, 28, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Yiu, H.H.P.; Wright, P.A. Enzymes supported on ordered mesoporous solids: A special case of an inorganic-organic hybrid. J. Mater. Chem. 2005, 15, 3690–3700. [Google Scholar] [CrossRef]
- Washmon-Kriel, L.; Jimenez, V.L.; Balkus, K.J., Jr. Cytochrome c immobilization into mesoporous molecular sieves. J. Mol. Catal. B 2000, 10, 453–469. [Google Scholar] [CrossRef]
- Gascón, V.; Márquez-Álvarez, C.; Blanco, R.M. Efficient retention of laccase by non-covalent immobilization on amino-functionalized ordered mesoporous silica. Appl. Catal. A Gen. 2014, 482, 116–126. [Google Scholar] [CrossRef]
- Zhan, W.; Lü, Y.; Yang, L.; Guo, Y.; Wang, Y.; Guo, Y.; Lu, G. Epoxidation of vinyl functionalized cubic Ia3d mesoporous silica for immobilization of penicillin G acylase. Chin. J. Catal. 2014, 35, 1709–1715. [Google Scholar] [CrossRef]
- Yoshiyuki, T.; Ryota, Y.; Atsushi, I.; Michael, L.; Markus, B.L.; Tetsuya, H. Solid-support immobilization of a “swing” fusion protein for enhanced glucose oxidase catalytic activity. Colloids Surf. B Biointerfaces 2013, 112, 186–191. [Google Scholar]
- Mannar, R.; Amit, K.; Costa Pessoa, J. Vanadium complexes immobilized on solid supports and their use as catalysts for oxidation and functionalization of alkanes and alkenes. Coord. Chem. Rev. 2011, 255, 2315–2344. [Google Scholar]
- Talha Gokmen, M.; Filip, E.; Du, P. Porous polymer particles—A comprehensive guide to synthesis, characterization, functionalization and applications. Prog. Polym. Sci. 2012, 37, 365–405. [Google Scholar] [CrossRef]
- Thomas, D.; Lazzara, D.N.; Kliesch, T.T.; Janshoff, A.; Steinem, C. Phospholipids as an alternative to direct covalent coupling: Surface functionalization of nanoporous alumina for protein recognition and purification. J. Colloid Interface Sci. 2012, 366, 57–63. [Google Scholar]
- Wahab, M.A.; Imae, I.; Kawakami, Y.; Ha, C.H. Periodic mesoporous organosilica materials incorporating various organic functional groups: Synthesis, structural characterization, and morphology. Chem. Mater. 2005, 17, 2165–2174. [Google Scholar] [CrossRef]
- Hudson, S.; Cooney, J.; Hodnett, B.K.; Magner, E. Chloroperoxidase on periodic mesoporous organosilanes: Immobilization and reuse. Chem. Mater. 2007, 19, 2049–2055. [Google Scholar] [CrossRef]
- Lei, C.; Shin, Y.; Liu, S.; Ackerman, E.J. Entrapping enzyme in a functionalized nanoporous support. J. Am. Chem. Soc. 2002, 124, 11242–11243. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.; Fernandez, A. Engineering productive enzyme confinement. Trends Biotechnol. 2007, 25, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Blanco, R.M.; Terreros, P.; Fernandez-Perez, M.; Otero, C.; Diaz- Gonzalez, G. Functionalization of mesoporous silica for lipase immobilization: Characterization of the support and the catalysts. J. Mol. Catal. B 2004, 30, 83–93. [Google Scholar] [CrossRef]
- Vinu, A.; Murugesan, V.; Tangermann, O.; Hartmann, M. Adsorption of cytochrome c on mesoporous molecular sieves: Influence of pH, pore diameter, and aluminum incorporation. Chem. Mater. 2004, 16, 3056–3065. [Google Scholar] [CrossRef]
- Wang, Y.; Caruso, F. Enzyme encapsulation in nanoporous silica spheres. Chem. Commun. 2004, 13, 1528–1529. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Caruso, F. Mesoporous silica spheres as supports for enzyme immobilization and encapsulation. Chem. Mater. 2005, 17, 953–961. [Google Scholar] [CrossRef]
- Johansson, E.; Zink, J.I. Nanostructured silica thin films self-assembled with electron donors and acceptors to measure electron tunneling. J. Am. Chem. Soc. 2007, 129, 14437–14443. [Google Scholar] [CrossRef] [PubMed]
- Coombs, N.; Khushalani, D.; Ozin, G.A.; Oliver, S.; Shen, G.C.; Sokolov, I.; Yang, H. Blueprints for inorganic materials with natural form: Inorganic liquid crystals. J. Chem. Soc. Dalton Trans. 1997, 3941–3952. [Google Scholar] [CrossRef]
- Yang, M.; Sokolov, I.Y.U.; Coombs, N.; Kresge, C.T.; Ozin, G.A. Formation of hollow helicoids in mesoporous silica. Adv. Mater. 1999, 11, 1427–1431. [Google Scholar] [CrossRef]
- Sokolov, I.Y.U.; Ozin, G.A.; Henderson, G.S.; Yang, H.; Coombs, N. Beyond the hemicylindrical micellar monolayer on graphite AFM evidence for a lyotropic liquid crystal film. Adv. Mater 1997, 9, 917–921. [Google Scholar] [CrossRef]
- Lei, J.; Fan, J.; Yu, C.; Zhang, L.; Jiang, S.; Tu, B.; Zhao, D. Immobilization of enzymes in mesoporous materials: Controlling the entrance to nanospace. Microporous Mesoporous Mater. 2004, 73, 121–128. [Google Scholar] [CrossRef]
- Kleitz, F.; Choi, S.H.; Ryoo, R. Cubic Ia3d large mesoporous silica, synthesis, replication to platinum nanowires, carbon nanorods, carbon nanotubes. Chem. Commun. 2003, 17, 2136–2137. [Google Scholar] [CrossRef]
- Konpidis, G.; Holt, C.; Sawyer, L. Beta-Lactoglobulin: Binding properties, structure, and function. J. Dairy Sci. 2004, 87, 785–796. [Google Scholar] [CrossRef]
- Creamer, L.K.; Parry, D.A.D.; Malcom, G.N. Secondary structure of bovine b-lactoglobulin B. Arch. Biochem. Biophys. 1983, 227, 98–105. [Google Scholar] [CrossRef]
- Hoffman, M.; Sala, G.; Olieman, C.; de Kruif, G.C. Molecular mass distributions of heat induced beta-lactoglobulin. J. Agric. Food Chem. 1997, 45, 2949–2957. [Google Scholar] [CrossRef]
- Cairoli, S.; Iametti, S.; Bonomi, F. Reversible and irreversible modifications of beta lactoglobulin upon exposure to heat. J. Protein Chem. 1994, 13, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Moitzi, C.; Donato, L.; Schmitt Bovetto, L.; Gillies, G.; Stradner, A. Structure of β-lactoglobulinmicrogels formed during heating as revealed by small-angle X-ray scattering and light scattering. Food Hydrocoll. 2011, 25, 1766–1774. [Google Scholar] [CrossRef]
- Falahati, M.; Ma’mani, L.; Saboury, A.A.; Shafiee, A.; Foroumadi, A.R.; Badiei, A.R. Aminopropyl-functionalized cubic Ia3d mesoporous silica nanoparticle as an efficient support for immobilization of superoxide dismutase. Biochim. Biophys. Acta 2011, 1814, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Falahati, M.; Saboury, A.A.; Shafiee, A.; Rezayat Sorkhabadi, S.M.; Kachooei, E.; Ma’mani, L.; Haertlé, T. Highly efficient immobilization of beta-lactoglobulin in functionalized mesoporous nanoparticles: A simple and useful approach for enhancement of protein stability. Biophys. Chem. 2012, 165, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Kao, K.C.; Lin, T.S.; Mou, C.Y. Enhanced activity and stability of lysozyme by immobilization in the matching nanochannels of mesoporous silica. Nanoparticles 2014, 118, 6734–6743. [Google Scholar] [CrossRef]
- Dufour, E.; Haertle, T. Temperature-induced folding changes of beta-lactoglobulin in hydro-methanolic solutions. Int. J. Biol. Macromol. 1993, 15, 293–297. [Google Scholar]
- Relkin, P. Reversibility of heat-induced conformational changes, surface exposed hydrophobic clusters of beta-lactoglobulin, their role in heat-induced sol-gel state transition. Int. J. Biol. Macromol. 1998, 22, 59–66. [Google Scholar] [CrossRef]
- Apenten, R.K.O.; Khokhar, S.; Galani, D. Stability parameters for beta-lactoglobulin thermal dissociation, unfolding in phosphate buffer at pH 7.0. Food Hydrocoll. 2002, 16, 95–103. [Google Scholar] [CrossRef]
- Simion, A.N.; Aprodu, I.; Dumitrașcu, L.; Bahrim, G.E.; Alexe, P.; Stănciuc, N. Probing thermal stability of the β-lactoglobulin–oleic acid complex by fluorescence spectroscopy and molecular modeling. J. Mol. Struct. 2015, 1095, 26–33. [Google Scholar] [CrossRef]
- Leeb, E.; Götz, A.; Letzel, T.; Chelulei Cheison, C.; Kulozik, U. Influence of denaturation and aggregation of β-lactoglobulin on its tryptic hydrolysis and the release of functional peptides. Food Chem. 2015, 187, 545–554. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadi, S.; Farokhi, M.; Padidar, P.; Falahati, M. Combined Spectroscopic and Calorimetric Studies to Reveal Absorption Mechanisms and Conformational Changes of Protein on Nanoporous Biomaterials. Int. J. Mol. Sci. 2015, 16, 17289-17302. https://doi.org/10.3390/ijms160817289
Ahmadi S, Farokhi M, Padidar P, Falahati M. Combined Spectroscopic and Calorimetric Studies to Reveal Absorption Mechanisms and Conformational Changes of Protein on Nanoporous Biomaterials. International Journal of Molecular Sciences. 2015; 16(8):17289-17302. https://doi.org/10.3390/ijms160817289
Chicago/Turabian StyleAhmadi, Saharnaz, Maryam Farokhi, Parisa Padidar, and Mojtaba Falahati. 2015. "Combined Spectroscopic and Calorimetric Studies to Reveal Absorption Mechanisms and Conformational Changes of Protein on Nanoporous Biomaterials" International Journal of Molecular Sciences 16, no. 8: 17289-17302. https://doi.org/10.3390/ijms160817289
APA StyleAhmadi, S., Farokhi, M., Padidar, P., & Falahati, M. (2015). Combined Spectroscopic and Calorimetric Studies to Reveal Absorption Mechanisms and Conformational Changes of Protein on Nanoporous Biomaterials. International Journal of Molecular Sciences, 16(8), 17289-17302. https://doi.org/10.3390/ijms160817289





