Effects of a Short Physical Exercise Intervention on Patients with Multiple Sclerosis (MS)
Abstract
:1. Introduction
2. Results
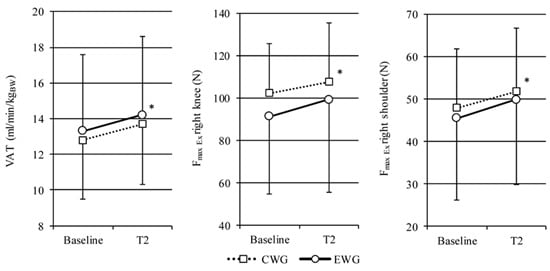
2.1. Aerobic Capacity
| Parameter | CWG (n = 30) | EWG (n = 30) | p-Value |
|---|---|---|---|
| Age (years) | 42.3 ± 9.0 | 45.6 ± 11.4 | 0.21 |
| Height (cm) | 170 ± 5 | 169 ± 4 | 0.66 |
| Body weight (kg) | 71.4 ± 12.1 | 70.8 ± 11.9 | 0.84 |
| BMI (kg/m2) | 24.5 ± 3.6 | 24.7 ± 4.0 | 0.86 |
| EDSS | 2.6 ± 1.1 | 3.1 ± 1.3 | 0.09 |
| MS specific medication | 20/30 | 21/30 | |
| Female/male | 24/6 | 20/10 |
| Parameter | CWG | EWG | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | T2 | Baseline | T2 | Time | η2 | Time × Group | |
| Load (W) | 124 ± 48 | 131 ± 53 | 119 ± 44 | 124 ± 41 | <0.01 | 0.17 | 0.78 |
| Load (W/kg) | 1.75 ± 0.61 | 1.84 ± 0.68 | 1.71 ± 0.66 | 1.78 ± 0.60 | <0.01 | 0.17 | 0.78 |
| Lactate50W (mmol/min) | 1.45 ± 0.51 | 1.31 ± 0.51 | 1.57 ± 0.79 | 1.35 ± 0.52 | <0.01 | 0.14 | 0.54 |
| Lactatemax (mmol/min) | 5.43 ± 2.03 | 5.90 ± 1.97 | 4.80 ± 2.89 | 5.14 ± 2.50 | <0.01 | 0.12 | 0.66 |
| Heart raterest (bpm) | 92 ± 12 | 90 ± 11 | 88 ± 12 | 85 ± 13 | 0.02 | 0.09 | 0.63 |
| Heart rate50W (bpm) | 120 ± 15 | 115 ± 15 | 116 ± 15 | 110 ± 14 | <0.01 | 0.31 | 0.74 |
| Heart ratemax (bpm) | 161 ± 17 | 162 ± 18 | 152 ± 24 | 152 ± 24 | 0.53 | – | 0.85 |
| VO2peak (mL/min) | 1684 ± 601 | 1756 ± 599 | 1632 ± 539 | 1676 ± 494 | 0.12 | – | 0.71 |
| VO2peak (mL/min/kgBW) | 23.8 ± 7.8 | 24.6 ± 7.4 | 23.5 ± 8.2 | 23.7 ± 7.1 | 0.24 | – | 0.72 |
| Borg scale | 16.5 ± 1.4 | 16.0 ± 1.9 | 16.3 ± 1.4 | 15.7 ± 1,70 | 0.02 | 0.09 | 0.68 |
| VAT (W) | 51.0 ± 23.4 | 55.8 ± 24.8 | 50.5 ± 23.1 | 57.6 ± 25.5 | <0.01 | 0.26 | 0.39 |
| VAT (mL/min/kgBW) | 12.8 ± 3.3 | 13.7 ± 3.4 | 13.3 ± 4.3 | 14.2 ± 4.4 | <0.01 | 0.35 | 0.79 |
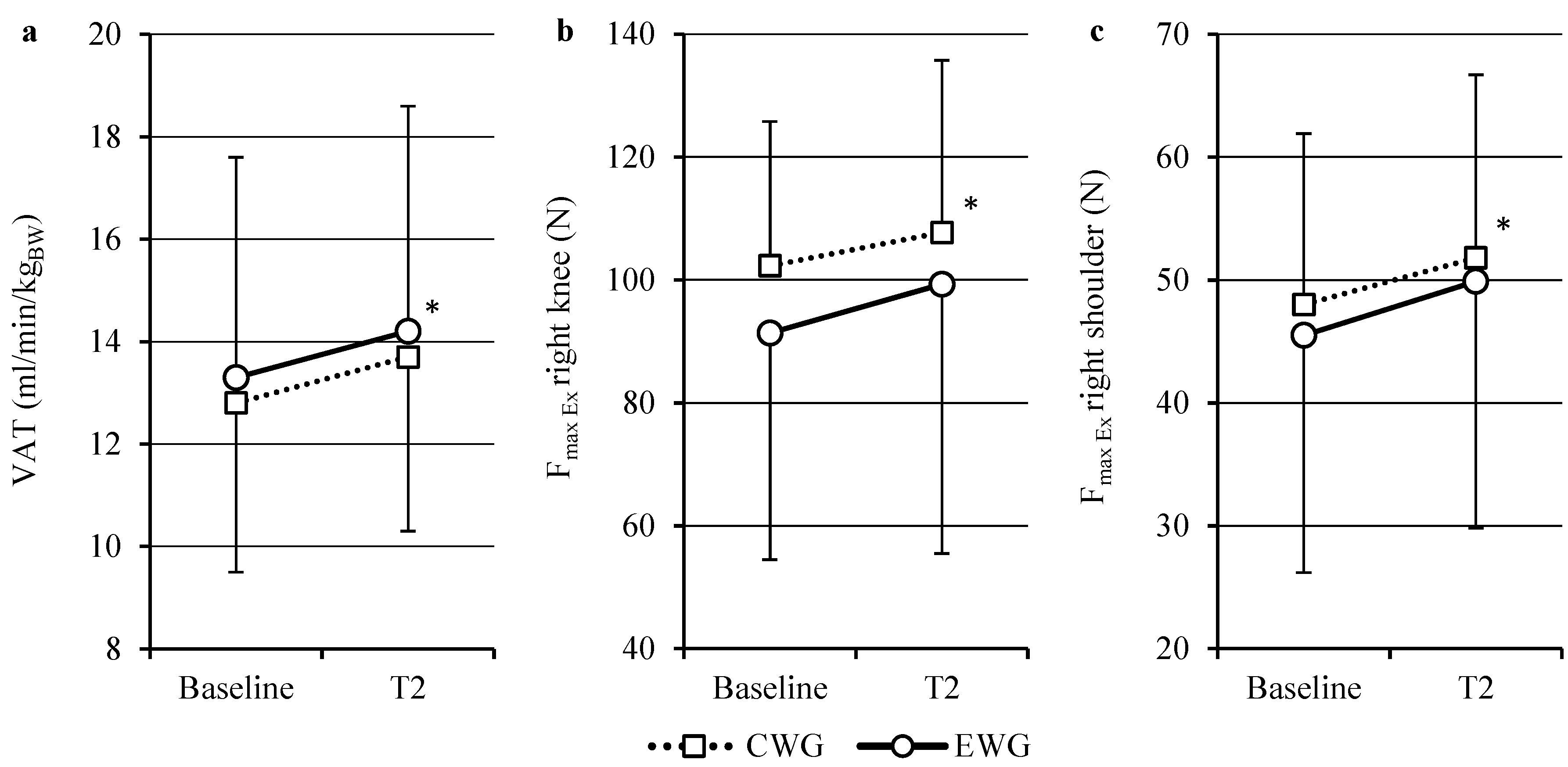
2.2. Isokinetics
| Parameter | CWG | EWG | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | T2 | Baseline | T2 | Time | η2 | Time × Group | |
| Knee | |||||||
| Fmax Ex right (N) | 102.3 ± 23.5 | 107.7 ± 28.0 | 91.4 ± 36.9 | 99.3 ± 42.3 | <0.01 | 0.15 | 0.50 |
| Fmax Ex left (N) | 105.5 ± 28.1 | 108.2 ± 33.1 | 92.7 ± 39.3 | 95.6 ± 43.8 | 0.23 | - | 0.95 |
| Fmax Fl right (N) | 55.3 ± 16.0 | 61.3 ± 18.7 | 51.0 ± 21.0 | 55.9 ± 24.6 | 0.01 | 0.18 | 0.72 |
| Fmax Fl left (N) | 58.2 ± 20.2 | 64.0 ± 23.7 | 48.7 ± 23.5 | 51.7 ± 24.85 | <0.01 | 0.16 | 0.31 |
| Shoulder | |||||||
| Fmax Ex right (N) | 48.0 ± 13.9 | 51.8 ± 14.9 | 45.5 ± 19.3 | 49.9 ± 20.1 | <0.01 | 0.14 | 0.85 |
| Fmax Ex left (N) | 46.3 ± 17.5 | 50.0 ± 18.9 | 43.3 ± 17.3 | 46.9 ± 18.6 | <0.01 | 0.19 | 0.98 |
| Fmax Fl right (N) | 34.2 ± 9.6 | 36.5 ± 10.0 | 35.3 ± 12.6 | 36.9 ± 14.1 | 0.02 | 0.10 | 0.67 |
| Fmax Fl left (N) | 35.8 ± 13.9 | 36.9 ± 12.4 | 34.0 ± 12.1 | 35.9 ± 12.5 | 0.04 | 0.07 | 0.60 |
2.3. Questionnaires
2.3.1. SF-36
2.3.2. MFIS
2.4. Training Program
| Parameter | CWG | EWG | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | T2 | Baseline | T2 | Time | η2 | Time × Group | |
| MFIS score | 35.5 ± 17.0 | 30.6 ± 16.7 | 35.1 ± 17.4 | 30.3 ± 18.1 | <0.01 | 0.24 | 0.97 |
| SF 36 | |||||||
| Scale 1 (physical functioning) | 71.7 ± 21.3 | 71.5 ± 22.9 | 60.7 ± 27.1 | 62.7 ± 26.6 | 0.43 | – | 0.33 |
| Scale 2 (role limitations due to physical limitations) | 50.0 ± 44.9 | 62.5 ± 42.3 | 42.1 ± 47.2 | 50.0 ± 42.0 | 0.03 | 0.10 | 0.63 |
| Scale 3 (bodily pain) | 87.9 ± 19.6 | 87.4 ± 17.4 | 71.3 ± 24.4 | 72.4 ± 23.7 | 0.90 | – | 0.67 |
| Scale 4 (general health perceptions) | 46.9 ± 19.3 | 49.6 ± 22.4 | 43.6 ± 24.0 | 48.8 ± 25.6 | 0.03 | 0.10 | 0.48 |
| Scale 5 (vitality) | 47.5 ± 18.7 | 49.0 ± 20.4 | 44.8 ± 23.8 | 50.7 ± 22.3 | <0.01 | 0.18 | 0.07 |
| Scale 6 (social functioning) | 72.9 ± 28.9 | 76.6 ± 24.3 | 68.2 ± 30.6 | 80.1 ± 24.3 | <0.01 | 0.21 | 0.07 |
| Scale 7 (role limitations caused by emotional problems) | 66.7 ± 48.2 | 65.3 ± 52.5 | 78.8 ± 40.6 | 90.9 ± 25.6 | 0.36 | – | 0.25 |
| Scale 8 (mental health) | 65.8 ± 20.7 | 67.2 ± 19.1 | 62.0 ± 20.1 | 67.8 ± 18.0 | <0.01 | 0.17 | 0.07 |
| Physical health | 44.7 ± 9.1 | 46.2 ± 9.1 | 39.0 ± 10.8 | 39.6 ± 11.3 | 0.16 | – | 0.56 |
| Mental health | 44.9 ± 13.6 | 45.4 ± 13.4 | 46.7 ± 11.7 | 51.4 ± 8.6 | 0.04 | 0.09 | 0.01 (η2 = 0.13) |
3. Discussion
3.1. Aerobic Capacity
3.2. Muscle Strength
3.3. Quality of Life
3.4. Fatigue
3.5. EDSS
3.6. Dropout
4. Patients and Methods
4.1. Patients and Study Design
4.2. Neurological Examination
4.2.1. EDSS
4.2.2. Aerobic Capacity/Spiroergometry
4.3. Isokinetics
4.4. Questionnaires
4.4.1. Short Form-36 Health Survey (SF-36)
4.4.2. MFIS (Modified Fatigue Impact Scale)
4.5. Training Program
4.6. Statistics
5. Conclusions and Limitations
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mostert, S.; Kesselring, J. Effects of a short-term exercise training program on aerobic fitness, fatigue, health perception and activity level of subjects with multiple sclerosis. Mult. Scler. 2002, 8, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Bjarnadottir, O.H.; Konradsdottir, A.D.; Reynisdottir, K.; Olafsson, E. Multiple sclerosis and brief moderate exercise. A randomised study. Mult. Scler. 2007, 13, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Petajan, J.H.; Gappmaier, E.; White, A.T.; Spencer, M.K.; Mino, L.; Hicks, R.W. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann. Neurol. 1996, 39, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Gallien, P.; Nicolas, B.; Robineau, S.; Petrilli, S.; Houedakor, J.; Durufle, A. Physical training and multiple sclerosis. Ann. Readapt. Med. Phys. 2007, 50, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Dalgas, U.; Stenager, E.; Ingemann-Hansen, T. Multiple sclerosis and physical exercise: Recommendations for the application of resistance-, endurance- and combined training. Mult. Scler. 2008, 14, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, A.K.; Stenager, E.; Dalgas, U. The effect of exercise therapy on fatigue in multiple sclerosis. Mult. Scler. 2011, 17, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Rietberg, M.B.; Brooks, D.; Uitdehaag, B.M.; Kwakkel, G. Exercise therapy for multiple sclerosis. Cochrane Database Syst. Rev. 2005, 3, CD003980. [Google Scholar]
- Latimer-Cheung, A.E.; Martin Ginis, K.A.; Hicks, A.L.; Motl, R.W.; Pilutti, L.A.; Duggan, M.; Wheeler, G.; Persad, R.; Smith, K.M. Development of evidence-informed physical activity guidelines for adults with multiple sclerosis. Arch. Phys. Med. Rehabil. 2013, 94, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.; Dieberg, G.; Smart, N. Exercise as a Therapy for improvement of walking ability in adults with multiple sclerosis: A meta-analysis. Arch. Phys. Med. Rehabil. 2015, 96, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Waschbisch, A.; Tallner, A.; Pfeifer, K.; Maurer, M. Multiple sclerosis and exercise: Effects of physical activity on the immune system. Nervenarzt 2009, 80, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Kerling, A.; Keweloh, K.; Tegtbur, U.; Kuck, M.; Grams, L.; Horstmann, H.; Windhagen, A. Physical capacity and quality of life in patients with multiple sclerosis. NeuroRehabilitation 2014, 35, 97–104. [Google Scholar] [PubMed]
- Tallner, A.; Waschbisch, A.; Wenny, I.; Schwab, S.; Hentschke, C.; Pfeifer, K.; Maurer, M. Multiple sclerosis relapses are not associated with exercise. Mult. Scler. 2012, 18, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Flensner, G.; Ek, A.C.; Soderhamn, O.; Landtblom, A.M. Sensitivity to heat in MS patients: A factor strongly influencing symptomology—An explorative survey. BMC Neurol. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Skjerbaek, A.G.; Moller, A.B.; Jensen, E.; Vissing, K.; Sorensen, H.; Nybo, L.; Stenager, E.; Dalgas, U. Heat sensitive persons with multiple sclerosis are more tolerant to resistance exercise than to endurance exercise. Mult. Scler. 2013, 19, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA 2009, 301, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Blair, S.N. Physical inactivity: The biggest public health problem of the 21st century. Br. J. Sports Med. 2009, 43, 1–2. [Google Scholar] [PubMed]
- Wens, I.; Dalgas, U.; Vandenabeele, F.; Krekels, M.; Grevendonk, L.; Eijnde, B.O. Multiple sclerosis affects skeletal muscle characteristics. PLoS ONE 2014, 9, e108158. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010; p. 58. [Google Scholar]
- Ponichtera, J.A.; Rodgers, M.M.; Glaser, R.M.; Mathews, T.A.; Camaione, D.N. Concentric and eccentric isokinetic lower extremity strength in persons with multiple sclerosis. J. Orthop. Sports Phys. Ther. 1992, 16, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Heine, M.; Hoogervorst, E.L.; Hacking, H.G.; Verschuren, O.; Kwakkel, G. Validity of maximal exercise testing in people with multiple sclerosis and low to moderate levels of disability. Phys. Ther. 2014, 94, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Langeskov-Christensen, M.; Langeskov-Christensen, D.; Overgaard, K.; Moller, A.B.; Dalgas, U. Validity and reliability of VO(2)-max measurements in persons with multiple sclerosis. J. Neurol. Sci. 2014, 342, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Faude, O.; Kindermann, W.; Meyer, T. Lactate threshold concepts: How valid are they? Sports Med. 2009, 39, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; Smith, D.C.; Elliott, J.; Weikert, M.; Dlugonski, D.; Sosnoff, J.J. Combined training improves walking mobility in persons with significant disability from multiple sclerosis: A pilot study. J. Neurol. Phys. Ther. 2012, 36, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Romberg, A.; Virtanen, A.; Ruutiainen, J.; Aunola, S.; Karppi, S.L.; Vaara, M.; Surakka, J.; Pohjolainen, T.; Seppanen, A. Effects of a 6-month exercise program on patients with multiple sclerosis: A randomized study. Neurology 2004, 63, 2034–2038. [Google Scholar] [CrossRef] [PubMed]
- Wens, I.; Hansen, D.; Verboven, K.; Deckx, N.; Kosten, L.; Stevens, A.L.; Cools, N.; Eijnde, B.O. Impact of 24 weeks of resistance and endurance exercise on glucose tolerance in persons with multiple sclerosis. Am. J. Phys. Med. Rehabil. 2015. [Google Scholar] [CrossRef] [PubMed]
- Dalgas, U.; Stenager, E.; Jakobsen, J.; Petersen, T.; Overgaard, K.; Ingemann-Hansen, T. Muscle fiber size increases following resistance training in multiple sclerosis. Mult. Scler. 2010, 16, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Cakt, B.D.; Nacir, B.; Genc, H.; Saracoglu, M.; Karagoz, A.; Erdem, H.R.; Ergun, U. Cycling progressive resistance training for people with multiple sclerosis: A randomized controlled study. Am. J. Phys. Med. Rehabil. 2010, 89, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Oken, B.S.; Kishiyama, S.; Zajdel, D.; Bourdette, D.; Carlsen, J.; Haas, M.; Hugos, C.; Kraemer, D.F.; Lawrence, J.; Mass, M. Randomized controlled trial of yoga and exercise in multiple sclerosis. Neurology 2004, 62, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- McCullagh, R.; Fitzgerald, A.P.; Murphy, R.P.; Cooke, G. Long-term benefits of exercising on quality of life and fatigue in multiple sclerosis patients with mild disability: A pilot study. Clin. Rehabil. 2008, 22, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Rampello, A.; Franceschini, M.; Piepoli, M.; Antenucci, R.; Lenti, G.; Olivieri, D.; Chetta, A. Effect of aerobic training on walking capacity and maximal exercise tolerance in patients with multiple sclerosis: A randomized crossover controlled study. Phys. Ther. 2007, 87, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Golzari, Z.; Shabkhiz, F.; Soudi, S.; Kordi, M.R.; Hashemi, S.M. Combined exercise training reduces IFN-gamma and IL-17 levels in the plasma and the supernatant of peripheral blood mononuclear cells in women with multiple sclerosis. Int. Immunopharmacol. 2010, 10, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Dalgas, U.; Stenager, E. Exercise and disease progression in multiple sclerosis: Can exercise slow down the progression of multiple sclerosis? Ther. Adv. Neurol. Disord. 2012, 5, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Disability rating scales in multiple sclerosis. Ann. N. Y. Acad. Sci. 1984, 436, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998; p. 104. [Google Scholar]
- Scharhag-Rosenberger, F. Spiroergometrie zur Ausdauerleistungsdiagnostik. Dtsch. Z. Sportmed. 2010, 61, 146–147. [Google Scholar]
- Roecker, K.; Niess, A.M.; Horstmann, T.; Striegel, H.; Mayer, F.; Dickhuth, H.H. Heart rate prescriptions from performance and anthropometrical characteristics. Med. Sci. Sports Exerc. 2002, 34, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, K.; Whipp, B.J.; Koyl, S.N.; Beaver, W.L. Anaerobic threshold and respiratory gas exchange during exercise. J. Appl. Physiol. 1973, 35, 236–243. [Google Scholar] [PubMed]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Fisk, J.D.; Ritvo, P.G.; Ross, L.; Haase, D.A.; Marrie, T.J.; Schlech, W.F. Measuring the functional impact of fatigue: Initial validation of the fatigue impact scale. Clin. Infect. Dis. 1994, 18, S79–S83. [Google Scholar] [CrossRef] [PubMed]
- Flachenecker, P.; Kumpfel, T.; Kallmann, B.; Gottschalk, M.; Grauer, O.; Rieckmann, P.; Trenkwalder, C.; Toyka, K.V. Fatigue in multiple sclerosis: A comparison of different rating scales and correlation to clinical parameters. Mult. Scler. 2002, 8, 523–526. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerling, A.; Keweloh, K.; Tegtbur, U.; Kück, M.; Grams, L.; Horstmann, H.; Windhagen, A. Effects of a Short Physical Exercise Intervention on Patients with Multiple Sclerosis (MS). Int. J. Mol. Sci. 2015, 16, 15761-15775. https://doi.org/10.3390/ijms160715761
Kerling A, Keweloh K, Tegtbur U, Kück M, Grams L, Horstmann H, Windhagen A. Effects of a Short Physical Exercise Intervention on Patients with Multiple Sclerosis (MS). International Journal of Molecular Sciences. 2015; 16(7):15761-15775. https://doi.org/10.3390/ijms160715761
Chicago/Turabian StyleKerling, Arno, Karin Keweloh, Uwe Tegtbur, Momme Kück, Lena Grams, Hauke Horstmann, and Anja Windhagen. 2015. "Effects of a Short Physical Exercise Intervention on Patients with Multiple Sclerosis (MS)" International Journal of Molecular Sciences 16, no. 7: 15761-15775. https://doi.org/10.3390/ijms160715761
APA StyleKerling, A., Keweloh, K., Tegtbur, U., Kück, M., Grams, L., Horstmann, H., & Windhagen, A. (2015). Effects of a Short Physical Exercise Intervention on Patients with Multiple Sclerosis (MS). International Journal of Molecular Sciences, 16(7), 15761-15775. https://doi.org/10.3390/ijms160715761