Interferon Beta-1a (AVONEX®) as a Treatment Option for Untreated Patients with Multiple Sclerosis (AXIOM): A Prospective, Observational Study
Abstract
:1. Introduction
2. Results
2.1. Subjects
2.2. Previous MS-Specific Treatment (Retrospective Part)
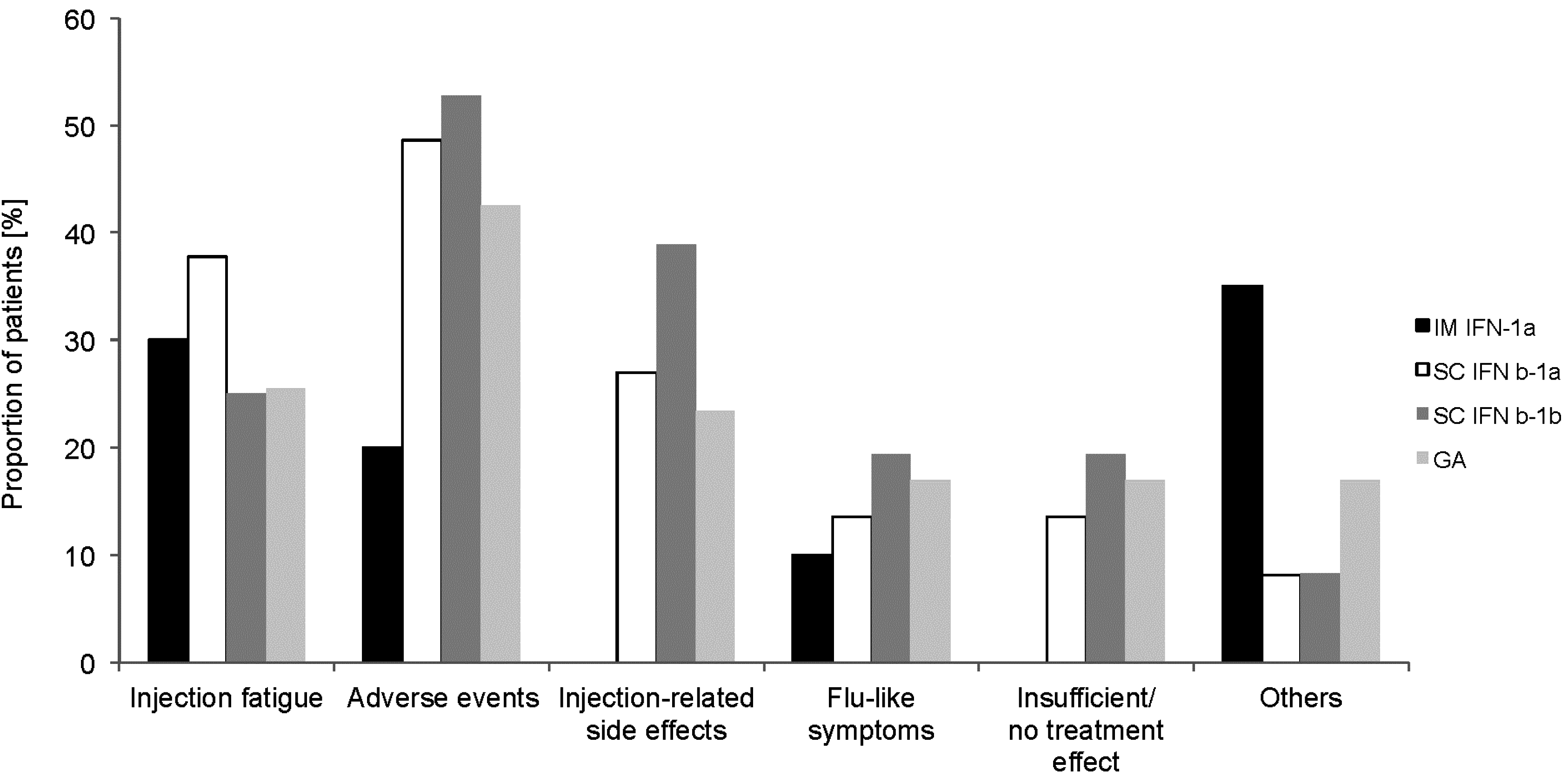
2.3. Treatment with IM IFNb-1a (Prospective Part)

| Value | IM IFNb-1a (n = 15/20) 1 | SC IFNb-1a (n = 33/37) 1 | SC IFNB-1b (n = 32/36) 1 | GA (n = 44/46) 1 | Natalizumab (n = 6/8) 1 |
|---|---|---|---|---|---|
| Mean (SD) | 33.8 (32.2) | 23.0 (31.5) | 26.1 (36.5) | 13.5 (22.3) | 14.8 (12.1) |
| 95% CI | 16.0–51.7 | 11.8–34.1 | 13.0–39.3 | 6.8–20.3 | 2.0–27.5 |
| Range | 2.5–103.2 | 0.0–112.2 | 0.0–171.5 | 0.2–94.6 | 1.5–31.9 |

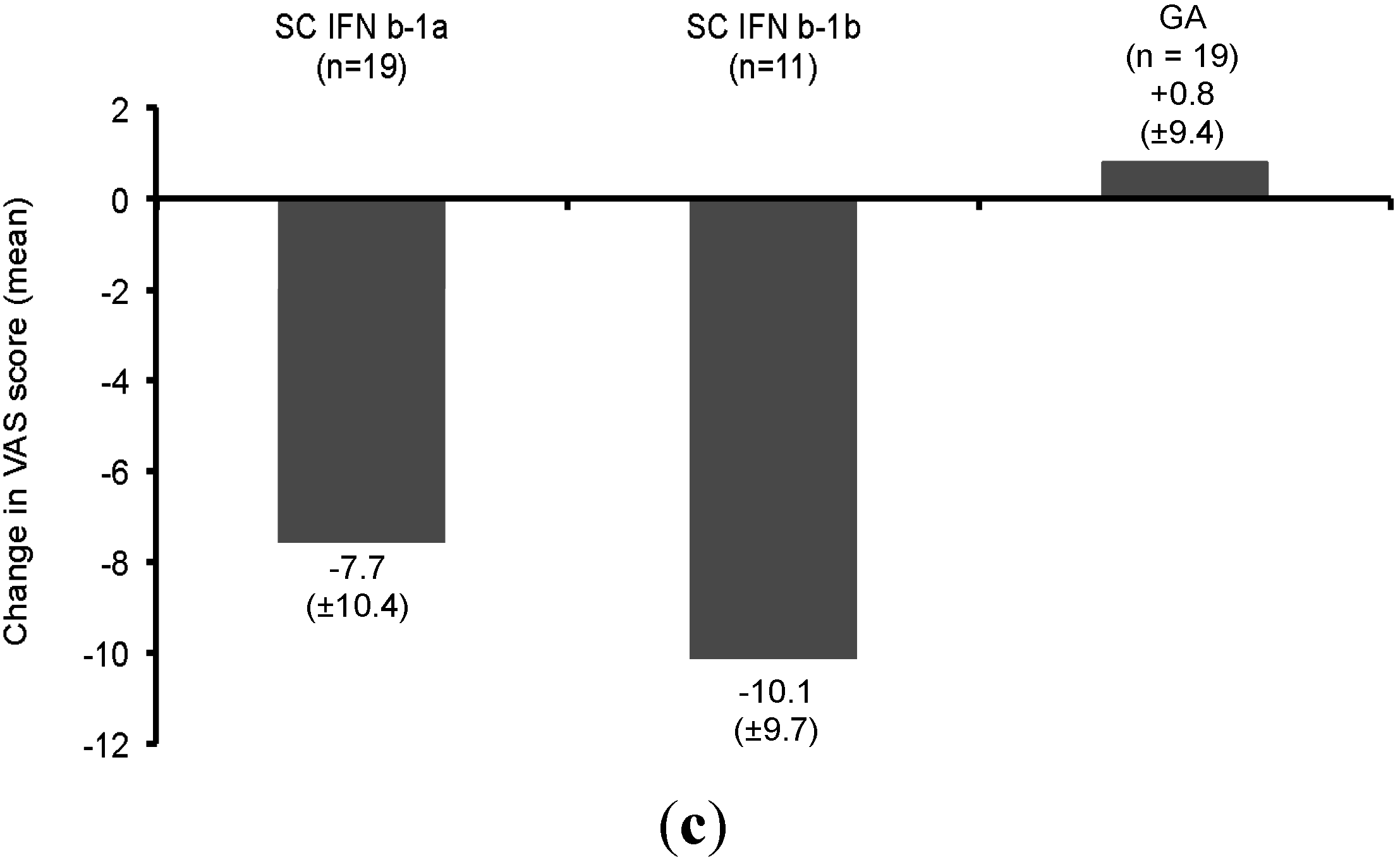
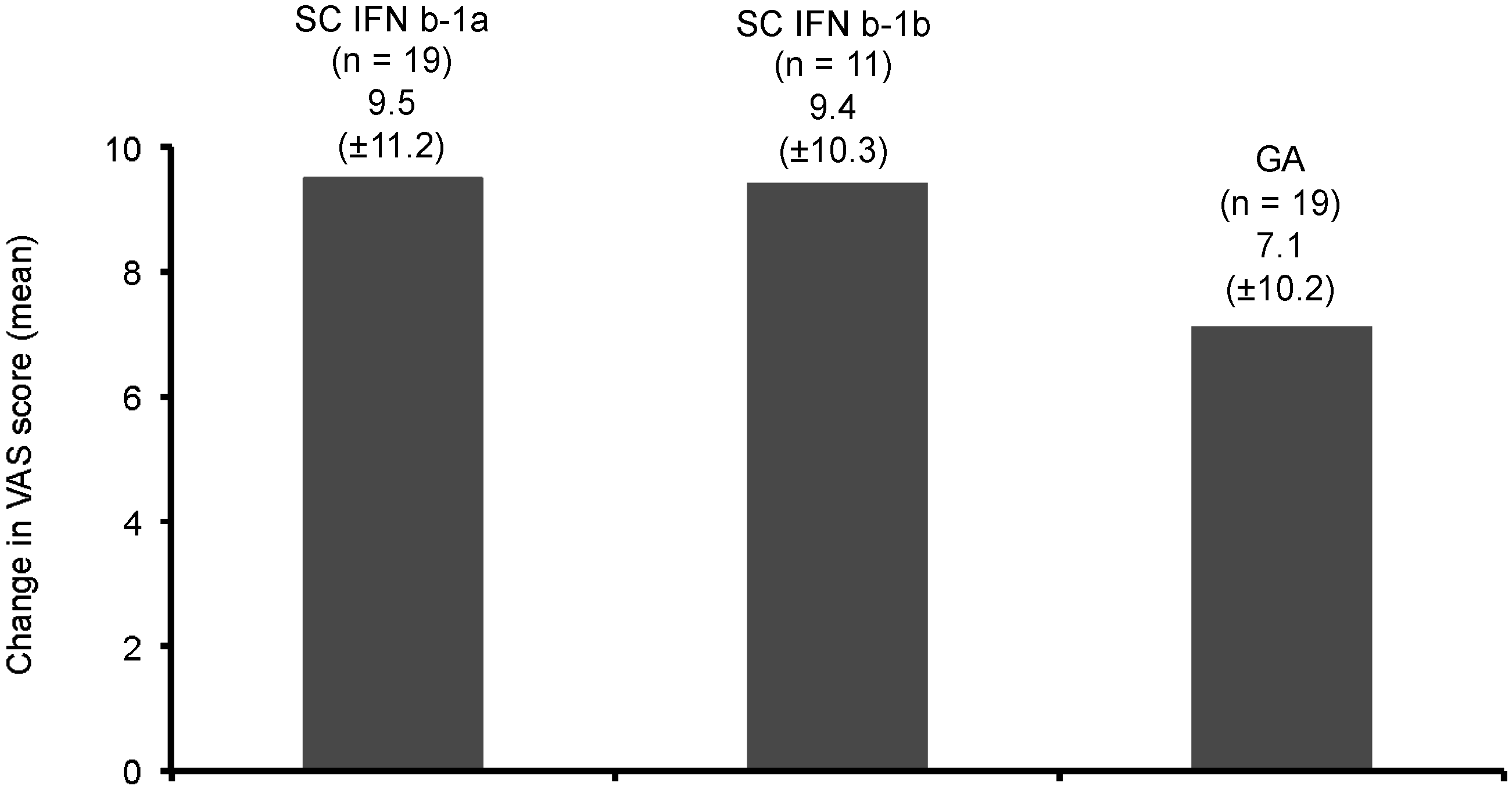
| Value | Number of Relapses per 3 Months of Time | |||||
|---|---|---|---|---|---|---|
| Retrospective Observational Period Prior to Study Entry | Prospective Observational Period after Study Entry | |||||
| 12 Months Preceding Baseline (n = 177) | 3 Months Preceding Baseline (n = 123) | 3 Months Preceding Visit 2 (n = 158/168) 1 | 3 Months Preceding Visit 3 (n = 142/148) 1 | 3 Months Preceding Visit 4 (n = 123/129) 1 | 3 Months Preceding Visit 5 (n = 103/107) 1 | |
| Mean (SD) | 0.45 (0.35) | 1.1 (0.3) | 0.1 (0.4) | 0.2 (0.4) | 0.2 (0.4) | 0.1 (0.4) |
| 95% CI | 0.4–0.5 | 1.0–1.1 | 0.1–0.2 | 0.1–0.2 | 0.1–0.2 | 0.1–0.2 |
| Range | 0.25–3.0 | 1.0–2.0 | 0.0–2.0 | 0.0–2.0 | 0.0–1.0 | 0.0–2.0 |
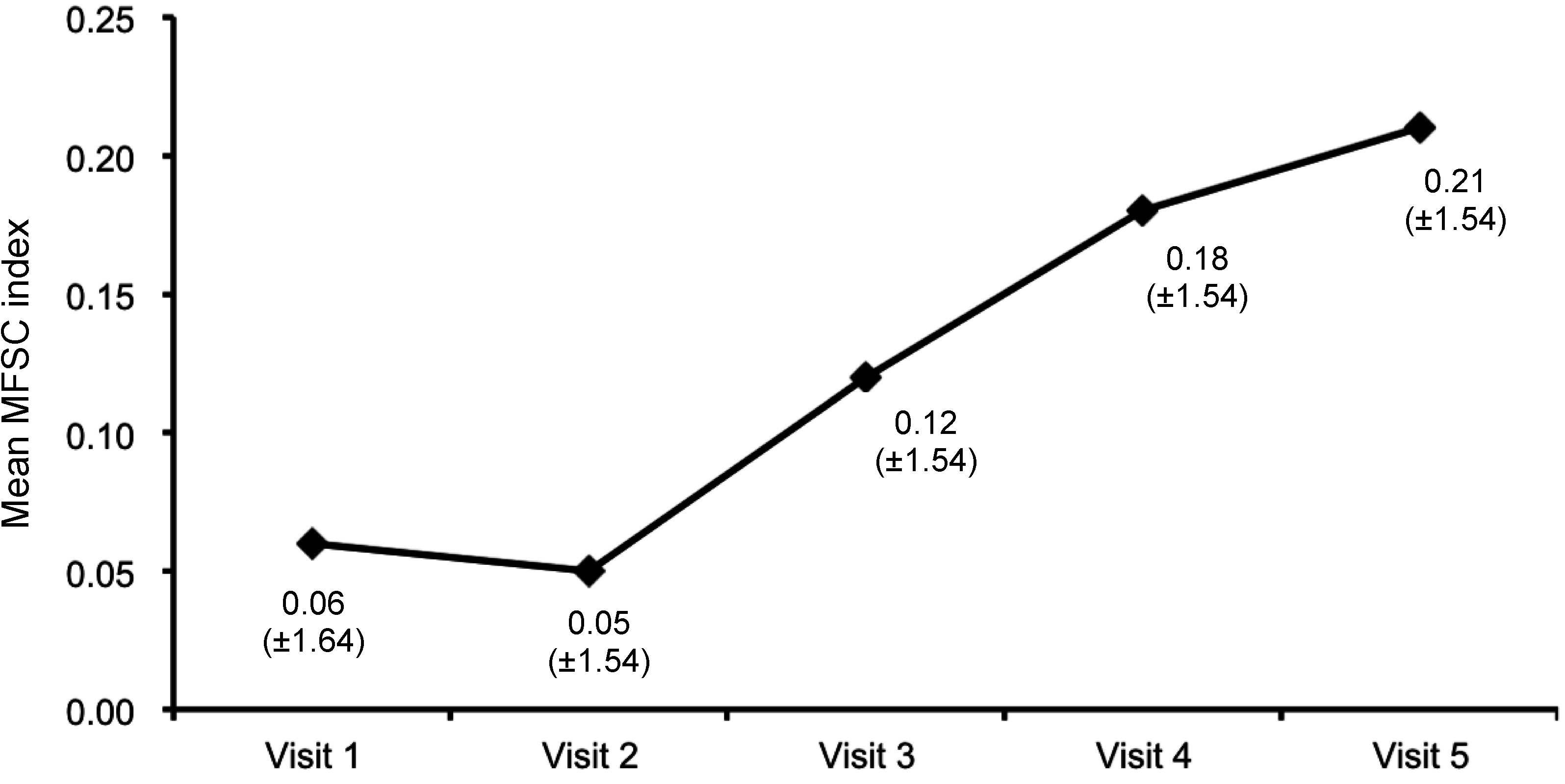
3. Discussion
4. Methods
4.1. Study Design and Objectives
4.2. Subjects
4.3. Treatment
4.4. Assessment and Documentation
4.5. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Putzki, N.; Limmroth, V. QUASIMS: An observational study in Germany on interferon beta preparations as therapy for relapsing multiple sclerosis. Aktuelle Neurol. 2007, 34, 1–7. [Google Scholar] [CrossRef]
- Al-Sabbagh, A.; Bennet, R.; Kozma, C.; Dickson, M.; Meletiche, D. Medication gaps in disease-modifying therapy for multiple sclerosis are associated with an increased risk of relapse: Findings from a national managed care database. J. Neurol. 2008, 255, S79. [Google Scholar]
- Siger, M.; Durko, A.; Nicpan, A.; Konarska, M.; Grudziecka, M.; Selmaj, K. Discontinuation of interferon beta therapy in multiple sclerosis patients with high pre-treatment disease activity leads to prompt return to previous disease activity. J. Neurol. Sci. 2011, 303, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.T.; Sá, M.J. Switching and escalating therapy in long-lasting multiple sclerosis: Not always necessary. ISRN Neurol. 2012, 2012, 451457. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Blodau, A.; Zessack, N.; Lang, M.; High Dose, High Frequency Study Group. Optimizing immunomodulatory therapy with interferon beta in patients with multiple sclerosis. A prospective study. Int. J. MS Care 2010, 12, 147–154. [Google Scholar] [CrossRef]
- Río, J.; Porcel, J.; Téllez, N.; Sánchez-Betancourt, A.; Tintoré, M.; Arévalo, M.J.; Nos, C.; Montalban, X. Factors related with treatment adherence to interferon beta and glatiramer acetate therapy in multiple sclerosis. Mult. Scler. J. 2005, 11, 306–309. [Google Scholar] [CrossRef]
- Freedman, M.S.; Patry, D.G.; Grand’Maison, F.; Myles, M.L.; Paty, D.W.; Selchen, D.H. Treatment optimization in multiple sclerosis. Can. J. Neurol. Sci. 2004, 31, 157–168. [Google Scholar] [PubMed]
- Caon, C.; Din, M.; Ching, W.; Tselis, A.; Lisak, R.; Khan, O. Clinical course after change of immunomodulating therapy in relapsing-remitting multiple sclerosis. Eur. J. Neurol. 2006, 13, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Coyle, P.K. Switching Therapies in Multiple Sclerosis. CNS Drugs 2013, 27, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Kleinschnitz, C.; Niemczyk, G.; Rehberg-Weber, K.; Wernsdörfer, C.; AXIOM Study Group. Interferon beta 1a (AVONEX®) as Treatment Option for Untreated MS-Patients. Poster 1007. In Proceedings of the 28th Congress of the European Committee for Treatment and Research in Multiple Sclerosis, Lyon, France, 10–13 October 2012.
- Polman, C.H.; Rudick, R.A. The multiple sclerosis functional composite: A clinically meaningful measure of disability. Neurology 2010, 74, S8–S15. [Google Scholar] [CrossRef] [PubMed]
- Beer, K.; Müller, M.; Hew-Winzeler, A.M.; Bont, A.; Maire, P.; You, X.; Foulds, P.; Marlin, J.; Curtius, D. The prevalence of injection-site reactions with disease-modifying therapies and their effect on adherence in patients with multiple sclerosis: An observational study. BMC Neurol. 2011, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, K.K.; Butler, J.S.; Mattingly, M.; Ryan, M. Factors leading patients to discontinue multiple sclerosis therapies. J. Am. Pharm. Assoc. 2004, 45, 371–375. [Google Scholar]
- Tremlett, H.L.; Oger, J. Interrupted therapy: Stopping and switching of the beta-interferons prescribed for MS. Neurology 2003, 61, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Carrá, A.; Onaha, P.; Luetic, G.; Burgos, M.; Crespo, E.; Deri, N.; Halfon, M.; Jaacks, G.; Lopez, A.; Sinay, V. Therapeutic outcome 3 years after switching of immunomodulatory therapies in patients with relapsing-remitting multiple sclerosis in Argentina. Eur. J. Neurol. 2008, 15, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Lanzillo, R.; Florio, C.; Orefice, G.; Vivo, P.; Ascione, S.; Schiavone, V.; Pagano, A.; Vacca, G.; De Michele, G.; et al. Long-term clinical experience with weekly interferon beta-1a in relapsing multiple sclerosis. Eur. J. Neurol. 2006, 13, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Milanese, C.; La Mantia, L.; Palumbo, R.; Martinelli, V.; Murialdo, A.; Zaffaroni, M.; Caputo, D.; Capra, R.; Bergamaschi, R.; North Italy Multiple Sclerosis Group. A post-marketing study on interferon beta 1b and 1a treatment in relapsing-remitting multiple sclerosis: Different response in drop-outs and treated patients. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1689–1692. [Google Scholar] [CrossRef] [PubMed]
- Panitch, H.; Goodin, D.S.; Francis, G.; Chang, P.; Coyle, P.K.; O’Connor, P.; Monaghan, E.; Li, D.; Weinshenker, B.; EVIDENCE Study Group; EVidence of Interferon Dose-response: Europian North American Compartative Efficacy; University of British Columbia MS/MRI Research Group. Randomized, comparative study of interferon beta-1a treatment regimens in MS: The EVIDENCE Trial. Neurology 2002, 59, 1496–1506. [Google Scholar] [PubMed]
- Bermel, R.A.; Weinstock-Guttman, B.; Bourdette, D.; Foulds, P.; You, X.; Rudick, R.A. Intramuscular interferon beta-1a therapy in patients with relapsing-remitting multiple sclerosis: A 15-year follow-up study. Mult. Scler. J. 2010, 16, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.F.; Barnes, C.J.; Nair, K.V. Emerging methods for evaluating the effectiveness of intramuscular interferon beta-1a for relapsing-remitting multiple sclerosis. J. Manag. Care Pharm. 2013, 19, S16–S23. [Google Scholar] [PubMed]
- Putzki, N.; Fischer, J.; Gottwald, K.; Reifschneider, G.; Ries, S.; Siever, A.; Hoffmann, F.; Kafferlein, W.; Kausch, U.; Liedtke, M.; et al.; “Mensch im Mittelpunkt” Study Group Quality of life in 1000 patients with early relapsing-remitting multiple sclerosis. Eur. J. Neurol. 2009, 16, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Gajofatto, A.; Bacchetti, P.; Grimes, B.; High, A.; Waubant, E. Switching first-line disease-modifying therapy after failure: Impact on the course of relapsing-remitting multiple sclerosis. Mult. Scler. J. 2009, 15, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Limmroth, V.; Putzki, N.; Kachuck, N.J. The interferon beta therapies for treatment of relapsing-remitting multiple sclerosis: Are they equally efficacious? A comparative review of open-label studies evaluating the efficacy, safety, or dosing of different interferon beta formulations alone or in combination. Ther. Adv. Neurol. Disord. 2011, 4, 281–296. [Google Scholar] [PubMed]
- Prosperini, L.; Borriello, G.; de Giglio, L.; Leonardi, L.; Barletta, V.; Pozzilli, C. Management of breakthrough disease in patients with multiple sclerosis: When an increasing of Interferon beta dose should be effective? BMC Neurol. 2011, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Applebee, A.; Panitch, H. Early stage and long term treatment of multiple sclerosis with interferon-beta. Biologics 2009, 3, 257–271. [Google Scholar] [PubMed]
- Ebers, G.C.; Traboulsee, A.; Li, D.; Langdon, D.; Reder, A.T.; Goodin, D.S.; Bogumil, T.; Beckmann, K.; Wolf, C.; Konieczny, A.; Investigators of the 16-year Long-Term Follow-Up Study. Analysis of clinical outcomes according to original treatment groups 16 years after the pivotal IFNB-1b trial. J. Neurol. Neurosurg. Psychiatry 2010, 81, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.F.; Zerkowski, K.; Nair, K.V. Evidence for long-term use of intramuscular interferon beta-1a: An overview of relapse, disability, and MRI data from selected clinical trials. J. Manag. Care Pharm. 2013, 19, S4–S15. [Google Scholar] [PubMed]
- Jeffery, D.R. Early intervention with immunomodulatory agents in the treatment of multiple sclerosis. J. Neurol. Sci. 2002, 197, 1–8. [Google Scholar] [CrossRef]
- Shirani, A.; Zhao, Y.; Karim, M.E.; Evans, C.; Kingwell, E.; van der Kop, M.L.; Oger, J.; Gustafson, P.; Petkau, J.; Tremlett, H. Association between use of interferon beta and progression of disability in patients with relapsing-remitting multiple sclerosis. J. Am. Med. Assoc. 2012, 308, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Trojano, M.; Pellegrini, F.; Paolicelli, D.; Fuiani, A.; Zimatore, G.B.; Tortorella, C.; Simone, I.L.; Patti, F.; Ghezzi, A.; Zipoli, V.; Italian Multiple Sclerosis Database Network (MSDN) Group. Real-life impact of early interferon beta therapy in relapsing multiple sclerosis. Ann. Neurol. 2009, 66, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Drake, A.S.; Weinstock-Guttman, B.; Morrow, S.A.; Hojnacki, D.; Munschauer, F.E.; Benedict, R.H. Psychometrics and normative data for the Multiple Sclerosis Functional Composite: Replacing the PASAT with the Symbol Digit Modalities Test. Mult. Scler. J. 2010, 16, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Rudick, R.A.; Cutter, G.; Reingold, S. The multiple sclerosis functional composite: A new clinical outcome measure for multiple sderosis trials. Mult. Scler. J. 2002, 8, 359–365. [Google Scholar] [CrossRef]
- Miller, D.M.; Rudick, R.A.; Cutter, G.; Baier, M.; Fischer, J.S. Clinical significance of the multiple sclerosis functional composite: Relationship to patient-reported quality of life. Arch. Neurol. 2000, 57, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.S.; Rudick, R.A.; Cutter, G.R.; Reingold, S.C. The Multiple Sclerosis Functional Composite Measure (MSFC): An integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult. Scler. J. 1999, 5, 244–250. [Google Scholar] [CrossRef]
- Cohen, J.A.; Cutter, G.R.; Fischer, J.S.; Goodman, A.D.; Heidenreich, F.R.; Jak, A.J.; Kniker, J.E.; Kooijmans, M.F.; Lull, J.M.; Sandrock, A.W.; et al. Use of the multiple sclerosis functional composite as an outcome measure in a phase 3 clinical trial. Arch. Neurol. 2011, 58, 961–967. [Google Scholar] [CrossRef]
- Goldman, M.D.; Motl, R.W.; Rudick, R.A. Possible clinical outcome measures for clinical trials in patients with multiple sclerosis. Ther. Adv. Neurol. Disord. 2010, 3, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Rudick, R.A.; Polman, C.H.; Cohen, J.A.; Walton, M.K.; Miller, A.E.; Confavreux, C.; Lublin, F.D.; Hutchinson, M.; O’Connor, P.W.; Schwid, S.R.; et al. Assessing disability progression with the Multiple Sclerosis Functional Composite. Mult. Scler. J. 2009, 15, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Schwid, S.R.; Goodman, A.D.; McDermott, M.P.; Bever, C.F.; Cook, S.D. Quantitative functional measures in MS: What is a reliable change? Neurology 2002, 58, 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Kersten, P.; Küçükdeveci, A.A.; Tennant, A. The use of the Visual Analogue Scale (VAS) in rehabilitation outcomes. J. Rehabil. Med. 2012, 44, 609–610. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Meyer, D.; Herrman, C.E.; Sheppard, C.; Murray, R.; Fox, E.J.; Mathena, J.; Conner, J.; Buck, P.O. Tolerability and safety of novel half milliliter formulation of glatiramer acetate for subcutaneous injection: An open-label, multicenter, randomized comparative study. J. Neurol. 2010, 257, 1917–1923. [Google Scholar] [CrossRef] [PubMed]
- Jaber, A.; Bozzato, G.B.; Vedrine, L.; Prais, W.A.; Berube, J.; Laurent, P.E. A novel needle for subcutaneous injection of interferon beta-1a: Effect on pain in volunteers and satisfaction in patients with multiple sclerosis. BMC Neurol. 2008, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Cutter, G.C.; Baier, M.L.; Rudick, R.A.; Cookfair, D.L.; Fischer, J.S.; Petkau, J.; Syndulko, K.; Weinshenker, B.G.; Antel, J.P.; Confavreux, C. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain 1999, 122, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.S.; Jak, A.J.; Kniker, J.E.; Rudick, R.A.; Cutter, G.; National MS Society’s Clinical Outcomes Assessment Task Force. Multiple sclerosis functional composite (MSFC). Administration and scoring manual; revised October 2001. Available online: http://www.mstrust.org.uk/competencies/downloads/MSFC.pdf (accessed on 29 May 2015).
- Rudick, R.; Antel, J.; Confavreux, C.; Cutter, G.; Ellison, G.; Fischer, J.; Lublin, F.; Miller, A.; Petkau, J.; Rao, S. Recommendations from the national multiple sclerosis society clinical outcomes assessment task force. Ann. Neurol. 1997, 42, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Edgar, C.; Jongen, P.J.; Sanders, E.; Sindic, C.; Goffette, S.; Dupuis, M.; Jacquerye, P.; Guillaume, D.; Reznik, R.; Wesnes, K. Cognitive performance in relapsing remitting multiple sclerosis: A longitudinal study in daily practice using a brief computerized cognitive battery. BMC Neurol. 2011, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- National Multiple Sclerosis Society. Disease Management Consensus Statement (2008). Available online: www.nationalmssociety.org/download.aspx?id=8 (accessed on 1 April 2013).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleinschnitz, C.; Niemczyk, G.; Rehberg-Weber, K.; Wernsdörfer, C. Interferon Beta-1a (AVONEX®) as a Treatment Option for Untreated Patients with Multiple Sclerosis (AXIOM): A Prospective, Observational Study. Int. J. Mol. Sci. 2015, 16, 15271-15286. https://doi.org/10.3390/ijms160715271
Kleinschnitz C, Niemczyk G, Rehberg-Weber K, Wernsdörfer C. Interferon Beta-1a (AVONEX®) as a Treatment Option for Untreated Patients with Multiple Sclerosis (AXIOM): A Prospective, Observational Study. International Journal of Molecular Sciences. 2015; 16(7):15271-15286. https://doi.org/10.3390/ijms160715271
Chicago/Turabian StyleKleinschnitz, Christoph, Gabriele Niemczyk, Karin Rehberg-Weber, and Colin Wernsdörfer. 2015. "Interferon Beta-1a (AVONEX®) as a Treatment Option for Untreated Patients with Multiple Sclerosis (AXIOM): A Prospective, Observational Study" International Journal of Molecular Sciences 16, no. 7: 15271-15286. https://doi.org/10.3390/ijms160715271
APA StyleKleinschnitz, C., Niemczyk, G., Rehberg-Weber, K., & Wernsdörfer, C. (2015). Interferon Beta-1a (AVONEX®) as a Treatment Option for Untreated Patients with Multiple Sclerosis (AXIOM): A Prospective, Observational Study. International Journal of Molecular Sciences, 16(7), 15271-15286. https://doi.org/10.3390/ijms160715271





