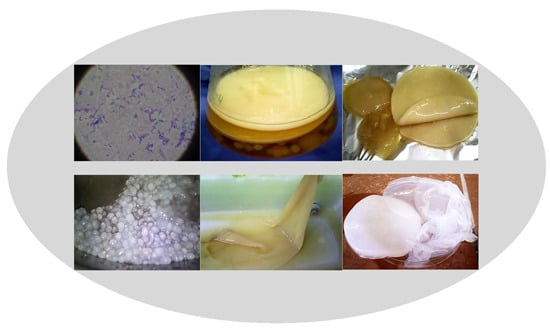
Bacterial Cellulose Production from Industrial Waste and by-Product Streams
Abstract
:1. Introduction
2. Results and Discussion
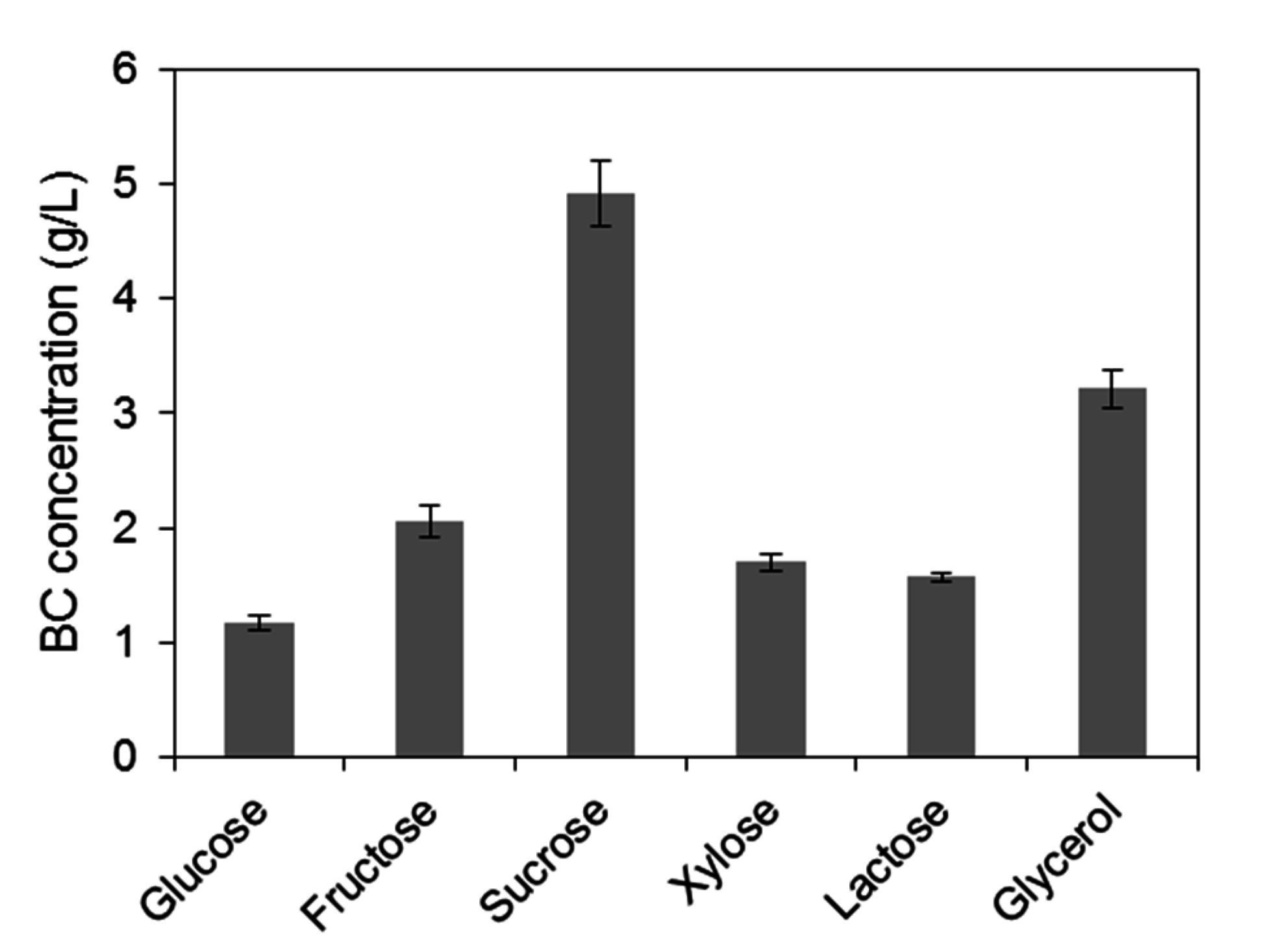
2.1. Assessment of Carbon Sources for Bacterial Cellulose Production
2.2. Evaluation of Different Free Amino Nitrogen Concentrations for BC Production
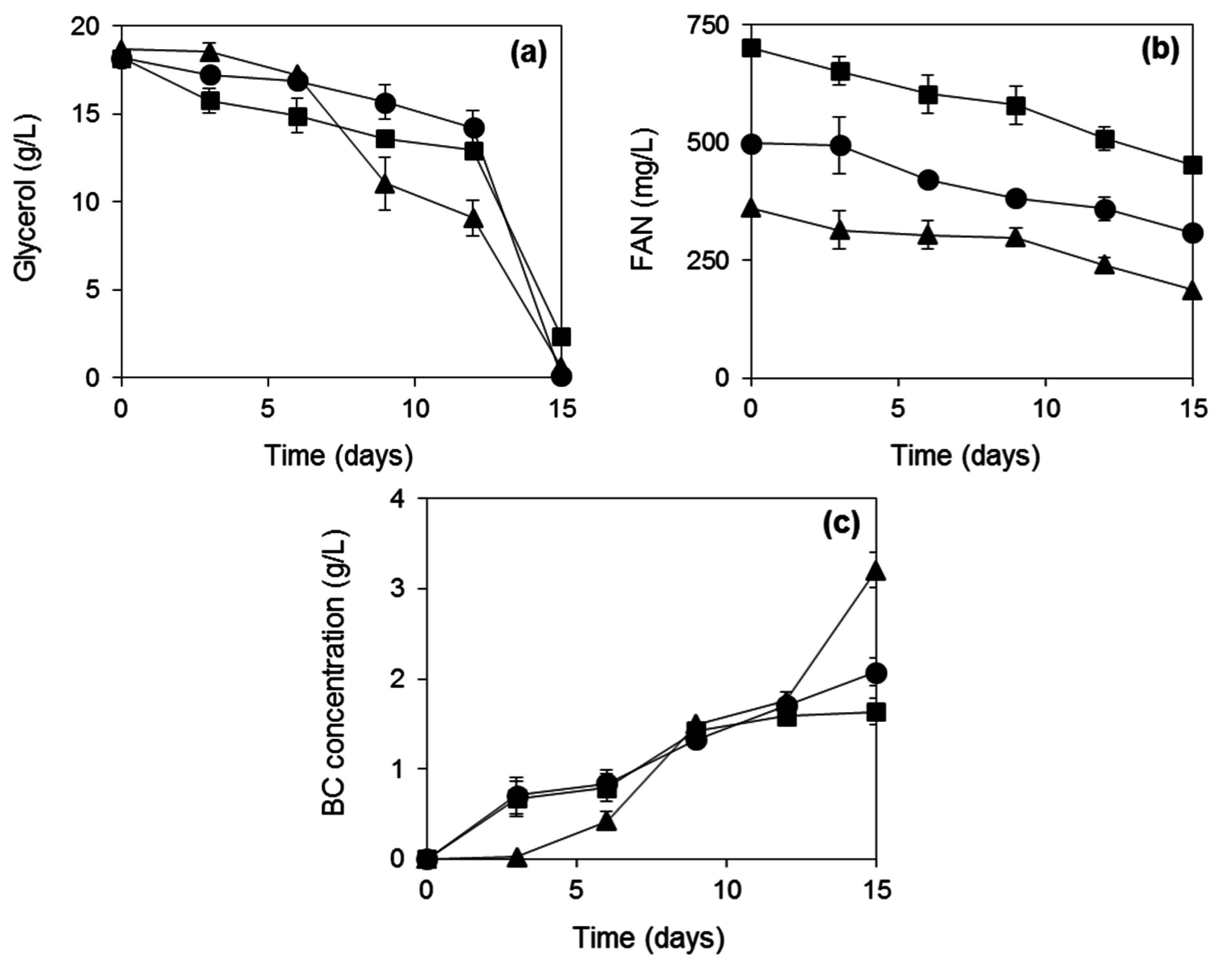
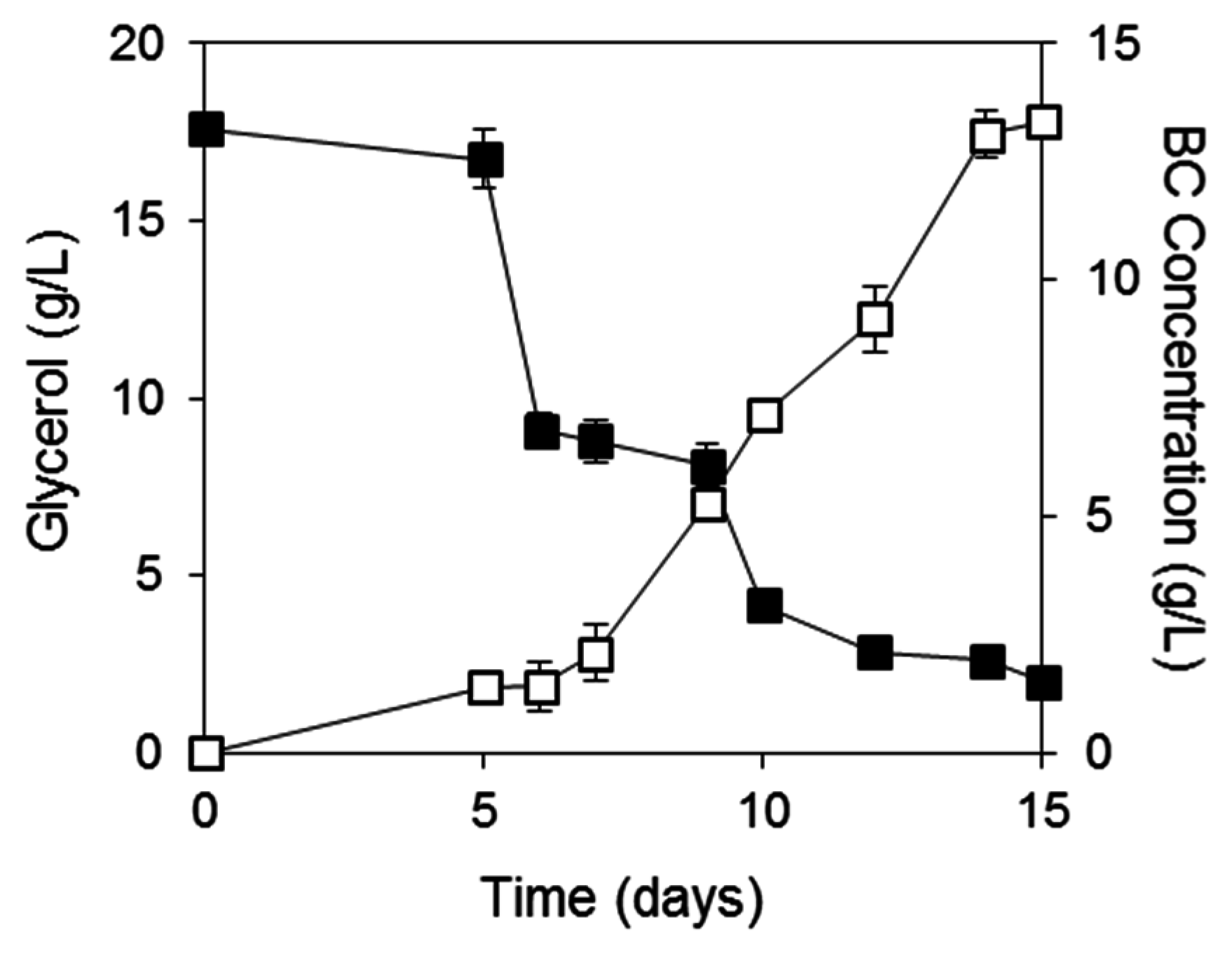
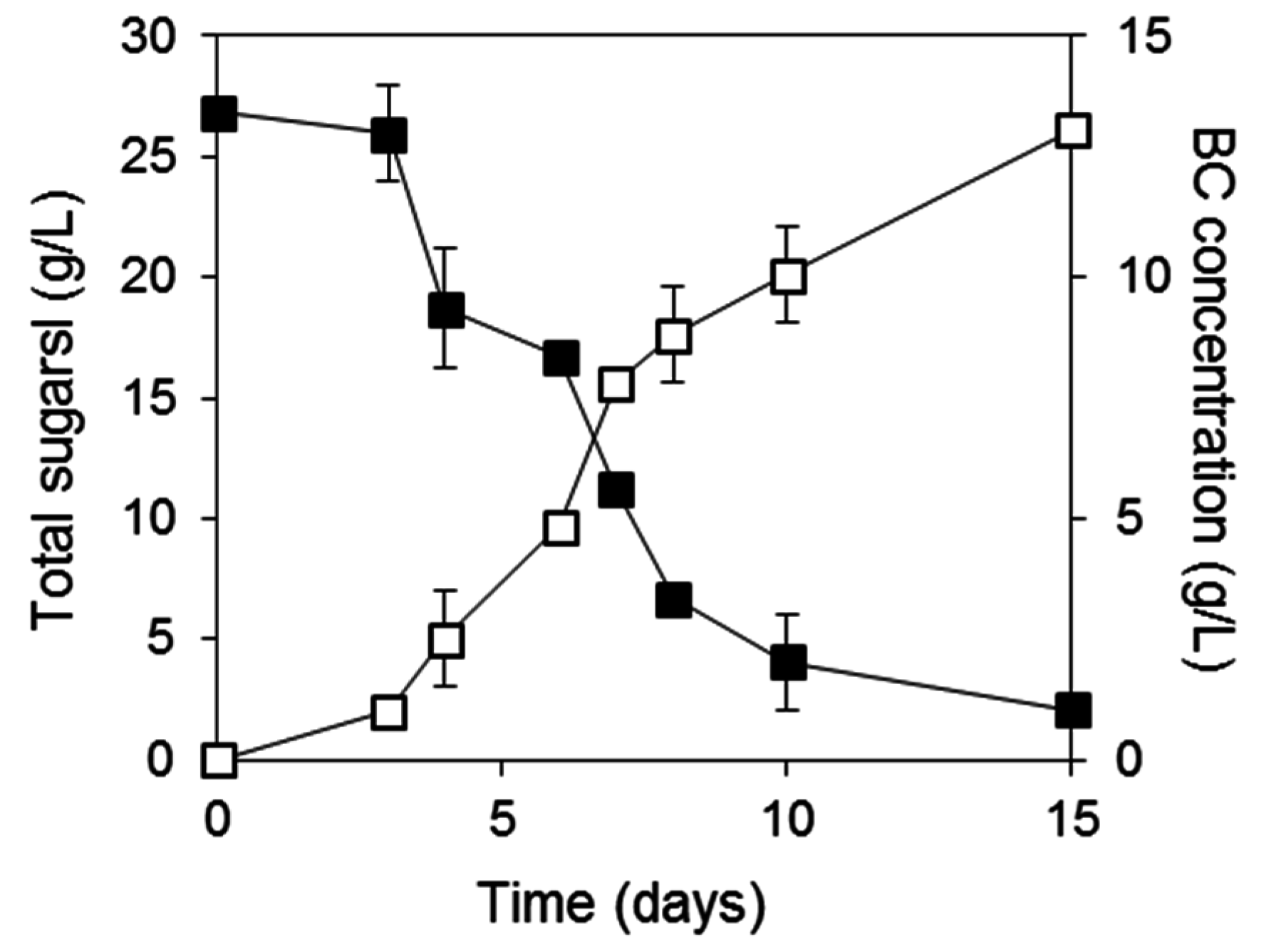
2.3. BC Production from Biodiesel and Confectionery Industry Side Streams
| Strain | Fermentation Mode | Carbon Source | BC (g/L) | Productivity (g/L/d) | Reference |
|---|---|---|---|---|---|
| Acetobacter aceti subsp. xylinus ATCC 23770 | Static batch fermentations | Konjac powder | 2.1 | 0.26 | [4] |
| A. xylinum NBRC 13693 | Static batch fermentations | Fruit juices | 5.9 | 0.42 | [5] |
| A. xylinum BPR 2001 (ATCC 700178) | Agitated batch cultures (135 rpm) | Maple syrup | 1.5 | 0.07 | [6] |
| Gluconacetobacter xylinus BCRC 12334 | Static batch fermentations | Thin stillage from rice wine distillery | 10.4 | 1.48 | [7] |
| G. xylinus ATCC 23770 | Static batch fermentations | Wheat straw hydrolysates | 8.3 | 1.18 | [8] |
| G. xylinus ATCC 23770 | Static batch fermentations | Spruce hydrolysates | 8.2 | 0.59 | [9] |
| G. xylinus NRRL B-42 | Static batch fermentations | Glycerol from biodiesel production process | 10 | 0.71 | [10] |
| Grape bagasse from wine production | 8 | 0.57 | |||
| G. xylinum CGMCC 2955 | Static batch fermentations | Wastewater of candied jujube processing industry | 2.2 | 0.37 | [11] |
| G. xylinus CH001 | Static batch fermentations | Acetone-butanol-ethanol fermentation wastewater | 1.3 | 0.19 | [12] |
| A. xylinum KJ1 | Static cultures in 30 L fermentor | Saccharified food wastes | 18 | 3.6 | [27] |
| Agitated culture in 10 L jar fermentor | 16.8 | 3.36 | |||
| G. xylinus ATCC 23770 | Static batch fermentations | Cotton cloth hydrolysates | 10.8 | 0.77–1.54 | [28] |
| G. hansenii CGMCC 3917 | Static batch fermentations | Waste beer yeast hydrolysates | 7 | 0.5 | [29] |
| A. xylinum KJ1 | 50 L spherical type bubble column bioreactor | Saccharified food wastes | 5.6 | 1.87 | [30] |
| G. medellinensis | Static batch fermentations | Pineapple waste and sugar cane juice | 4 | 0.3 | [31] |
| Agitated batch fermentations | 0.8 | 0.11 | |||
| G. sacchari | Static batch fermentations | Grape skins aqueous extract | 0.6 | 0.15 | [32] |
| Industrial hardwood spent sulfite liquor | 0.3 | 0.07 |
2.4. Water Holding Capacity (WHC)
| Properties | BC1 | BC2 | BC3 | BC4 |
|---|---|---|---|---|
| Stress at break [σ] (MPa) | 139.5 ± 12.6 | 79.8 ± 7.6 | 94.5 ± 8.2 | 72.3 ± 6 |
| Elongation at break [ε] (%) | 8.5 ± 0.2 | 7.1 ± 0.0 | 9.2 ± 0.4 | 7.05 ± 0.02 |
| Young’s modulus [Ε] (GPa) | 1.64 ± 0.2 | 1.13 ± 0.11 | 1.02 ± 0.09 | 0.97 ± 0.05 |
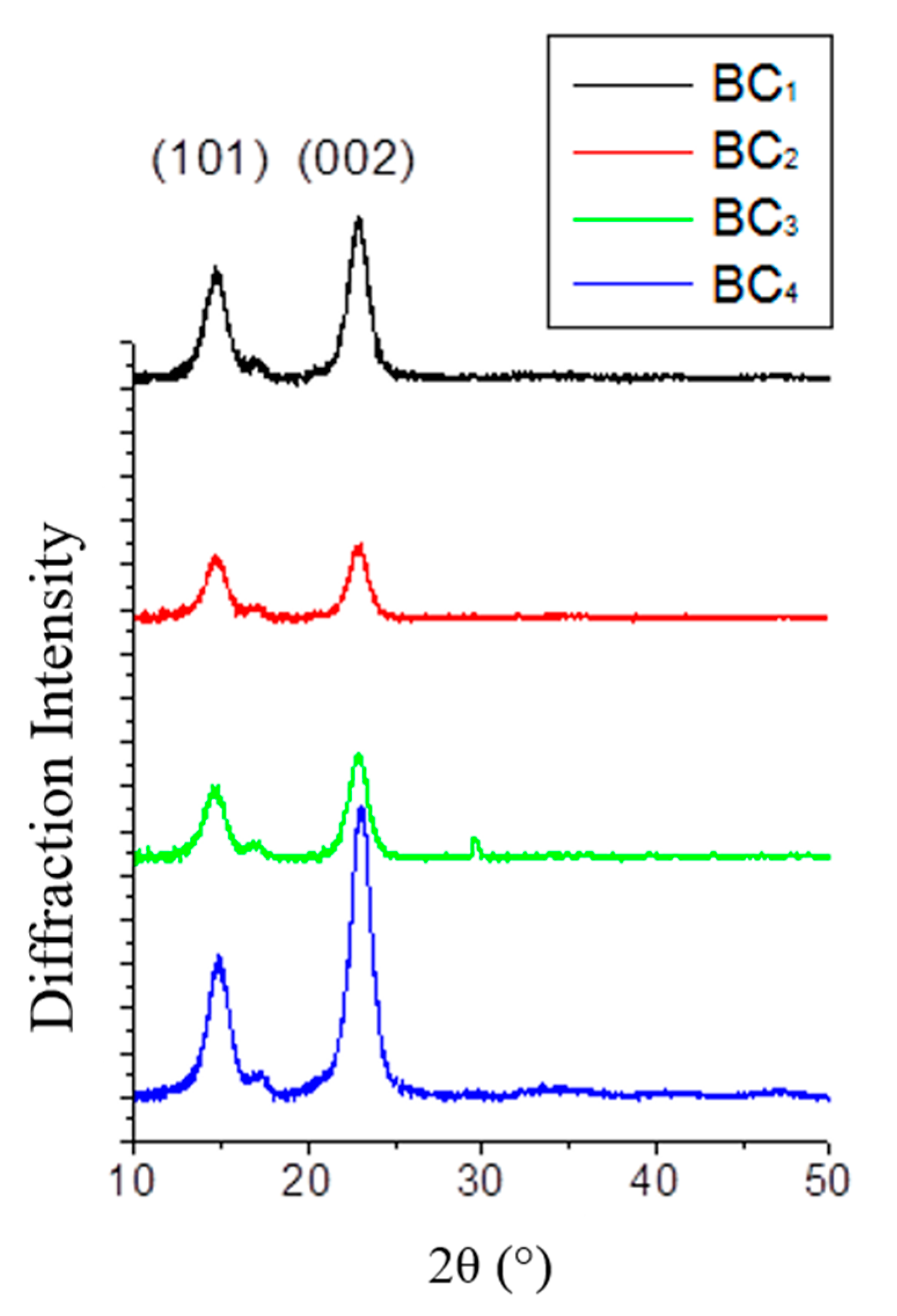
| Crystallinity index [CrI] (%) | 88 | 74 | 81 | 89 |
| Mean crystallite size [CrS] (nm) | 5.9 | 6.4 | 6.1 | 5.7 |
| Intrinsic viscosity [η] (dL/g) | 9.3 | 7.5 | 4.7 | 6.2 |
| Molecular weight [MW] (106 g·mol−1) | 0.43 | 0.39 | 0.31 | 0.35 |
| Degree of polymerization [DP] | 2672.8 | 2391.2 | 1889.1 | 2176.1 |
| Water holding capacity [WHC] (g·water/g·dry BC) | 138 ± 9 | 124 ± 5 | 131 ± 4 | 102 ± 6 |
2.5. Effect of Different Culture Media on the Intrinsic Viscosity and Degree of Polymerization
2.6. Mechanical Properties
2.7. XRD Analysis
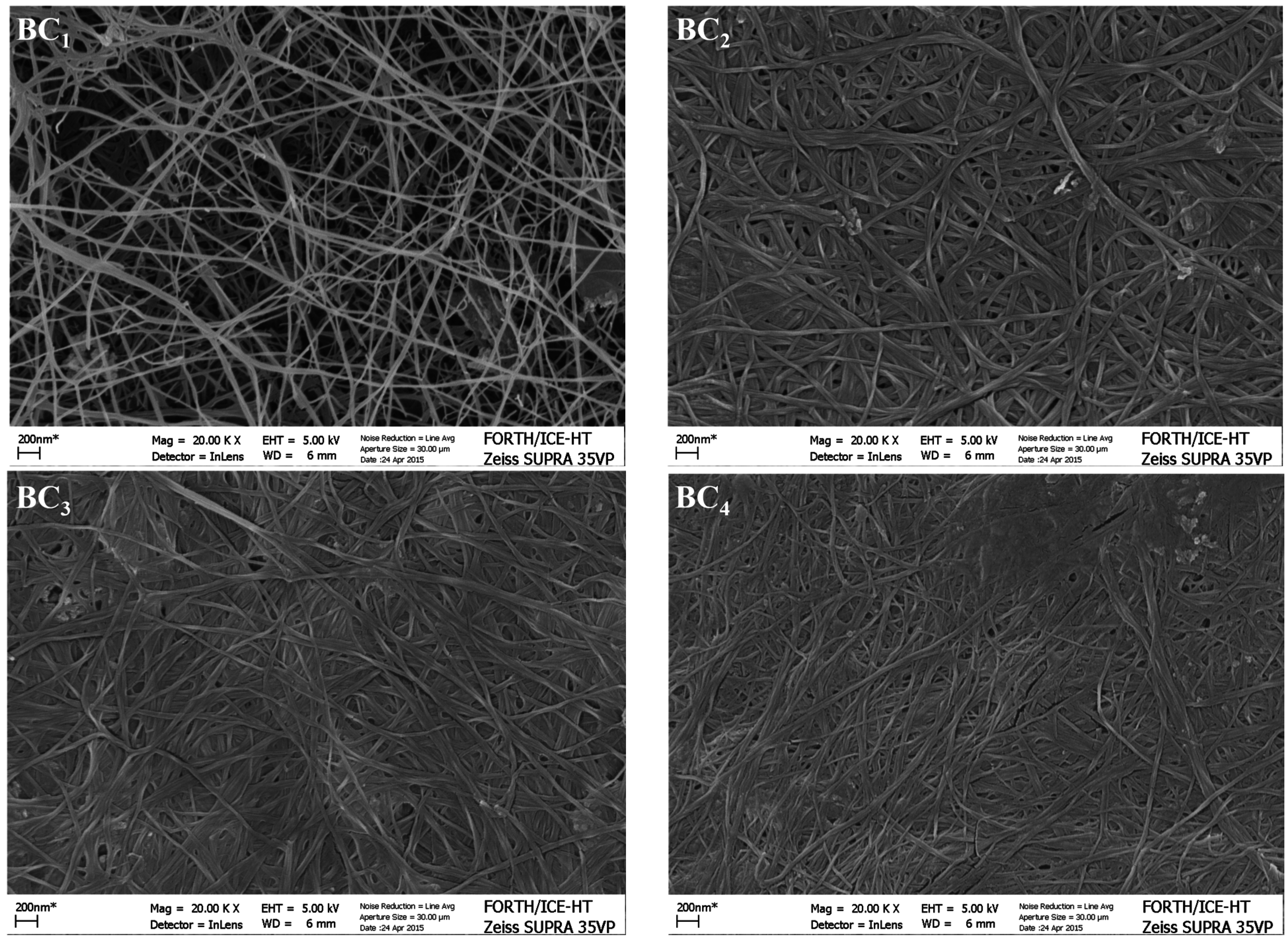
2.8. BC Morphology Characterization
3. Experimental Section
3.1. Raw Materials
3.2. Microorganisms
3.3. Solid State Fermentation
3.4. Production of SFM and FRW Hydrolysates
3.5. Fermentative Production of Bacterial Cellulose
3.6. Characterization of BC Samples
3.6.1. Water Holding Capacity (WHC)
3.6.2. Intrinsic Viscosity and Degree of Polymerization (DP)
3.6.3. X-ray Diffraction Analysis (XRD)
3.6.4. Mechanical Strength
3.6.5. Scanning Electron Microscopy (SEM)
3.7. Analytical Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Phisalaphong, M.; Jatupaiboon, N. Biosynthesis and characterization of bacteria cellulose-chitosan film. Carbohydr. Polym. 2008, 74, 482–488. [Google Scholar] [CrossRef]
- Chawla, P.R.; Bajaj, I.B.; Survase, S.A.; Singhal, R.S. Microbial cellulose: Fermentative production and applications. Food Technol. Biotechnol. 2009, 47, 107–124. [Google Scholar]
- Paximada, P.; Tsouko, E.; Kopsahelis, N.; Koutinas, A.A.; Mandala, I. Bacterial cellulose as stabilizer of o/w emulsions. Food Hydrocoll. 2014. [Google Scholar] [CrossRef]
- Hong, F.; Qiu, K.Y. An alternative carbon source from konjac powder for enhancing production of bacterial cellulose in static cultures by a model strain Acetobacteraceti subsp. xylinus ATCC 23770. Carbohydr. Polym. 2008, 72, 545–549. [Google Scholar] [CrossRef]
- Kurosumi, A.; Sasaki, C.; Yamashita, Y.; Nakamura, Y. Utilization of various fruit juices as carbon source for production of bacterial cellulose by Acetobacter xylinum NBRC 13693. Carbohydr. Polym. 2009, 76, 333–335. [Google Scholar] [CrossRef]
- Zeng, X.; Small, D.P.; Wan, W. Statistical optimization of culture conditions for bacterial cellulose production by Acetobacter xylinum BPR 2001 from maple syrup. Carbohydr. Polym. 2011, 85, 506–513. [Google Scholar] [CrossRef]
- Wu, J.M.; Liu, R.H. Thin stillage supplementation greatly enhances bacterial cellulose production by Gluconacetobacter xylinus. Carbohydr. Polym. 2012, 90, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hong, F.; Yang, X.X.; Han, S.F. Biotransformation of wheat straw to bacterial cellulose and its mechanism. Bioresour. Technol. 2013, 135, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cavka, A.; Jönsson, L.J.; Hong, F. Comparison of methods for detoxification of spruce hydrolysate for bacterial cellulose production. Microb. Cell Factories 2013, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, A.; Foresti, M.L.; Cerrutti, P.; Galvagno, M. Bacterial cellulose from simple and low cost production media by Gluconacetobacter xylinus. J. Polym. Environ. 2013, 21, 545–554. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Hua, J.; Jia, S.; Zhang, J.; Liu, H. Production of nanobacterial cellulose from waste water of candied jujube-processing industry using Acetobacter xylinum. Carbohydr. Polym. 2015, 120, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yang, X.Y.; Xiong, L.; Guo, H.J.; Luo, J.; Wang, B.; Zhang, H.R.; Lin, X.Q.; Chen, X.D. Evaluating the possibility of using acetone-butanol-ethanol (ABE) fermentation wastewater for bacterial cellulose production by Gluconacetobacter xylinus. Lett. Appl. Microbiol. 2015, 60, 491–496. [Google Scholar] [CrossRef] [PubMed]
- US Energy Information Administration (EIA). Available online: http://www.eia.gov/cfapps/ipdbproject/iedindex3.cfm?tid=79&pid=81&aid=1&id=regions, &syid=2008&eyid =2012&unit=TBPD (accessed on 10 March 2015).
- European Biomass Industry Association. Available online: http://www.eubia.org/index.php/about-biomass/biofuels-for-transport/biodiesel (accessed on 15 February 2015).
- Foreign Agricultural Service USDA. Available online: http://apps.fas.usda.gov/psdonline/psdReport.aspx?hidReportRetrievalName=Table+13%3a+Sunflowerseed+and+Products%3a+World+Supply+and+Distribution&hidReportRetrievalID=712&hidReportRetrievalTemplateID=11 (accessed on 10 March 2015).
- Food and Agriculture Organization of the United Nations (FAO). Available online: http://www.fao.org/food-loss-and-food-waste/en/ (accessed on 5 April 2015).
- Ishihara, M.; Matsunaga, M.; Hayashi, N.; Tisler, V. Utilization of d-xylose as carbon source for production of bacterial cellulose. Enzym. Microb. Technol. 2002, 31, 986–991. [Google Scholar] [CrossRef]
- Battad-Bernardo, E.; Mc Crindle, S.L.; Couperwhite, I.; Neilan, B.A. Insertion of an E. coli lacZ gene in Acetobacter xylinus for the production of cellulose in whey. FEMS Microbiol. Lett. 2004, 231, 253–260. [Google Scholar] [CrossRef]
- Son, H.J.; Kim, H.G.; Kim, K.K.; Kim, H.S.; Kim, Y.G.; Lee, S.J. Increased production of bacterial cellulose by Acetobacter sp. V6 in synthetic media under shaking culture conditions. Bioresour. Technol. 2003, 86, 215–219. [Google Scholar] [CrossRef]
- Czaja, W.; Romanowicz, D.; Brown, R.M. Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose 2004, 11, 403–411. [Google Scholar] [CrossRef]
- Masaoka, S.; Ohe, T.; Sakota, N. Production of cellulose from glucose by Acetobacter xylinum. J. Ferment. Bioeng. 1993, 75, 18–22. [Google Scholar] [CrossRef]
- Dahman, Y.; Jayasuriya, K.E.; Kalis, M. Potential of biocellulose nanofibers production from agricultural renewable resources: Preliminary study. Appl. Biochem. Biotechnol. 2010, 162, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Ramana, K.V.; Tomar, A.; Singh, L. Effect of various carbon and nitrogen sources on cellulose synthesis by Acetobacter xylinum. World J. Microbiol. Biotechnol. 2000, 16, 245–248. [Google Scholar] [CrossRef]
- Mikkelsen, D.; Flanagan, B.M.; Dykes, G.A.; Gidley, M.J. Influence of different carbon sources on bacterial cellulose production by Gluconacetobacter xylinus strain ATCC 53524. J. Appl. Microbiol. 2009, 107, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Kachrimanidou, V.; Kopsahelis, N.; Papanikolaou, S.; Kookos, I.K.; de bruyn, M.; Clark, J.H.; Koutinas, A.A. Sunflower-based biorefinery: Poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate -co-3-hydroxyvalerate) production from crude glycerol, sunflower meal and levulinic acid. Bioresour. Technol. 2014, 172, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Tsakona, S.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A.A. Formulation of fermentation media from flour-rich waste streams for microbial lipid production by Lipomyces starkeyi. J. Biotechnol. 2014, 189, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Park, J.M.; Chun, H.Y.; Kim, S.J. Comparisons of physical properties of bacterial celluloses produced in different culture conditions using saccharified food wastes. Biotechnol. Bioprocess Eng. 2006, 11, 26–31. [Google Scholar] [CrossRef]
- Hong, F.; Guo, X.; Zhang, S.; Han, S.F.; Yang, G.; Jönsson, L.J. Bacterial cellulose production from cotton-based waste textiles: Enzymatic saccharification enhanced by ionic liquid pretreatment. Bioresour. Technol. 2012, 104, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Lopez-Sanchez, P.; Li, R.; Li, Z. Production of bacterial cellulose by Gluconacetobacter hansenii CGMCC 3917 using only waste beer yeast as nutrient source. Bioresour. Technol. 2014, 151, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Li, H.; Seo, J.H.; Kim, M.J.; Kim, S.J. Pilot-scale production of bacterial cellulose by a spherical type bubble column bioreactor using saccharified food wastes. Korean J. Chem. Eng. 2009, 26, 141–146. [Google Scholar] [CrossRef]
- Alga, I.; Fernandes, S.C.M.; Mondragon, G.; Castro, C.; Garcia-Astrain, C.; Gabilondo, N.; Retegi, A.; Eceiza, A. Pineapple agroindustrial residues for the production of high value bacterial cellulose with different morphologies. J. Appl. Polym. Sci. 2015, 132, 41237. [Google Scholar] [CrossRef]
- Carreira, P.; Mendes, J.A.S.; Trovatti, E.; Serafim, L.S.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Utilization of residues from agro-forest industries in the production of high value bacterial cellulose. Bioresour. Technol. 2011, 102, 7354–7360. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Tabuchi, M.; Morinaga, Y.; Yoshinaga, F. Structural features and properties of bacterial cellulose produced in agitated culture. Cellulose 1998, 5, 187–200. [Google Scholar] [CrossRef]
- Tuinier, R.; ten Grotenhuis, E.; Holt, C.; Timmins, P.A.; de Kruif, C.G. Depletion interaction of casein micelles and an exocellular polysaccharide. Phys. Rev. E 1999, 60, 848–856. [Google Scholar] [CrossRef]
- Morris, E.R.; Ross-Murphy, S.B. Techniques in Carbohydrate Metabolism; Elsevier: London, UK, 1981; pp. 201–246. [Google Scholar]
- Jonas, R.; Farah, L. Production and application of microbial cellulose. Polym. Degrad. Stab. 1998, 59, 101–106. [Google Scholar] [CrossRef]
- Shi, Q.S.; Feng, J.; Li, W.R.; Zhou, G.; Chen, A.M.; Ouyang, Y.S.; Chen, Y.B. Effect of different conditions on the average degree of polymerization of bacterial cellulose produced by Gluconacetobacter intermedius BC-41. Cell. Chem. Technol. 2013, 47, 503–508. [Google Scholar]
- Keshk, S. Physical properties of bacterial cellulose sheets produced in presense of lignosulfonate. Enzym. Microb. Technol. 2006, 40, 9–12. [Google Scholar] [CrossRef]
- Cheng, K.C.; Catchmark, J.M.; Demirci, A. Effect of different additives on bacterial cellulose production by Acetobacter xylinum and analysis of material property. Cellulose 2009, 16, 1033–1045. [Google Scholar] [CrossRef]
- George, J.; Ramana, K.V.; Sabapathy, S.N.; Bawa, A.S. Physico-mechanical properties of chemically treated bacterial (Acetobacter xylinum) cellulose membrane. World J. Microbiol. Biotechnol. 2005, 21, 1323–1327. [Google Scholar] [CrossRef]
- Shezad, O.; Khan, S.; Khan, T.; Park, J.K. Physicochemical and mechanical properties characterization of bacterial cellulose produced with an excellent productivity in static conditions using simple fed-batch cultivation strategy. Carbohydr. Polym. 2010, 82, 173–180. [Google Scholar] [CrossRef]
- Gayathry, G.; Gopalaswamy, G. Production and characterization of microbial cellulosic fible from Acetobacter xylinum. Indian J. Fibre Text. Res. 2014, 39, 93–96. [Google Scholar]
- Mc Kenna, B.A.; Mikkelsen, D.; Wehr, J.B.; Gidley, M.J.; Menzies, N.W. Mechanical and structural properties of native and alkali-treated bacterial cellulose produced by Gluconacetobacter xylinus strain ATCC 53524. Cellulose 2009, 16, 1047–1055. [Google Scholar] [CrossRef]
- Clasen, C.; Sultanova, B.; Wilhelms, T.; Heisig, P.; Kulicke, W.M. Effects of different drying processes on the material properties of bacterial cellulose membranes. Macromol. Symp. 2006, 244, 48–58. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Yano, H.; Nogi, M.; Eichhorn, S.J. An estimation of the Young’s modulus of bacterial cellulose filaments. Cellulose 2008, 15, 507–513. [Google Scholar] [CrossRef]
- Park, S.; O Baker, J.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulose performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Jiang, X. Structure and properties of bacterial cellulose produced using a trickling bed reactor. Appl. Biochem. Biotechnol. 2014, 172, 3844–3861. [Google Scholar] [CrossRef] [PubMed]
- Mohammadkazemi, F.; Azin, M.; Ashori, A. Production of bacterial cellulose using different carbon sources and culture media. Carbohydr. Polym. 2015, 117, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Mohite, B.V.; Patil, S.V. Physical, structural, mechanical and thermal characterization of bacterial cellulose by G. hansenii NCIM 2529. Carbohydr. Polym. 2014, 106, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Kachrimanidou, V.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Yanniotis, S.; Kookos, I.; Koutinas, A.A. Utilisation of by-products from sunflower-based biodiesel production processes for the production of fermentation feedstock. Waste Biomass Valorization 2013, 4, 529–537. [Google Scholar] [CrossRef]
- Schrecker, S.T.; Gostomski, P.A. Determining the water holding capacity of microbial cellulose. Biotechnol. Lett. 2005, 27, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Mansencal, R. Polymer Data Handbook, 2nd ed.; Mark, J.E., Ed.; Oxford University Press: New York, NY, USA, 1999; pp. 3–48. [Google Scholar]
- Lie, S. The EBC-ninhydrin method for determination of free α amino nitrogen. J. Inst. Brew. 1973, 79, 37–41. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsouko, E.; Kourmentza, C.; Ladakis, D.; Kopsahelis, N.; Mandala, I.; Papanikolaou, S.; Paloukis, F.; Alves, V.; Koutinas, A. Bacterial Cellulose Production from Industrial Waste and by-Product Streams. Int. J. Mol. Sci. 2015, 16, 14832-14849. https://doi.org/10.3390/ijms160714832
Tsouko E, Kourmentza C, Ladakis D, Kopsahelis N, Mandala I, Papanikolaou S, Paloukis F, Alves V, Koutinas A. Bacterial Cellulose Production from Industrial Waste and by-Product Streams. International Journal of Molecular Sciences. 2015; 16(7):14832-14849. https://doi.org/10.3390/ijms160714832
Chicago/Turabian StyleTsouko, Erminda, Constantina Kourmentza, Dimitrios Ladakis, Nikolaos Kopsahelis, Ioanna Mandala, Seraphim Papanikolaou, Fotis Paloukis, Vitor Alves, and Apostolis Koutinas. 2015. "Bacterial Cellulose Production from Industrial Waste and by-Product Streams" International Journal of Molecular Sciences 16, no. 7: 14832-14849. https://doi.org/10.3390/ijms160714832
APA StyleTsouko, E., Kourmentza, C., Ladakis, D., Kopsahelis, N., Mandala, I., Papanikolaou, S., Paloukis, F., Alves, V., & Koutinas, A. (2015). Bacterial Cellulose Production from Industrial Waste and by-Product Streams. International Journal of Molecular Sciences, 16(7), 14832-14849. https://doi.org/10.3390/ijms160714832