Eriodictyol Protects Endothelial Cells against Oxidative Stress-Induced Cell Death through Modulating ERK/Nrf2/ARE-Dependent Heme Oxygenase-1 Expression
Abstract
:1. Introduction

2. Results
2.1. Effects of Induction of Heme Oxygenase-1 (HO-1) Expression by Eriodictyol in Human Umbilical Vein Endothelial Cells (HUVECs)
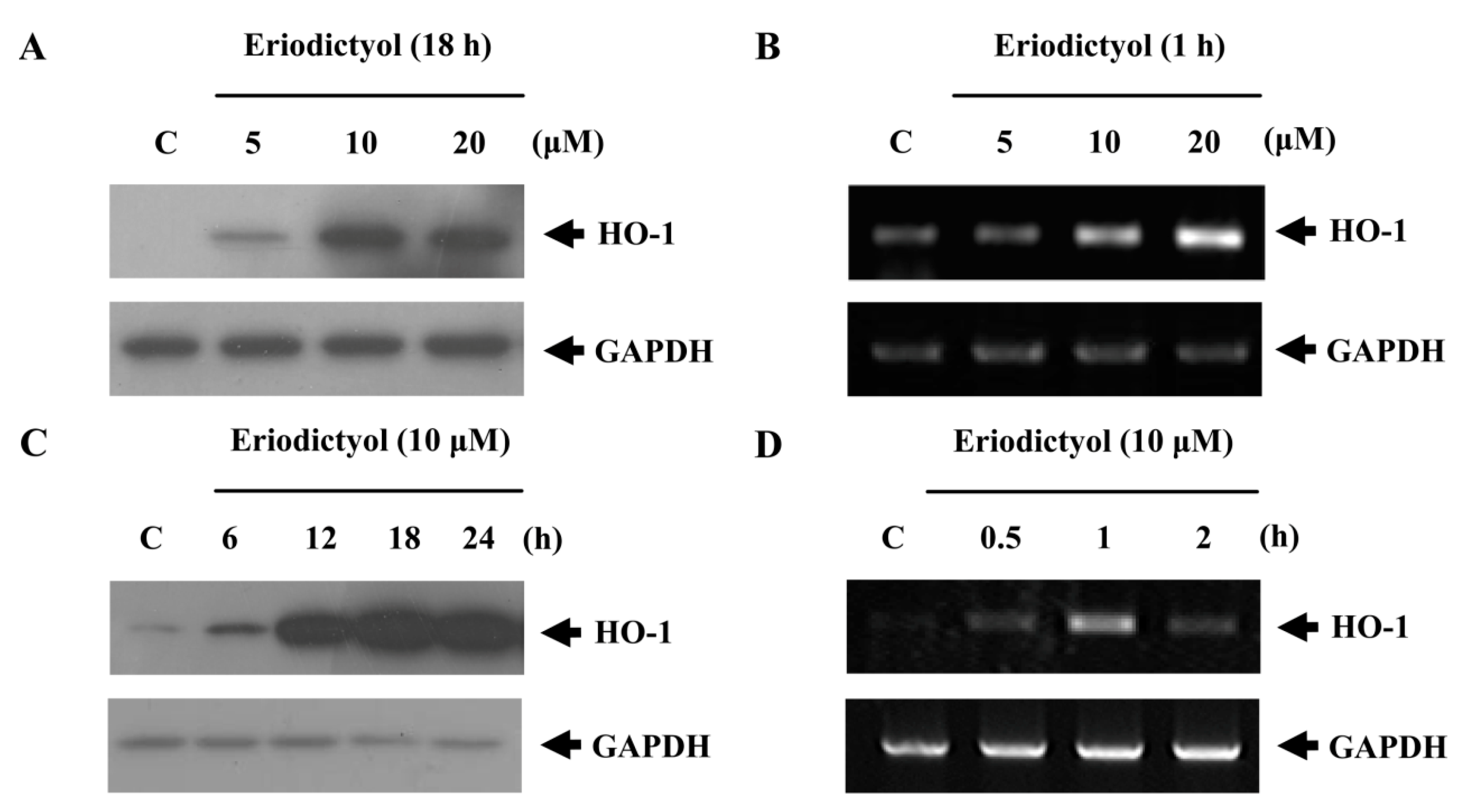
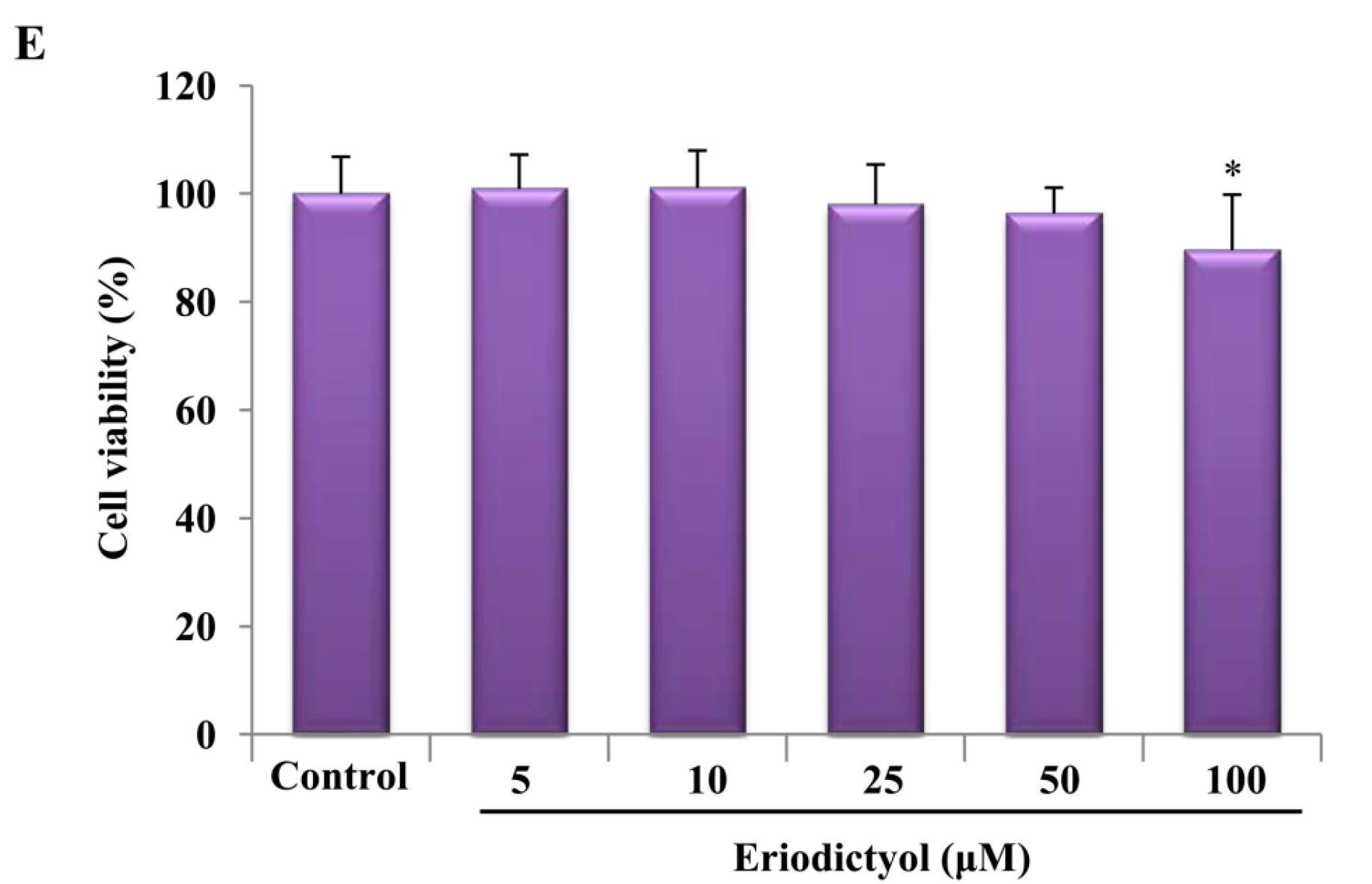
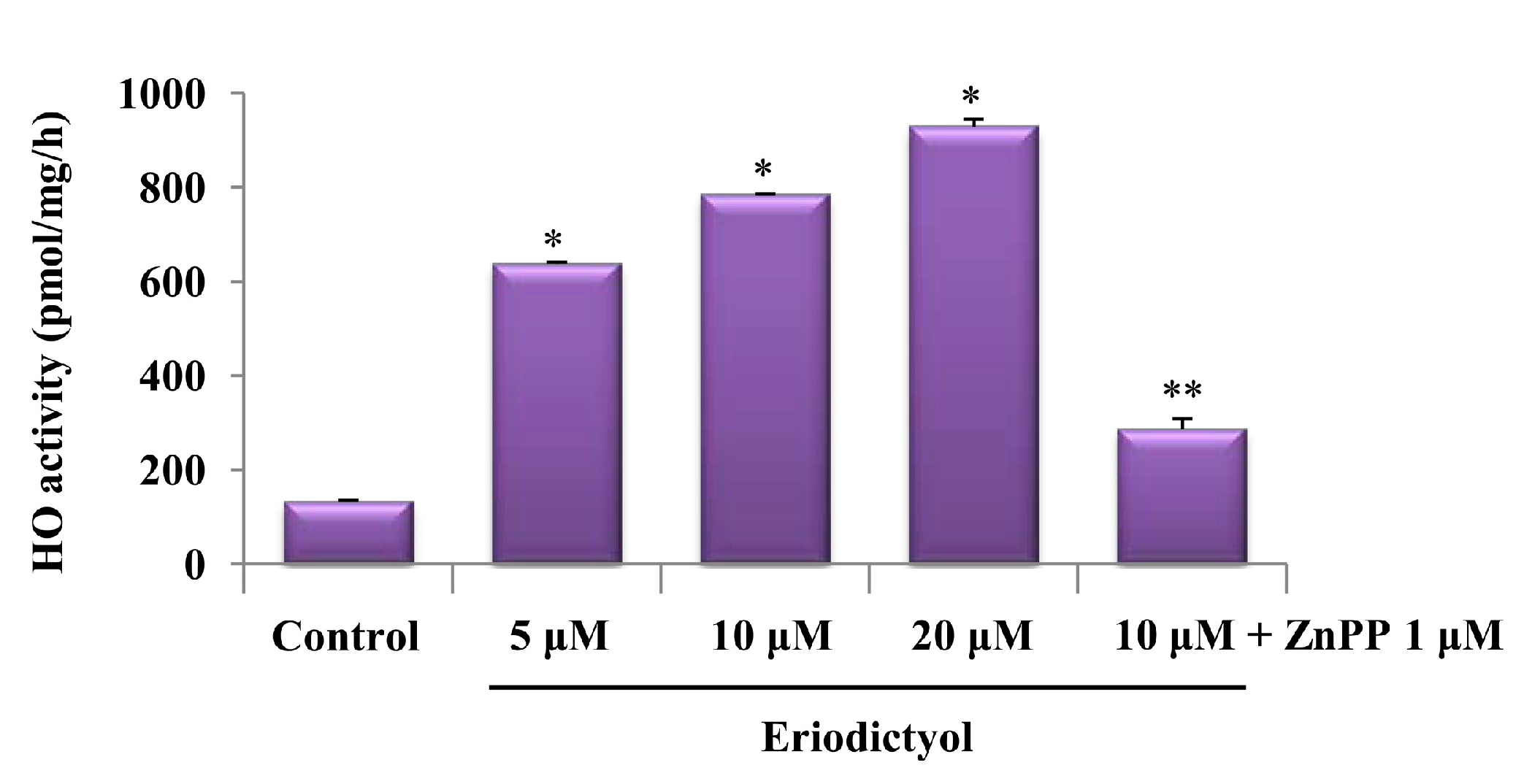
2.2. Blockage of Eriodictyol-Stimulated HO-1 Expression by ERK Inhibitor
 , dose increasing.
, dose increasing.
 , dose increasing.
, dose increasing.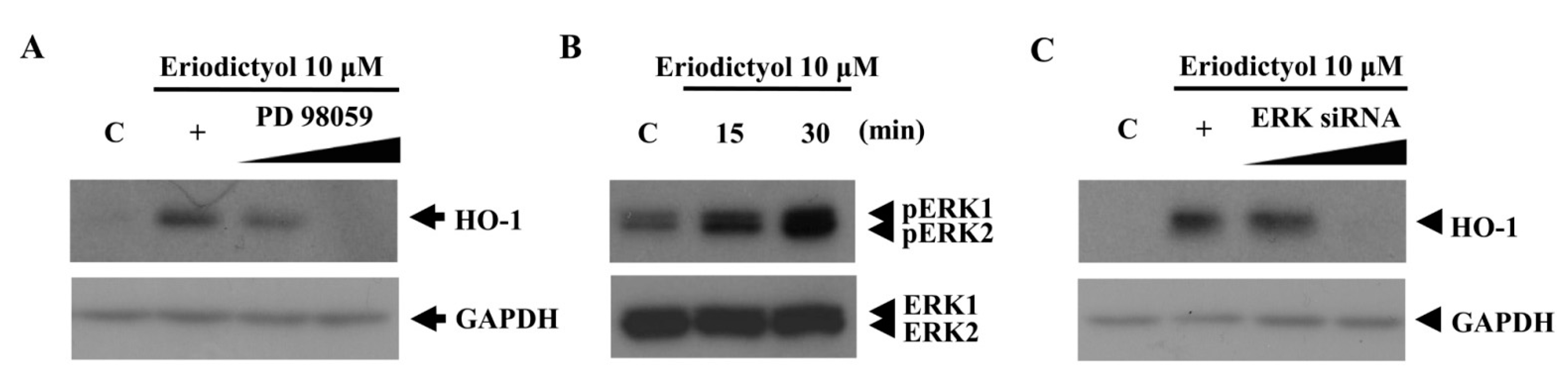
2.3. Eriodictyol Induced Nrf2 Nuclear Translocation and ARE-Luciferase Reporter Activity
 , dose increasing.
, dose increasing.
 , dose increasing.
, dose increasing.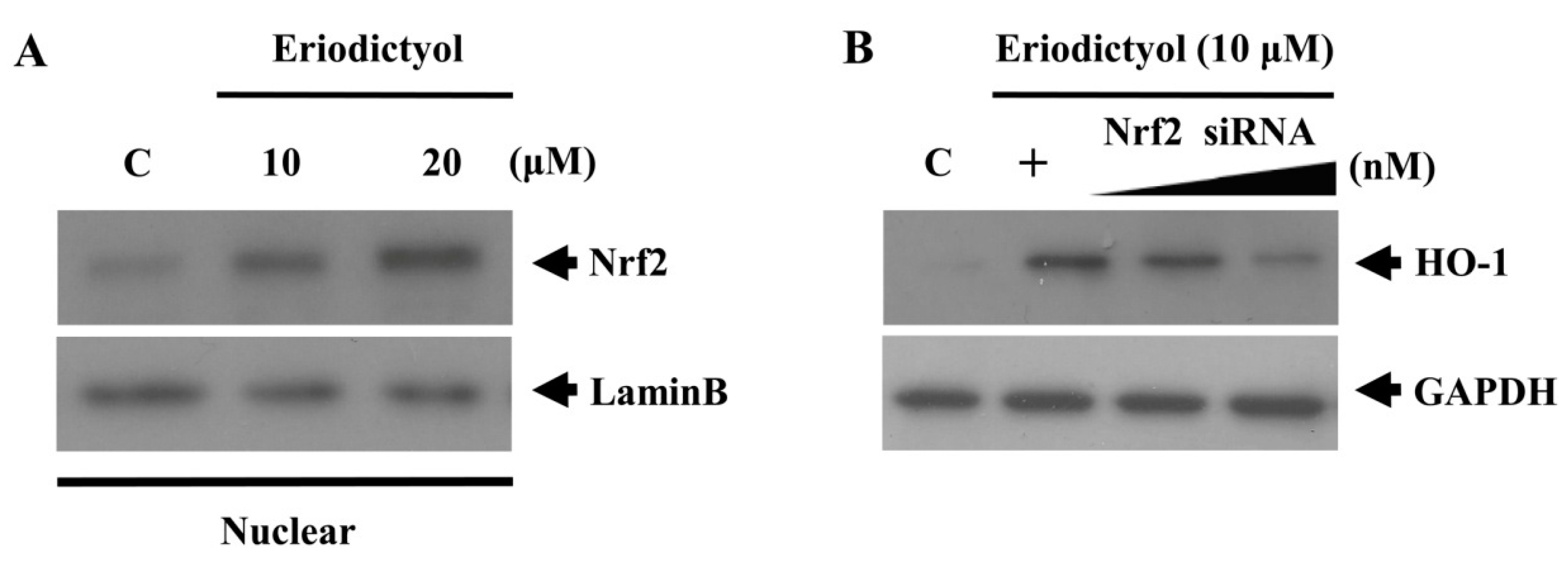
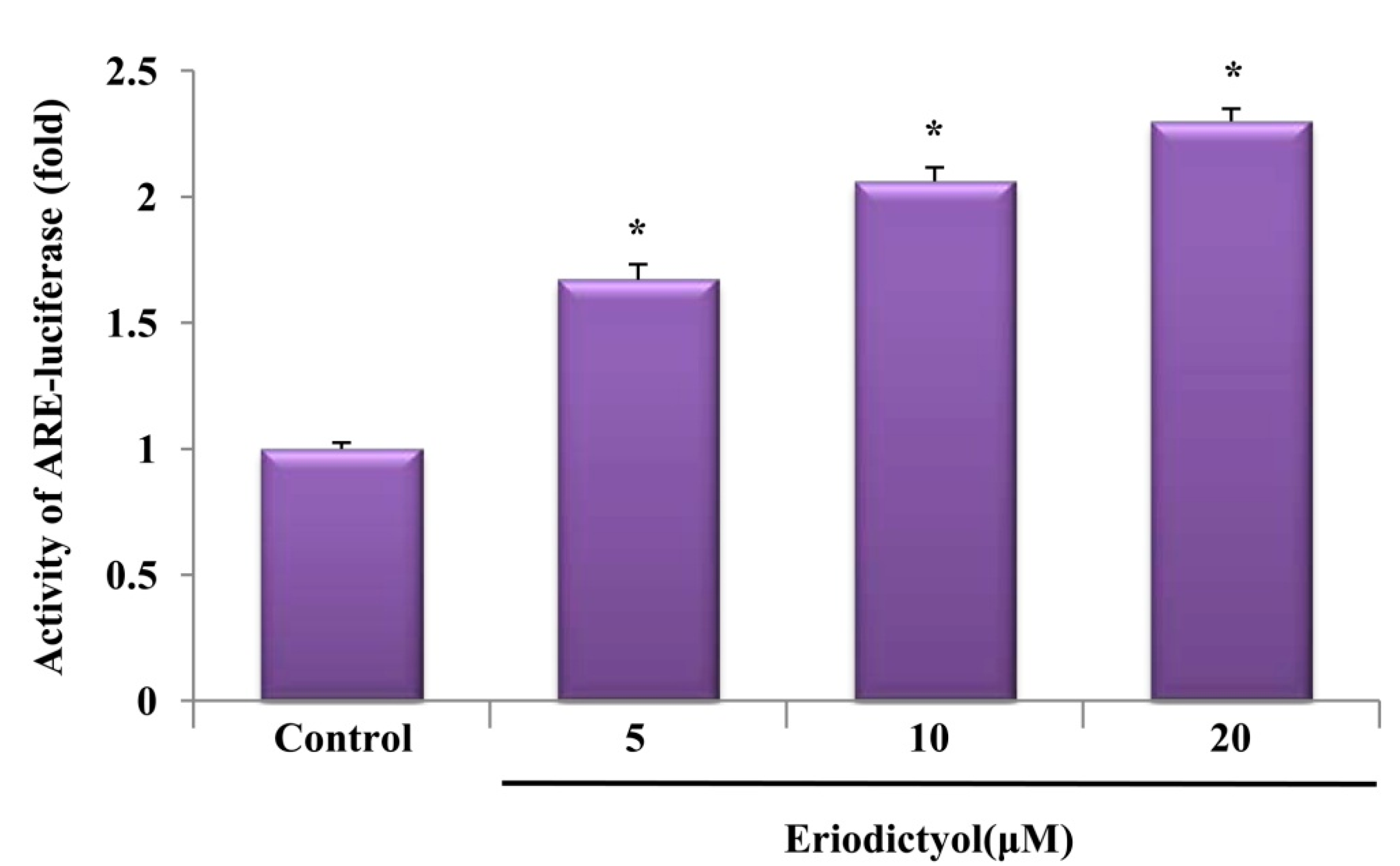
2.4. Protective Effect of Eriodictyol-Induced HO-1 against Oxidative Stress
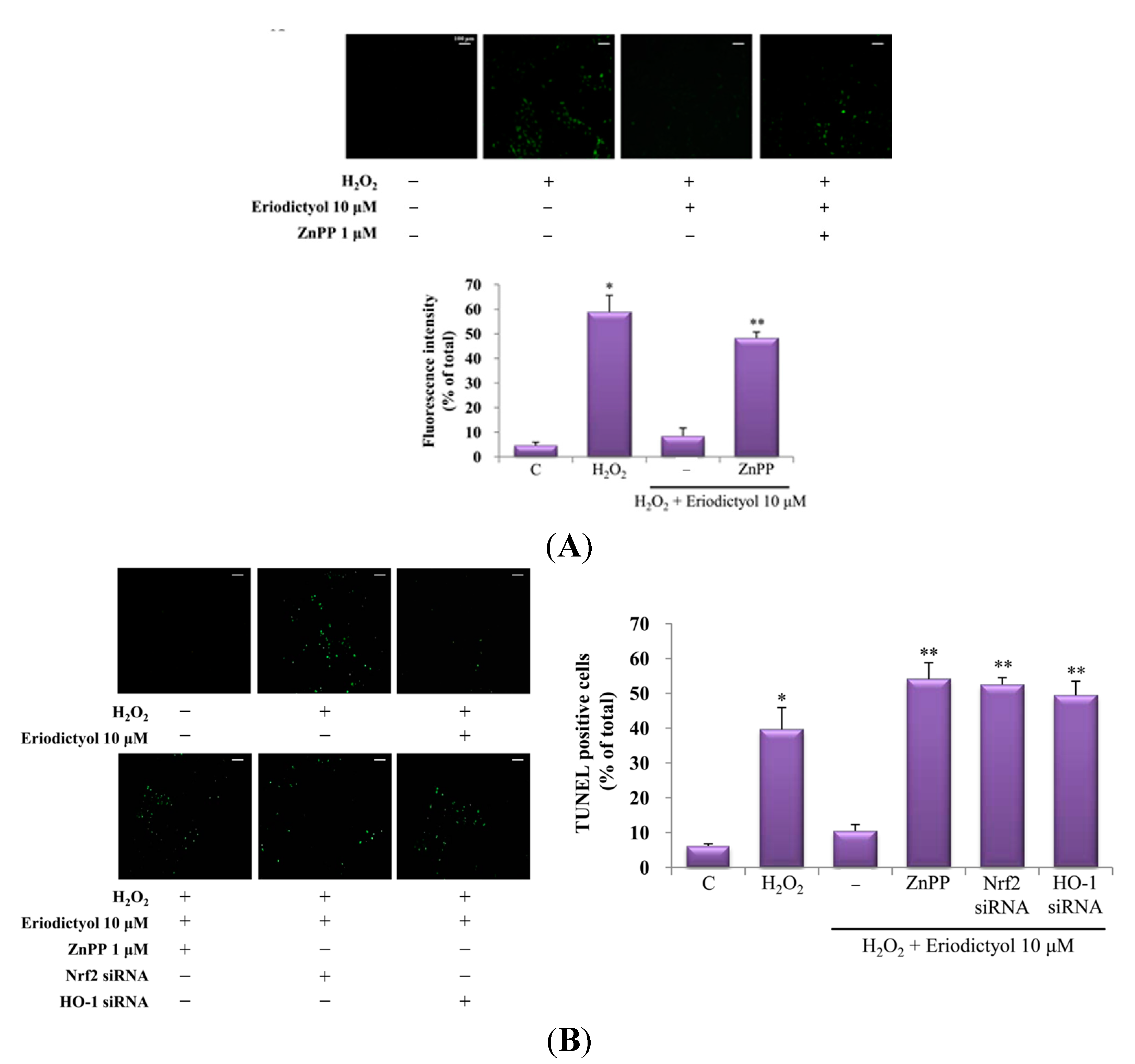
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Cell Culture and Viability Measurement
4.3. Western Blot Analysis
4.4. RNA Isolation and Reverse Transcriptase-Polymerase Chain Reaction
4.5. Assay for HO Activity
4.6. Nrf2 and ERK Silencing by siRNA
4.7. Preparation of Nuclear Proteins
4.8. Measurement of ARE Promoter Activity
4.9. Measurement of Intracellular Reactive Oxygen Species (ROS) Generation
4.10. Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End-Labeling (TUNEL) Assay
4.11. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Magenta, A.; Greco, S.; Gaetano, C.; Martelli, F. Oxidative stress and micrornas in vascular diseases. Int. J. Mol. Sci. 2013, 14, 17319–17346. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.; Morris, A.D.; Struthers, A.D. Lisinopril improves endothelial function in chronic cigarette smokers. Clin. Sci. 2001, 101, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Yang, C.S.; Pickett, C.B. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Radic. Biol. Med. 2004, 37, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ku, C.H.; Siow, R.C. Regulation of the Nrf2 antioxidant pathway by micrornas: New players in micromanaging redox homeostasis. Free Radic. Biol. Med. 2013, 64, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Ghattas, M.H.; Chuang, L.T.; Kappas, A.; Abraham, N.G. Protective effect of HO-1 against oxidative stress in human hepatoma cell line (HepG2) is independent of telomerase enzyme activity. Int. J. Biochem. Cell Biol. 2002, 34, 1619–1628. [Google Scholar] [CrossRef]
- Morse, D.; Choi, A.M. Heme oxygenase-1: The “Emerging molecule” has arrived. Am. J. Respir. Cell Mol. Biol. 2002, 27, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Onozuka, H.; Nakajima, A.; Matsuzaki, K.; Shin, R.W.; Ogino, K.; Saigusa, D.; Tetsu, N.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid, improves memory impairment and Aβ pathology in a transgenic mouse model of Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Barve, V.; Padhye, S.; Bhonde, R. Reduction of oxidative stress induced vanadium toxicity by complexing with a flavonoid, quercetin: A pragmatic therapeutic approach for diabetes. Biometals 2006, 19, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.R.; Liu, J.F.; Kang, J.; Chan, W.P. (2R,3S)-pinobanksin-3-cinnamate, a new flavonone from seeds of Alpinia galanga willd., presents in vitro neuroprotective effects. Mol. Cell. Toxicol. 2014, 10, 165–172. [Google Scholar] [CrossRef]
- Lee-Hilz, Y.Y.; Boerboom, A.M.; Westphal, A.H.; Berkel, W.J.; Aarts, J.M.; Rietjens, I.M. Pro-oxidant activity of flavonoids induces EpRE-mediated gene expression. Chem. Res. Toxicol. 2006, 19, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Ismaili, H.; Sosa, S.; Brkic, D.; Fkih-Tetouani, S.; Ilidrissi, A.; Touati, D.; Aquino, R.P.; Tubaro, A. Topical anti-inflammatory activity of extracts and compounds from thymus broussonettii. J. Pharm. Pharmacol. 2002, 54, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Minato, K.; Miyake, Y.; Fukumoto, S.; Yamamoto, K.; Kato, Y.; Shimomura, Y.; Osawa, T. Lemon flavonoid, eriocitrin, suppresses exercise-induced oxidative damage in rat liver. Life Sci. 2003, 72, 1609–1616. [Google Scholar] [CrossRef]
- Zhang, X.; Hung, T.M.; Phuong, P.T.; Ngoc, T.M.; Min, B.S.; Song, K.S.; Seong, Y.H.; Bae, K. Anti-inflammatory activity of flavonoids from populus davidiana. Arch. Pharm. Res. 2006, 29, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Narvaez-Mastache, J.M.; Novillo, F.; Delgado, G. Antioxidant aryl-prenylcoumarin, flavan-3-ols and flavonoids from Eysenhardtia subcoriacea. Phytochemistry 2008, 69, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Eriodictyol prevents early retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Biochem. Pharmacol. 2012, 84, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Yamamoto, K.; Tsujihara, N.; Osawa, T. Protective effects of lemon flavonoids on oxidative stress in diabetic rats. Lipids 1998, 33, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Hanneken, A.; Lin, F.F.; Johnson, J.; Maher, P. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress-induced death. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3164–3177. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Maher, P.; Hanneken, A. The flavonoid, eriodictyol, induces long-term protection in ARPE-19 cells through its effects on Nrf2 activation and phase II gene expression. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2398–2406. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K. Anti-inflammatory effects of eriodictyol in lipopolysaccharide-stimulated raw 264.7 murine macrophages. Arch. Pharm. Res. 2011, 34, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Jeong, K.W.; Shin, A.; Jin, B.; Jnawali, H.N.; Jun, B.H.; Lee, J.Y.; Heo, Y.S.; Kim, Y. Binding model for eriodictyol to Jun-N terminal kinase and its anti-inflammatory signaling pathway. Biochem. Mol. Biol. Rep. 2013, 46, 594–599. [Google Scholar] [CrossRef]
- Lou, H.; Jing, X.; Ren, D.; Wei, X.; Zhang, X. Eriodictyol protects against H2O2-induced neuron-like PC12 cell death through activation of Nrf2/ARE signaling pathway. Neurochem. Int. 2012, 61, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Cullinan, S.B.; Diehl, J.A. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 2004, 279, 20108–20117. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.A.; Jaiswal, A.K. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from Inrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H: Quinone oxidoreductase-1 gene expression. J. Biol. Chem. 2003, 278, 44675–44682. [Google Scholar] [PubMed]
- Kobayashi, M.; Yamamoto, M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid. Redox Signal. 2005, 7, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Park, Y.S. Korean Red Ginseng water extract inhibits COX-2 expression by suppressing p38 in acrolein-treated human endothelial cells. J. Ginseng Res. 2014, 38, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Joe, Y.; Zheng, M.; Kim, H.J.; Kim, S.; Uddin, M.J.; Park, C.; Ryu do, G.; Kang, S.S.; Ryoo, S.; Ryter, S.W.; et al. Salvianolic acid B exerts vasoprotective effects through the modulation of heme oxygenase-1 and arginase activities. J. Pharmacol. Exp. Ther. 2012, 341, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Jeong, S.I.; Kim, G.D.; Yang, H.; Park, C.S.; Jin, Y.H.; Park, Y.S. Upregulation of heme oxygenase-1 as an adaptive mechanism for protection against crotonaldehyde in human umbilical vein endothelial cells. Toxicol. Lett. 2011, 201, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Jeong, S.I.; Yang, H.; Jeong, S.H.; Jang, Y.P.; Park, C.S.; Kim, J.; Park, Y.S. Extract of Salvia miltiorrhiza (Danshen) induces Nrf2-mediated heme oxygenase-1 expression as a cytoprotective action in RAW 264.7 macrophages. J. Ethnopharmacol. 2012, 139, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Lian, T.W.; Wang, L.; Lo, Y.H.; Huang, I.J.; Wu, M.J. Fisetin, morin and myricetin attenuate CD36 expression and oxLDL uptake in U937-derived macrophages. Biochim. Biophys. Acta 2008, 1781, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Pullikotil, P.; Chen, H.; Muniyappa, R.; Greenberg, C.C.; Yang, S.; Reiter, C.E.; Lee, J.W.; Chung, J.H.; Quon, M.J. Epigallocatechin gallate induces expression of heme oxygenase-1 in endothelial cells via p38 MAPK and Nrf-2 that suppresses proinflammatory actions of TNF-α. J. Nutr. Biochem. 2012, 23, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Daleprane, J.B.; da Silva Freitas, V.; Pacheco, A.; Rudnicki, M.; Faine, L.A.; Dorr, F.A.; Ikegaki, M.; Salazar, L.A.; Ong, T.P.; Abdalla, D.S. Anti-atherogenic and anti-angiogenic activities of polyphenols from propolis. J. Nutr. Biochem. 2012, 23, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Hirose, E.; Matsushima, M.; Takagi, K.; Ota, Y.; Ishigami, K.; Hirayama, T.; Hayashi, Y.; Nakamura, T.; Hashimoto, N.; Imaizumi, K.; et al. Involvement of heme oxygenase-1 in kaempferol-induced anti-allergic actions in RBL-2H3 cells. Inflammation 2009, 32, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Juan, S.H.; Shen, S.C.; Hsu, F.L.; Chen, Y.C. Inhibition of lipopolysaccharide-induced nitric oxide production by flavonoids in RAW264.7 macrophages involves heme oxygenase-1. Biochem. Pharmacol. 2003, 66, 1821–1832. [Google Scholar] [CrossRef]
- Bower, A.M.; Real Hernandez, L.M.; Berhow, M.A.; de Mejia, E.G. Bioactive compounds from culinary herbs inhibit a molecular target for type 2 diabetes management, dipeptidyl peptidase IV. J. Agric. Food Chem. 2014, 62, 6147–6158. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.M.; Kim, J.H.; Park, S.J.; Kang, Y.J.; Kim, T.J. Inhibitory effect of eriodictyol on IgE/Ag-induced type I hypersensitivity. Biosci. Biotechnol. Biochem. 2012, 76, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, Y.H.; Lee, K.H.; Kim, T.J. Effect of eriodictyol on the development of atopic dermatitis-like lesions in ICR mice. Biol. Pharm. Bull. 2013, 36, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, C.; Shen, T.; Zhao, L.; Ren, D. R-eriodictyol and S-eriodictyol exhibited comparable effect against H2O2-induced oxidative stress in EA.Hy926 cells. Drug Discov. Ther. 2014, 8, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Kang, H.E.; Lee, M.G.; Hwang, S.J.; Kim, S.C.; Lee, C.H.; Kim, S.G. Liquiritigenin, a flavonoid aglycone from licorice, has a choleretic effect and the ability to induce hepatic transporters and phase-II enzymes. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G372–G381. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.E.; Hug, H.; Gossl, R.; Riss, G.; Mussler, B.; Elste, V.; Rimbach, G.; Barella, L. The natural compound ascorbigen modulates NADPH-quinone oxidoreductase (NQO1) mRNA and enzyme activity levels in cultured liver cells and in laboratory rats. Ann. Nutr. Metab. 2008, 53, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.M.; Alam, J. Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell Mol. Biol. 1996, 15, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.A.; Balla, G.; Vercellotti, G.M.; Balla, J.; Jacob, H.S.; Levitt, M.D.; Rosenberg, M.E. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J. Clin. Investig. 1992, 90, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Katsuki, H.; Unemura, K.; Izumi, Y.; Kume, T.; Takada-Takatori, Y.; Akaike, A. Heme oxygenase-1 contributes to pathology associated with thrombin-induced striatal and cortical injury in organotypic slice culture. Brain Res. 2010, 1347, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Jeong, S.I.; Yang, H.; Park, C.S.; Jin, Y.H.; Park, Y.S. Fisetin induces Nrf2-mediated HO-1 expression through PKC-delta and p38 in human umbilical vein endothelial cells. J. Cell. Biochem. 2011, 112, 2352–2360. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.M.; Shin, D.Y.; Lee, S.J.; Joe, Y.; Zheng, M.; Yim, J.H.; Callaway, Z.; Chung, H.T. Curcumin protects retinal pigment epithelial cells against oxidative stress via induction of heme oxygenase-1 expression and reduction of reactive oxygen. Mol. Vis. 2012, 18, 901–908. [Google Scholar] [PubMed]
- Alam, J.; Wicks, C.; Stewart, D.; Gong, P.; Touchard, C.; Otterbein, S.; Choi, A.M.; Burow, M.E.; Tou, J. Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells. Role of p38 kinase and Nrf2 transcription factor. J. Biol. Chem. 2000, 275, 27694–27702. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.E.; Yang, H.; Son, G.W.; Park, H.R.; Park, C.-S.; Jin, Y.-H.; Park, Y.S. Eriodictyol Protects Endothelial Cells against Oxidative Stress-Induced Cell Death through Modulating ERK/Nrf2/ARE-Dependent Heme Oxygenase-1 Expression. Int. J. Mol. Sci. 2015, 16, 14526-14539. https://doi.org/10.3390/ijms160714526
Lee SE, Yang H, Son GW, Park HR, Park C-S, Jin Y-H, Park YS. Eriodictyol Protects Endothelial Cells against Oxidative Stress-Induced Cell Death through Modulating ERK/Nrf2/ARE-Dependent Heme Oxygenase-1 Expression. International Journal of Molecular Sciences. 2015; 16(7):14526-14539. https://doi.org/10.3390/ijms160714526
Chicago/Turabian StyleLee, Seung Eun, Hana Yang, Gun Woo Son, Hye Rim Park, Cheung-Seog Park, Young-Ho Jin, and Yong Seek Park. 2015. "Eriodictyol Protects Endothelial Cells against Oxidative Stress-Induced Cell Death through Modulating ERK/Nrf2/ARE-Dependent Heme Oxygenase-1 Expression" International Journal of Molecular Sciences 16, no. 7: 14526-14539. https://doi.org/10.3390/ijms160714526
APA StyleLee, S. E., Yang, H., Son, G. W., Park, H. R., Park, C.-S., Jin, Y.-H., & Park, Y. S. (2015). Eriodictyol Protects Endothelial Cells against Oxidative Stress-Induced Cell Death through Modulating ERK/Nrf2/ARE-Dependent Heme Oxygenase-1 Expression. International Journal of Molecular Sciences, 16(7), 14526-14539. https://doi.org/10.3390/ijms160714526





