The Arginine/ADMA Ratio Is Related to the Prevention of Atherosclerotic Plaques in Hypercholesterolemic Rabbits When Giving a Combined Therapy with Atorvastatine and Arginine
Abstract
:1. Introduction

2. Results and Discussion
2.1. Results
2.1.1. Effect of Treatment on l-Arginine Levels
2.1.2. Effect of Treatment on ADMA and NO Levels
2.1.3. Effect of Treatment on Arginine/ADMA Ratio and Relation with Other Parameters
| Group | A (n = 9) | S (n = 8) | SA (n = 8) | C (n = 9) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | ||||
| Plasma levels l-arginine (µmol/L) | |||||||||||
| T0 | 463 | 39 | 367 | 33 | 371 | 13 | 460 | 51 | |||
| T8 | 388 a | 47 | 243 b | 28 | 476 a | 58 | 265 b | 14 | |||
| Plasma levels ADMA (µmol/L) | |||||||||||
| T0 ∞ | 1.14 | 0.11 | 1.02 | 0.03 | 1.15 | 0.05 | 1.11 | 0.05 | |||
| T8 | 0.88 | 0.05 | 0.97 | 0.05 | 1.03 | 0.05 | 0.99 | 0.03 | |||
| Arginine/ADMA ratio | |||||||||||
| T0 ∞ | 347 | 19 | 304 | 42 | 317 | 23 | 374 | 43 | |||
| T8 | 442 a | 46 | 252 b | 26 | 476 a | 54 | 261 b | 16 | |||
| NO levels | |||||||||||
| T0 | 110 | 20 | 88 | 9 | 103 | 10 | 108 | 16 | |||
| T8 | 156 | 35 | 82 | 8 | 104 | 10 | 105 | 10 | |||
| Total lesions in aorta (%) | |||||||||||
| – | 15.1 | 3.2 | 12.2 | 1.4 | 9.0 | 1.1 | 13.8 | 1.6 | |||
| Distal lesions in aorta (%) | |||||||||||
| – | 9.8 | 2.6 | 5.4 | 1.3 | 3.0 | 0.7 | 6.8 | 1.3 | |||
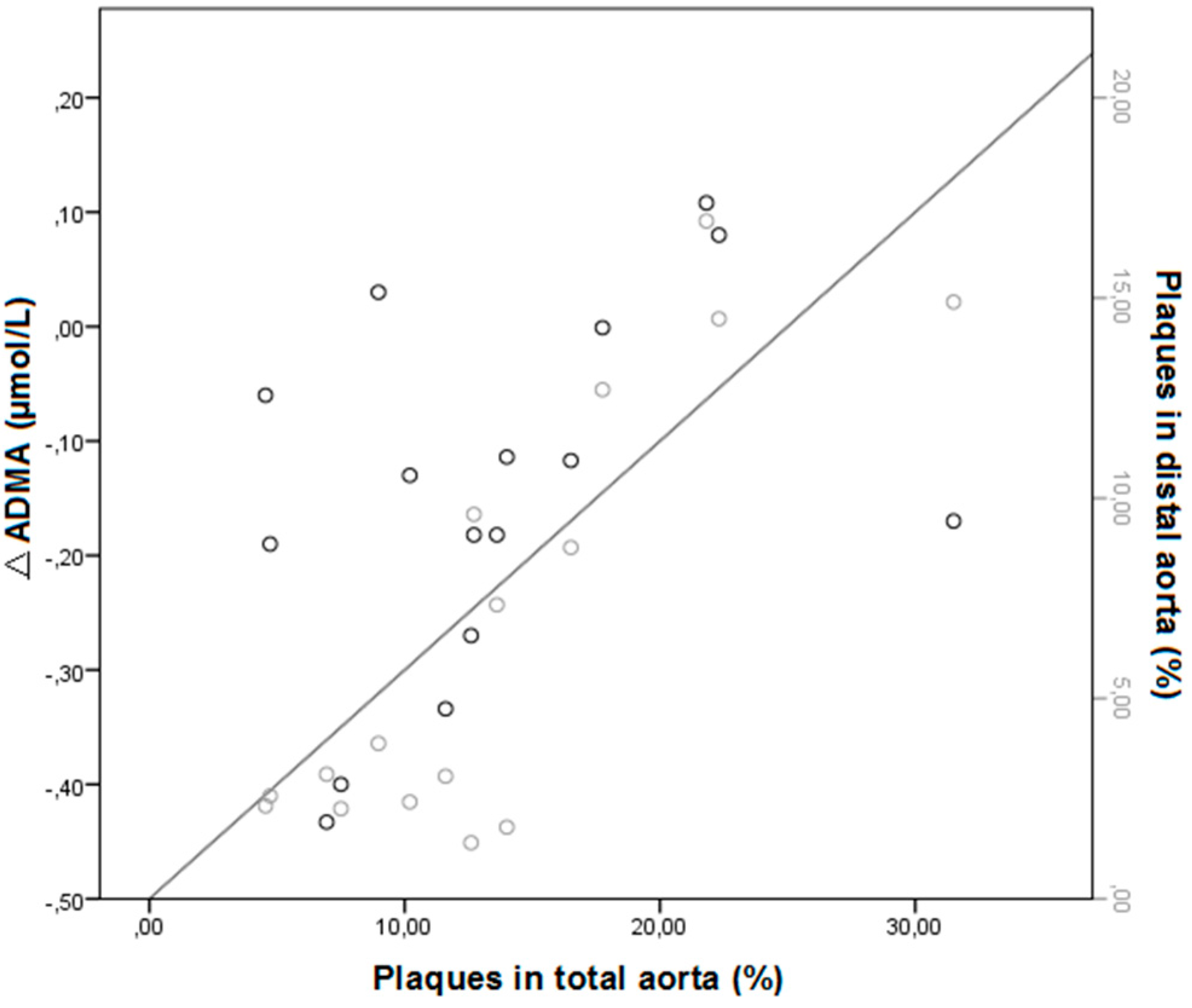
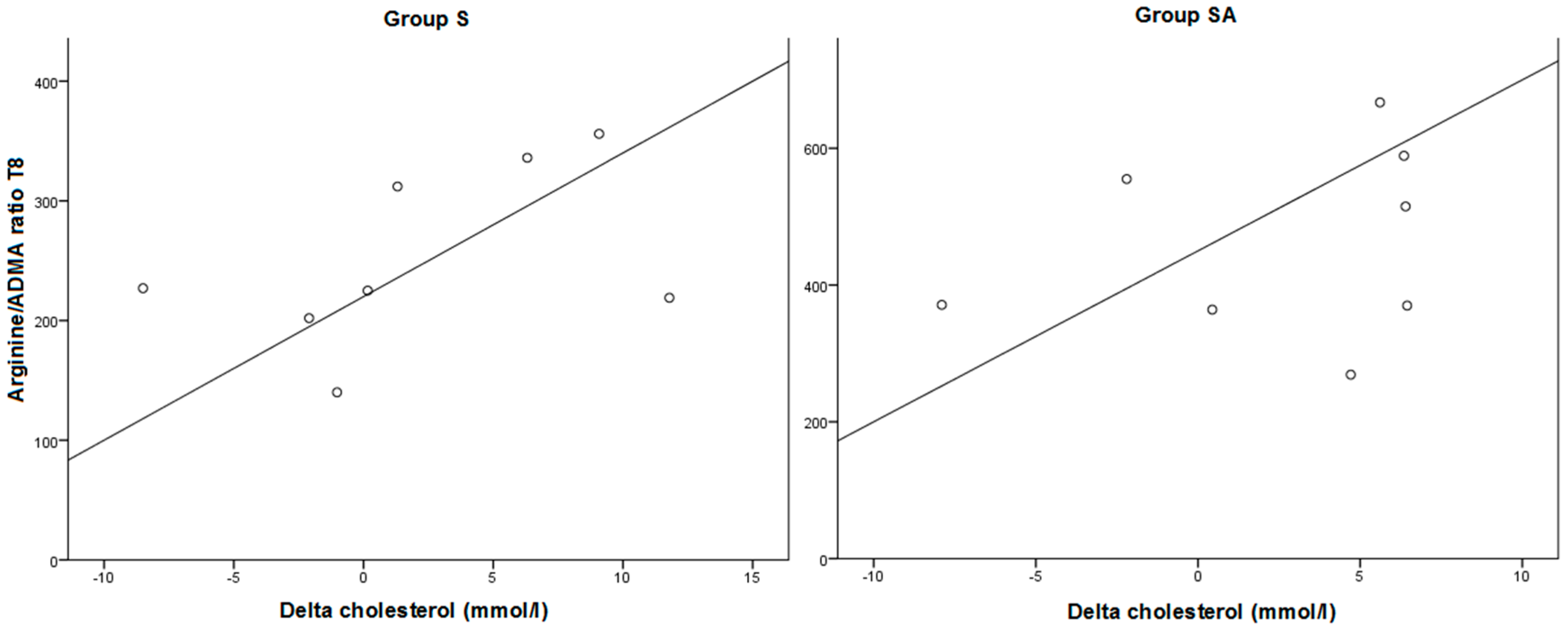
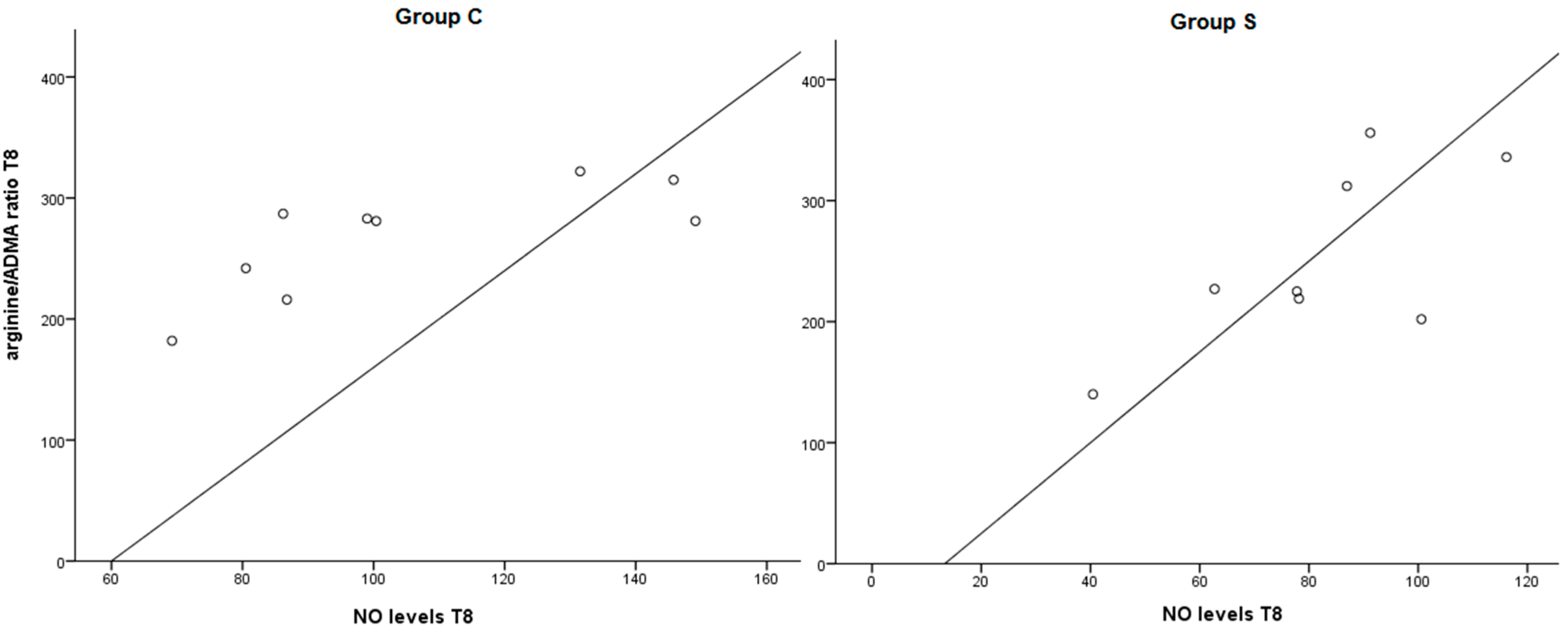
2.2. Discussion
3. Experimental Section
3.1. Treatment of Animals
3.2. Anatomy and Histological Analysis
3.3. Biochemical Analysis
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Tousoulis, D.; Kampoli, A.M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.G.; Negrao, C.E.; Krieger, M.H. Nitric oxide and the cardiovascular system: Cell activation, vascular reactivity and genetic variant. Arq. Bras. Cardiol. 2011, 96, 68–75. [Google Scholar] [PubMed]
- Napoli, C.; de Nigris, F.; Williams-Ignarro, S.; Pignalosa, O.; Sica, V.; Ignarro, L.J. Nitric oxide and atherosclerosis: An update. Nitric Oxide 2006, 15, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.M., Jr. Arginine metabolism: Boundaries of our knowledge. J. Nutr. 2007, 137, 1602S–1609S. [Google Scholar] [PubMed]
- Boger, R.H.; Bode-Boger, S.M.; Brandes, R.P.; Phivthong-Ngam, L.; Böhme, M.; Nafe, R.; Mügge, A.; Frölich, J.C. Dietary l-arginine reduces the progression of atherosclerosis in cholesterol-fed rabbits: Comparison with lovastatin. Circulation 1997, 96, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Leone, A.; Calver, A.; Collier, J.; Moncada, S. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. J. Cardiovasc. Pharmacol. 1992, 20 (Suppl. 12), S60–S62. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Leiper, J. Cardiovascular biology of the asymmetric dimethylarginine: Dimethylarginine dimethylaminohydrolase pathway. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Teerlink, T. ADMA metabolism and clearance. Vasc. Med. 2005, 10 (Suppl. 1), S73–S81. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, S. The biochemistry, measurement and current clinical significance of asymmetric dimethylarginine. Ann. Clin. Biochem. 2010, 47, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, S.J.; de Boer, M.C.; Buijs, N.; van Leeuwen, P.A. Asymmetric dimethylarginine and critical illness. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Bode-Boger, S.M.; Boger, R.H.; Kienke, S.; Junker, W.; Frolich, J.C. Elevated l-arginine/dimethylarginine ratio contributes to enhanced systemic NO production by dietary l-arginine in hypercholesterolemic rabbits. Biochem. Biophys. Res. Commun. 1996, 219, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; Voysey, M.; Gray, A.; Collins, R.; Baigent, C.; et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590. [Google Scholar] [PubMed]
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Simes, J.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [PubMed]
- Rasmusen, C.; Moinard, C.; Martin, C.; Tricottet, V.; Cynober, L.; Couderc, R. l-Arginine plus atorvastatin for prevention of atheroma formation in genetically hypercholesterolaemic rabbits. Br. J. Nutr. 2007, 97, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Bode-Boger, S.M.; Scalera, F.; Ignarro, L.J. The l-arginine paradox: Importance of the l-arginine/asymmetrical dimethylarginine ratio. Pharmacol. Ther. 2007, 114, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Notsu, Y.; Yano, S.; Shibata, H.; Nagai, A.; Nabika, T. Plasma arginine/ADMA ratio as a sensitive risk marker for atherosclerosis: Shimane CoHRE study. Atherosclerosis 2014, 239, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Vermeulen, M.A.; Richir, M.C.; Teerlink, T.; Houdijk, A.P.; Kostense, P.J.; Wisselink, W.; de Mol, B.A.; van Leeuwen, P.A.; Oudemans-van Straaten, H.M. Imbalance of arginine and asymmetric dimethylarginine is associated with markers of circulatory failure, organ failure and mortality in shock patients. Br. J. Nutr. 2012, 107, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.M.; Ding, Y.A.; Charng, M.J.; Lin, S.J. Asymmetrical dimethylarginine: A novel risk factor for coronary artery disease. Clin. Cardiol. 2003, 26, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Richir, M.C.; van Lambalgen, A.A.; Teerlink, T.; Wisselink, W.; Bloemena, E.; Prins, H.A.; de Vries, T.P.; van Leeuwen, P.A. Low arginine/asymmetric dimethylarginine ratio deteriorates systemic hemodynamics and organ blood flow in a rat model. Crit. Care Med. 2009, 37, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.; Adams, M.R.; Powe, A.J.; Donald, A.E.; McCredie, R.; Robinson, J.; McCarthy, S.N.; Keech, A.; Celermajer, D.S.; Deanfield, J.E. Oral l-arginine improves endothelium-dependent dilation in hypercholesterolemic young adults. J. Clin. Investig. 1996, 97, 1989–1994. [Google Scholar] [CrossRef] [PubMed]
- Boger, R.H.; Bode-Boger, S.M.; Mugge, A.; Kienke, S.; Brandes, R.; Dwenger, A.; Frölich, J.C. Supplementation of hypercholesterolaemic rabbits with l-arginine reduces the vascular release of superoxide anions and restores NO production. Atherosclerosis 1995, 117, 273–284. [Google Scholar] [CrossRef]
- Cooke, J.P.; Singer, A.H.; Tsao, P.; Zera, P.; Rowan, R.A.; Billingham, M.E. Antiatherogenic effects of l-arginine in the hypercholesterolemic rabbit. J. Clin. Investig. 1992, 90, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P.; Dzau, J.; Creager, A. Endothelial dysfunction in hypercholesterolemia is corrected by l-arginine. Basic Res. Cardiol. 1991, 86 (Suppl. 2), 173–181. [Google Scholar] [PubMed]
- Boger, R.H.; Bode-Boger, S.M.; Szuba, A.; Tsao, P.S; Chan, J.R.; Tangphao, O.; Blaschke, T.F.; Cooke, J.P. Asymmetric dimethylarginine (ADMA): A novel risk factor for endothelial dysfunction: Its role in hypercholesterolemia. Circulation 1998, 98, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Bode-Boger, S.M.; Muke, J.; Surdacki, A.; Brabant, G.; Boger, R.H.; Frolich, J.C. Oral l-arginine improves endothelial function in healthy individuals older than 70 years. Vasc. Med. 2003, 8, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Piatti, P.M.; Monti, L.D.; Valsecchi, G.; Magni, F.; Setola, E.; Marchesi, F.; Galli-Kienle, M.; Pozza, G.; Alberti, K.G. Long-term oral l-arginine administration improves peripheral and hepatic insulin sensitivity in type 2 diabetic patients. Diabetes Care 2001, 24, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, N.; Deb-Chatterji, M.; Dong, Q.; Kielstein, J.T.; Weissenborn, K.; Worthmann, H. Asymmetric dimethyarginine as marker and mediator in ischemic stroke. Int. J. Mol. Sci. 2012, 13, 15983–16004. [Google Scholar] [CrossRef] [PubMed]
- Boger, R.H.; Bode-Boger, S.M.; Sydow, K.; Heistad, D.D.; Lentz, S.R. Plasma concentration of asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, is elevated in monkeys with hyperhomocyst(e)inemia or hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Betz, B.; Moller-Ehrlich, K.; Kress, T.; Kniepert, J.; Schwedhelm, E.; Böger, R.H.; Wanner, C.; Sauvant, C.; Schneider, R. Increased symmetrical dimethylarginine in ischemic acute kidney injury as a causative factor of renal l-arginine deficiency. Transl. Res. 2013, 162, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ivashchenko, C.Y.; Bradley, B.T.; Ao, Z.; Leiper, J.; Vallance, P.; Johns, D.G. Regulation of the ADMA-DDAH system in endothelial cells: A novel mechanism for the sterol response element binding proteins, SREBP1c and -2. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H251–H258. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, W.; Feng, W.; Qu, X. Effects of rosuvastatin on serum asymmetric dimethylarginine levels and atrial structural remodeling in atrial fibrillation dogs. Pacing Clin. Electrophysiol. 2012, 35, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Vladimirova-Kitova, L.G.; Deneva-Koycheva, T.I. The effect of simvastatin on asymmetric dimethylarginine and flow-mediated vasodilation after optimizing the LDL level: A randomized, placebo-controlled study. Vascul. Pharmacol. 2012, 56, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Vladimirova-Kitova, L.G.; Deneva, T.I. Simvastatin and asymmetric dimethylarginine-homocysteine metabolic pathways in patients with newly detected severe hypercholesterolemia. Clin. Lab. 2010, 56, 291–302. [Google Scholar] [PubMed]
- Xia, W.; Yin, Z.; Li, J.; Song, Y.; Qu, X. Effects of rosuvastatin on asymmetric dimethylarginine levels and early atrial fibrillation recurrence after electrical cardioversion. Pacing Clin. Electrophysiol. 2009, 32, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Oguz, A.; Uzunlulu, M. Short term fluvastatin treatment lowers serum asymmetric dimethylarginine levels in patients with metabolic syndrome. Int. Heart J. 2008, 49, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Sicard, P.; Delemasure, S.; Korandji, C.; Segueira-Le Grand, A.; Lauzier, B.; Guilland, J.C.; Duvillard, L.; Zeller, M.; Cottin, Y.; Vergely, C.; et al. Anti-hypertensive effects of Rosuvastatin are associated with decreased inflammation and oxidative stress markers in hypertensive rats. Free Radic. Res. 2008, 42, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Katayama, Y.; Katsumata, T.; Otori, T.; Nishiyama, Y. Effects of long-term administration of HMG-CoA reductase inhibitor, atorvastatin, on stroke events and local cerebral blood flow in stroke-prone spontaneously hypertensive rats. Brain Res. 2007, 1169, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Xia, K.; Zhao, Z.; Deng, X.; Yang, T. Atorvastatin modulates the DDAH1/ADMA system in high-fat diet-induced insulin-resistant rats with endothelial dysfunction. Vasc. Med. 2012, 17, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Ueda, M.; Otsuka, T.; Katsura, K.; Abe, A.; Nagayama, H.; Katayama, Y. Statin treatment decreased serum asymmetric dimethylarginine (ADMA) levels in ischemic stroke patients. J. Atheroscler. Thromb. 2011, 18, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.M.; Ding, Y.A.; Leu, H.B.; Yin, W.H.; Sheu, W.H.; Chu, K.M. Effect of rosuvastatin on plasma levels of asymmetric dimethylarginine in patients with hypercholesterolemia. Am. J. Cardiol. 2004, 94, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Eritsland, J.; Larsen, J.; Arnesen, H.; Seljeflot, I. Increased levels of asymmetric dimethylarginine in populations at risk for atherosclerotic disease. Effects of pravastatin. Atherosclerosis 2003, 166, 279–284. [Google Scholar] [CrossRef]
- Young, J.M.; Strey, C.H.; George, P.M.; Florkowski, C.M.; Sies, C.W.; Frampton, C.M.; Scott, R.S. Effect of atorvastatin on plasma levels of asymmetric dimethylarginine in patients with non-ischaemic heart failure. Eur. J. Heart Fail. 2008, 10, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Valkonen, V.P.; Laakso, J.; Paiva, H.; Lehtimäki, T.; Lakka, T.A.; Isomustajärvi, M.; Ruokonen, I.; Salonen, J.T.; Laaksonen, R. Asymmetrical dimethylarginine (ADMA) and risk of acute coronary events. Does statin treatment influence plasma ADMA levels? Atheroscler. Suppl. 2003, 4, 19–22. [Google Scholar] [CrossRef]
- Paiva, H.; Laakso, J.; Lehtimaki, T.; Isomustajarvi, M.; Ruokonen, I.; Laaksonen, R. Effect of high-dose statin treatment on plasma concentrations of endogenous nitric oxide synthase inhibitors. J. Cardiovasc. Pharmacol. 2003, 41, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Laufs, U. Beyond lipid-lowering: Effects of statins on endothelial nitric oxide. Eur. J. Clin. Pharmacol. 2003, 58, 719–731. [Google Scholar] [PubMed]
- John, S.; Schneider, M.P.; Delles, C.; Jacobi, J.; Schmieder, R.E. Lipid-independent effects of statins on endothelial function and bioavailability of nitric oxide in hypercholesterolemic patients. Am. Heart J. 2005, 149, 473. [Google Scholar] [CrossRef] [PubMed]
- Maeso, R.; Aragoncillo, P.; Navarro-Cid, J.; Ruilope, L.M.; Diaz, C.; Hernández, G.; Lahera, V.; Cachofeiro, V. Effect of atorvastatin on endothelium-dependent constrictor factors in dyslipidemic rabbits. Gen. Pharmacol. 2000, 34, 263–272. [Google Scholar] [CrossRef]
- Neveux, N.; David, P.; Cynober, L. Measurement ofamino acid concentrations in biological fluids using ion exchange chromatography. In Metabolic and Therapeutic Aspects of Amino Acids in Clinical Nutrition, 2rd ed.; Cynober, L., Ed.; CRC Press: BocaRaton, FL, USA, 2004; pp. 17–28. [Google Scholar]
- Ricart-Jane, D.; Llobera, M.; Lopez-Tejero, M.D. Anticoagulants and other preanalytical factors interfere in plasma nitrate/nitrite quantification by the Griess method. Nitric Oxide 2002, 6, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brinkmann, S.J.H.; Wörner, E.A.; Buijs, N.; Richir, M.; Cynober, L.; Van Leeuwen, P.A.M.; Couderc, R. The Arginine/ADMA Ratio Is Related to the Prevention of Atherosclerotic Plaques in Hypercholesterolemic Rabbits When Giving a Combined Therapy with Atorvastatine and Arginine. Int. J. Mol. Sci. 2015, 16, 12230-12242. https://doi.org/10.3390/ijms160612230
Brinkmann SJH, Wörner EA, Buijs N, Richir M, Cynober L, Van Leeuwen PAM, Couderc R. The Arginine/ADMA Ratio Is Related to the Prevention of Atherosclerotic Plaques in Hypercholesterolemic Rabbits When Giving a Combined Therapy with Atorvastatine and Arginine. International Journal of Molecular Sciences. 2015; 16(6):12230-12242. https://doi.org/10.3390/ijms160612230
Chicago/Turabian StyleBrinkmann, Saskia J. H., Elisabeth A. Wörner, Nikki Buijs, Milan Richir, Luc Cynober, Paul A. M. Van Leeuwen, and Rémy Couderc. 2015. "The Arginine/ADMA Ratio Is Related to the Prevention of Atherosclerotic Plaques in Hypercholesterolemic Rabbits When Giving a Combined Therapy with Atorvastatine and Arginine" International Journal of Molecular Sciences 16, no. 6: 12230-12242. https://doi.org/10.3390/ijms160612230
APA StyleBrinkmann, S. J. H., Wörner, E. A., Buijs, N., Richir, M., Cynober, L., Van Leeuwen, P. A. M., & Couderc, R. (2015). The Arginine/ADMA Ratio Is Related to the Prevention of Atherosclerotic Plaques in Hypercholesterolemic Rabbits When Giving a Combined Therapy with Atorvastatine and Arginine. International Journal of Molecular Sciences, 16(6), 12230-12242. https://doi.org/10.3390/ijms160612230





