Anesthetic Propofol Overdose Causes Vascular Hyperpermeability by Reducing Endothelial Glycocalyx and ATP Production
Abstract
:1. Introduction
2. Results and Discussion
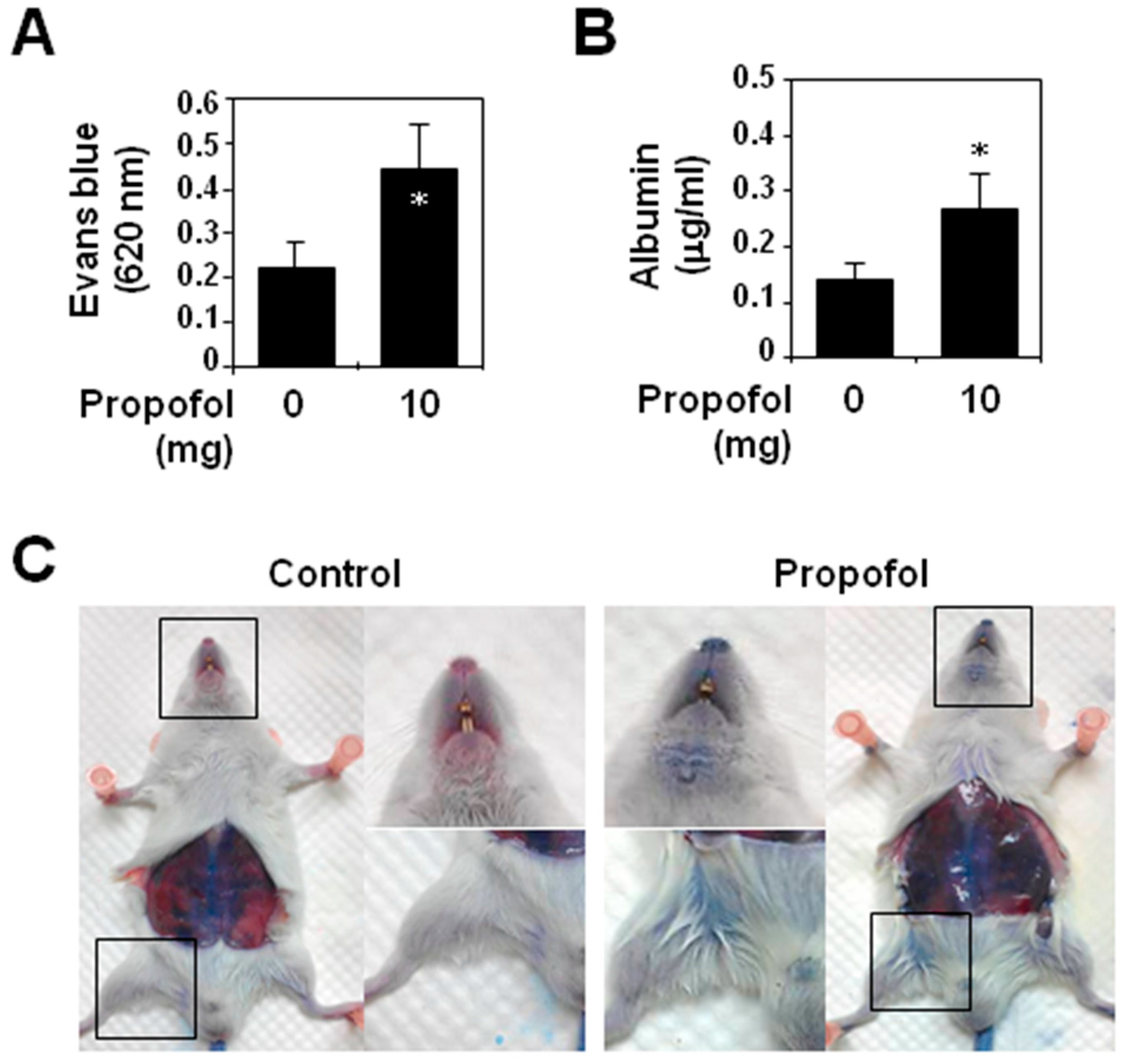
2.1. Analysis of Vascular Permeability in Propofol-Overdosed ICR Mice
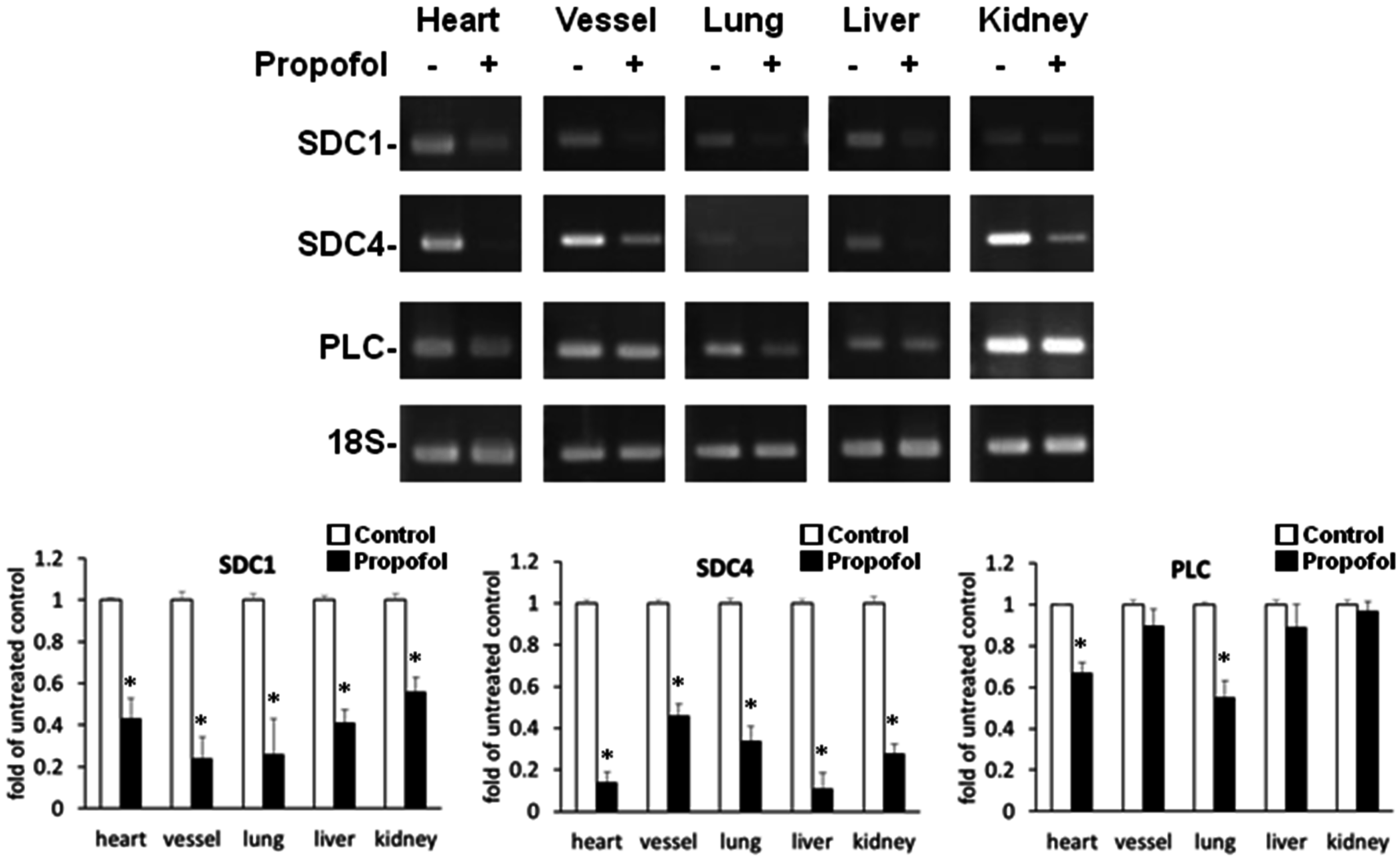
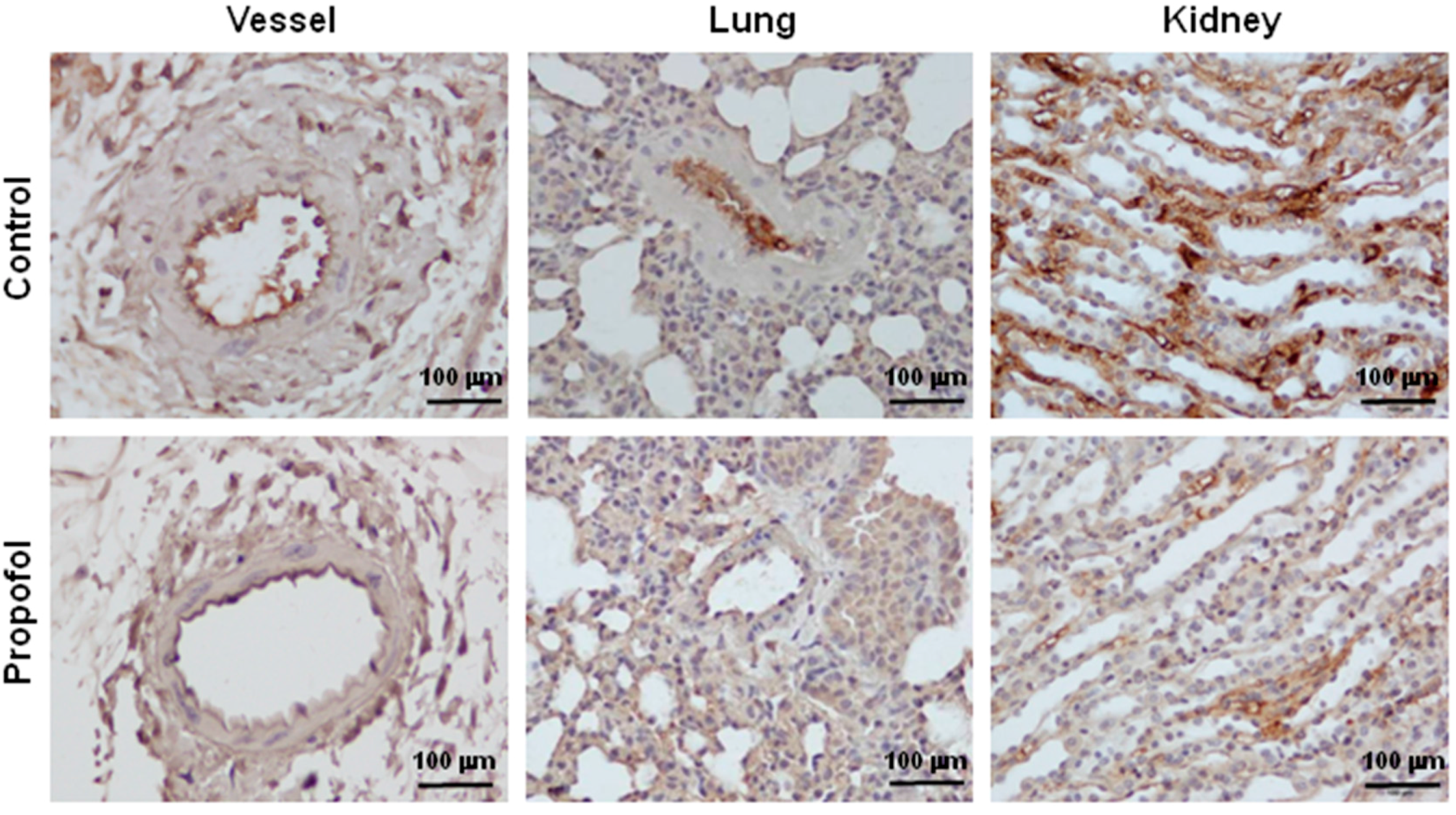
2.2. Endothelial Glycocalyx Expression in Propofol-Overdosed ICR Mice
2.3. Endothelial Glycocalyx in Propofol-Overdosed HMEC-1 Cells
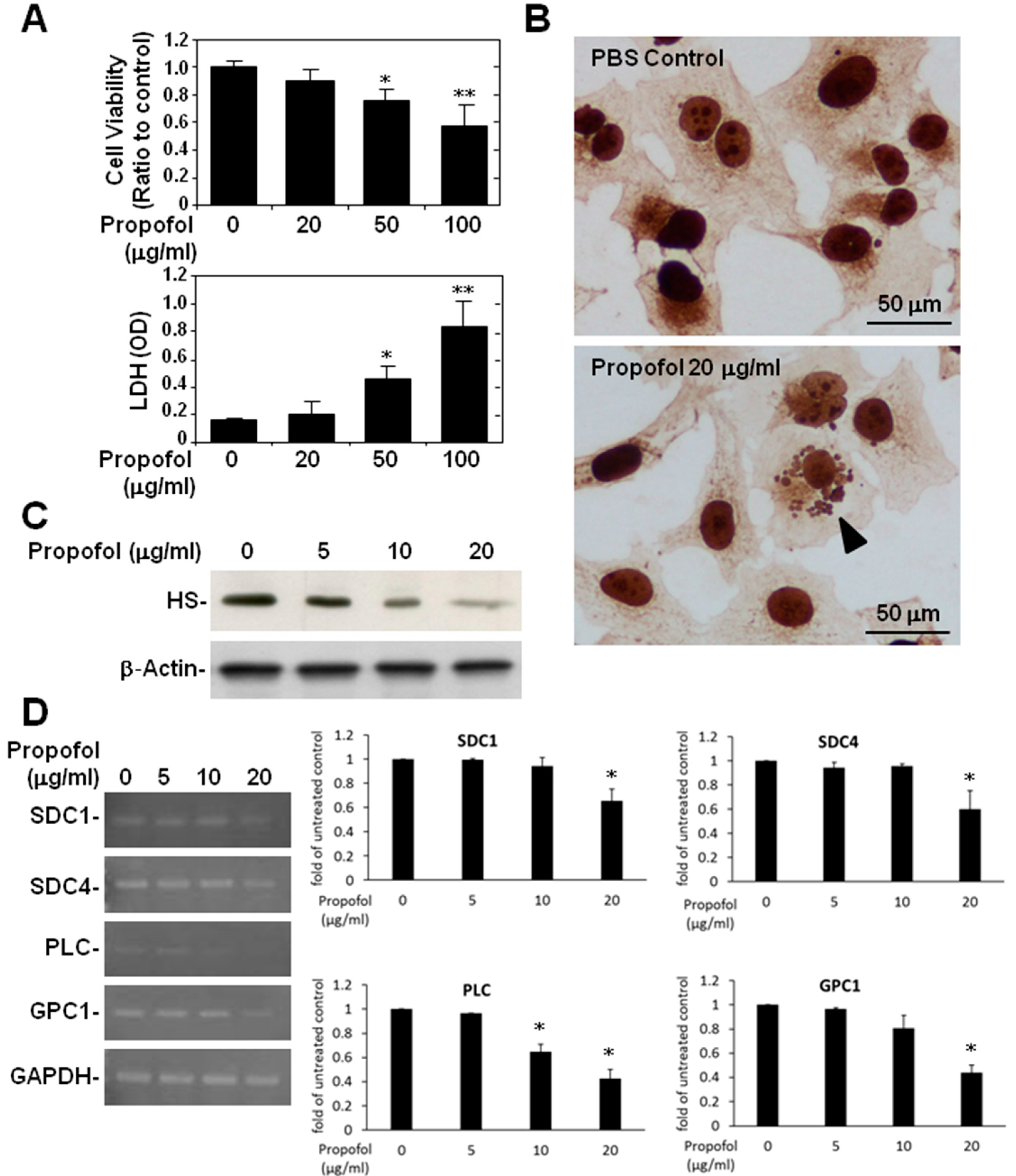
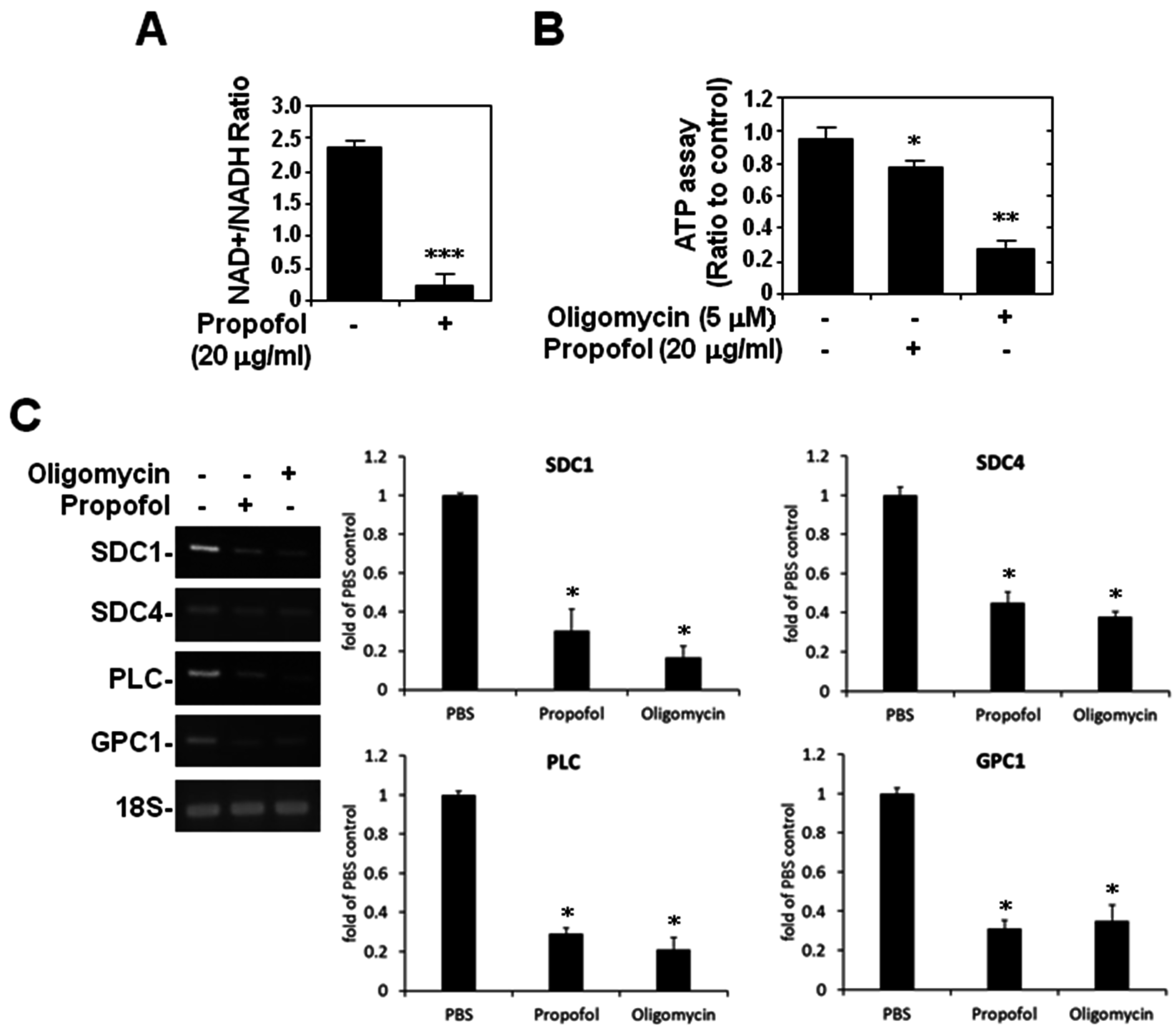
2.4. ATP Production and Glycocalyx Expression in Propofol-Overdosed HMEC-1 Cells
3. Experimental Section
3.1. Reagents and Antibodies
3.2. Cell Cultures
3.3. Animal Experiments
3.4. Reverse Transcriptase-Polymerase Chain Reaction
3.5. Viability Assay
| Gene | Forward | Reverse |
|---|---|---|
| 18S | AGCTCCCGAAAGCGACGTT | ATCTTGCAAAGCACCTGCAC |
| GAPDH | GGCGATGCTGGCGCTGAGTA | ACAGTTTCCCGGAGGGGCCA |
| SDC-1 (human) | GAGTTCTACGCCTGATGGGG | CGACAGGTGTGGTTGTGGTA |
| (mouse) | GGACCTCCTAGAAGGCCGATA | AGTTTCTTGGGTTCGGTGGG |
| SDC-4 (human) | CCCGGAGAGTCGATTCGAGA | GAGCTGCCAAGACCTCAGTT |
| (mouse) | TTGAGCTCGTCCCACAACGA | TGGTTCAGAGCACCAGGTTG |
| PLC (human) | CACACACCGACCACATACCA | CTCTGCCTAGCGATTCTGGG |
| (mouse) | AGTTCTGGGGTACATCGGGT | TCATGTCCGGCTTGGTGATG |
| GPC-1 (human) | CGGCTTTTGTTGTCTCCGC | GCATATAGGTCCCGGAAGGC |
| (mouse) | AGCTCCCGAAAGCGACGTT | ATCTTGCAAAGCACCTGCAC |
3.6. Cytotoxicity Assay
3.7. Immunohistochemistry and Immunocytochemistry
3.8. Western Blotting
3.9. NAD+/NADH Assay
3.10. ATP Assay
3.11. Transmission Electron Microscopy
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shafer, A.; Doze, V.A.; Shafer, S.L.; White, P.F. Pharmacokinetics and pharmacodynamics of propofol infusions during general anesthesia. Anesthesiology 1988, 69, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Fudickar, A.; Bein, B. Propofol infusion syndrome: Update of clinical manifestation and pathophysiology. Minerva Anestesiol. 2009, 75, 339–344. [Google Scholar] [PubMed]
- Branca, D.; Roberti, M.S.; Lorenzin, P.; Vincenti, E.; Scutari, G. Influence of the anesthetic 2,6-diisopropylphenol on the oxidative phosphorylation of isolated rat liver mitochondria. Biochem. Pharmacol. 1991, 42, 87–90. [Google Scholar] [CrossRef]
- Schenkman, K.A.; Yan, S. Propofol impairment of mitochondrial respiration in isolated perfused guinea pig hearts determined by reflectance spectroscopy. Crit. Care Med. 2000, 28, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Vanlander, A.V.; Okun, J.G.; de Jaeger, A.; Smet, J.; de Latter, E.; de Paepe, B.; Dacremont, G.; Wuyts, B.; Vanheel, B.; de Paepe, P.; et al. Possible pathogenic mechanism of propofol infusion syndrome involves coenzyme Q. Anesthesiology 2015, 122, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Vasile, B.; Rasulo, F.; Candiani, A.; Latronico, N. The pathophysiology of propofol infusion syndrome: A simple name for a complex syndrome. Intensive Care Med. 2003, 29, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Chen, K.H.; Liu, C.C.; Lee, C.T.; Yang, C.H.; Chuang, K.C.; Lin, C.R. Propofol-induced vascular permeability change is related to the nitric oxide signaling pathway and occludin phosphorylation. J. Biomed. Sci. 2007, 14, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, N.I.; Schuetz, P.; Yano, K.; Sorasaki, M.; Parikh, S.M.; Jones, A.E.; Trzeciak, S.; Ngo, L.; Aird, W.C. The association of endothelial cell signaling, severity of illness, and organ dysfunction in sepsis. Crit. Care 2010, 14, R182. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Chen, C.L.; Yang, T.T.; Choi, P.C.; Hsing, C.H.; Lin, C.F. Anesthetic propofol overdose causes endothelial cytotoxicity in vitro and endothelial barrier dysfunction in vivo. Toxicol. Appl. Pharmacol. 2012, 265, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Pries, A.R.; Kuebler, W.M. Normal endothelium. Handb. Exp. Pharmacol. 2006, 176, 1–40. [Google Scholar] [PubMed]
- Chappell, D.; Jacob, M.; Paul, O.; Rehm, M.; Welsch, U.; Stoeckelhuber, M.; Conzen, P.; Becker, B.F. The glycocalyx of the human umbilical vein endothelial cell: An impressive structure ex vivo but not in culture. Circ. Res. 2009, 104, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Chappell, D.; Jacob, M.; Hofmann-Kiefer, K.; Bruegger, D.; Rehm, M.; Conzen, P.; Welsch, U.; Becker, B.F. Hydrocortisone preserves the vascular barrier by protecting the endothelial glycocalyx. Anesthesiology 2007, 107, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Chappell, D.; Jacob, M.; Becker, B.F.; Hofmann-Kiefer, K.; Conzen, P.; Rehm, M. Expedition glycocalyx. A newly discovered “great barrier reef”. Anaesthesist 2008, 57, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Tarbell, J.M.; Pahakis, M.Y. Mechanotransduction and the glycocalyx. J. Intern. Med. 2006, 259, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Nieuwdorp, M.; Meuwese, M.C.; Vink, H.; Hoekstra, J.B.; Kastelein, J.J.; Stroes, E.S. The endothelial glycocalyx: A potential barrier between health and vascular disease. Curr. Opin. Lipidol. 2005, 16, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Rehm, M.; Zahler, S.; Lotsch, M.; Welsch, U.; Conzen, P.; Jacob, M.; Becker, B.F. Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology 2004, 100, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Kaji, T.; Yamada, A.; Miyajima, S.; Yamamoto, C.; Fujiwara, Y.; Wight, T.N.; Kinsella, M.G. Cell density-dependent regulation of proteoglycan synthesis by transforming growth factor-β1 in cultured bovine aortic endothelial cells. J. Biol. Chem. 2000, 275, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Lipowsky, H.H.; Lescanic, A. The effect of doxycycline on shedding of the glycocalyx due to reactive oxygen species. Microvasc. Res. 2013, 90, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Lan, Y.P.; Tang, H.F.; Zhu, S.M. Propofol pretreatment attenuates aquaporin-4 over-expression and alleviates cerebral edema after transient focal brain ischemia reperfusion in rats. Anesth. Analg. 2008, 107, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Zhang, J.; Xu, J.; Sheng, G.; Huang, G. Propofol administration modulates AQP-4 expression and brain edema after traumatic brain injury. Cell Biochem. Biophys. 2013, 67, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Marechal, X.; Favory, R.; Joulin, O.; Montaigne, D.; Hassoun, S.; Decoster, B.; Zerimech, F.; Neviere, R. Endothelial glycocalyx damage during endotoxemia coincides with microcirculatory dysfunction and vascular oxidative stress. Shock 2008, 29, 572–576. [Google Scholar] [PubMed]
- Becker, B.F.; Chappell, D.; Bruegger, D.; Annecke, T.; Jacob, M. Therapeutic strategies targeting the endothelial glycocalyx: Acute deficits, but great potential. Cardiovasc. Res. 2010, 87, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Freudenberger, T.; Melchior-Becker, A.; Rock, K.; Ter Braak, M.; Jastrow, H.; Kinzig, M.; Lucke, S.; Suvorava, T.; Kojda, G.; et al. Inhibition of hyaluronan synthesis accelerates murine atherosclerosis: Novel insights into the role of hyaluronan synthesis. Circulation 2010, 122, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Nieuwdorp, M.; Mooij, H.L.; Kroon, J.; Atasever, B.; Spaan, J.A.; Ince, C.; Holleman, F.; Diamant, M.; Heine, R.J.; Hoekstra, J.B.; et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 2006, 55, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.; Berkestedt, I.; Schmidtchen, A.; Ljunggren, L.; Bodelsson, M. Increased levels of glycosaminoglycans during septic shock: Relation to mortality and the antibacterial actions of plasma. Shock 2008, 30, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Chappell, D.; Westphal, M.; Jacob, M. The impact of the glycocalyx on microcirculatory oxygen distribution in critical illness. Curr. Opin. Anaesthesiol. 2009, 22, 155–162. [Google Scholar] [CrossRef] [PubMed]
- De Klaver, M.J.; Buckingham, M.G.; Rich, G.F. Isoflurane pretreatment has immediate and delayed protective effects against cytokine-induced injury in endothelial and vascular smooth muscle cells. Anesthesiology 2003, 99, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Annecke, T.; Chappell, D.; Chen, C.; Jacob, M.; Welsch, U.; Sommerhoff, C.P.; Rehm, M.; Conzen, P.F.; Becker, B.F. Sevoflurane preserves the endothelial glycocalyx against ischaemia-reperfusion injury. Br. J. Anaesth. 2010, 104, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Lucchinetti, E.; Ambrosio, S.; Aguirre, J.; Herrmann, P.; Harter, L.; Keel, M.; Meier, T.; Zaugg, M. Sevoflurane inhalation at sedative concentrations provides endothelial protection against ischemia-reperfusion injury in humans. Anesthesiology 2007, 106, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Chappell, D.; Heindl, B.; Jacob, M.; Annecke, T.; Chen, C.; Rehm, M.; Conzen, P.; Becker, B.F. Sevoflurane reduces leukocyte and platelet adhesion after ischemia-reperfusion by protecting the endothelial glycocalyx. Anesthesiology 2011, 115, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Bruegger, D.; Bauer, A.; Finsterer, U.; Bernasconi, P.; Kreimeier, U.; Christ, F. Microvascular changes during anesthesia: Sevoflurane compared with propofol. Acta Anaesthesiol. Scand. 2002, 46, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Annecke, T.; Rehm, M.; Bruegger, D.; Kubitz, J.C.; Kemming, G.I.; Stoeckelhuber, M.; Becker, B.F.; Conzen, P.F. Ischemia-reperfusion-induced unmeasured anion generation and glycocalyx shedding: Sevoflurane versus propofol anesthesia. J. Investig. Surg. 2012, 25, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Mulivor, A.W.; Lipowsky, H.H. Inflammation- and ischemia-induced shedding of venular glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1672–H1680. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Takino, T.; Miyamori, H.; Kinsen, H.; Yoshizaki, T.; Furukawa, M.; Sato, H. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J. Biol. Chem. 2003, 278, 40764–40770. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Hiebert, L.M. Alteration of endothelial proteoglycan and heparanase gene expression by high glucose, insulin and heparin. Vasc. Pharmacol. 2013, 59, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Gayosso, I.; Platts, S.H.; Duling, B.R. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2247–H2256. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.M.; Sloane, B.F. Cysteine cathepsins: Multifunctional enzymes in cancer. Nat. Rev. Cancer 2006, 6, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Hsing, C.H.; Chen, Y.H.; Chen, C.L.; Huang, W.C.; Lin, M.C.; Tseng, P.C.; Wang, C.Y.; Tsai, C.C.; Choi, P.C.; Lin, C.F. Anesthetic propofol causes glycogen synthase kinase-3β-regulated lysosomal/mitochondrial apoptosis in macrophages. Anesthesiology 2012, 116, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yang, Z.B.; Zhou, Q.H.; Huan, X.; Wang, L. Lipid metabolism disturbances and ampk activation in prolonged propofol-sedated rabbits under mechanical ventilation. Acta Pharmacol. Sin. 2012, 33, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.R.; Al Zaharna, M.; Wong, M.M.; Chiu, S.K.; Cheung, H.Y. Taxifolin enhances andrographolide-induced mitotic arrest and apoptosis in human prostate cancer cells via spindle assembly checkpoint activation. PLoS ONE 2013, 8, e54577. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.-C.; Lin, C.-F.; Li, C.-F.; Sun, D.-P.; Wang, L.-Y.; Hsing, C.-H. Anesthetic Propofol Overdose Causes Vascular Hyperpermeability by Reducing Endothelial Glycocalyx and ATP Production. Int. J. Mol. Sci. 2015, 16, 12092-12107. https://doi.org/10.3390/ijms160612092
Lin M-C, Lin C-F, Li C-F, Sun D-P, Wang L-Y, Hsing C-H. Anesthetic Propofol Overdose Causes Vascular Hyperpermeability by Reducing Endothelial Glycocalyx and ATP Production. International Journal of Molecular Sciences. 2015; 16(6):12092-12107. https://doi.org/10.3390/ijms160612092
Chicago/Turabian StyleLin, Ming-Chung, Chiou-Feng Lin, Chien-Feng Li, Ding-Ping Sun, Li-Yun Wang, and Chung-Hsi Hsing. 2015. "Anesthetic Propofol Overdose Causes Vascular Hyperpermeability by Reducing Endothelial Glycocalyx and ATP Production" International Journal of Molecular Sciences 16, no. 6: 12092-12107. https://doi.org/10.3390/ijms160612092
APA StyleLin, M.-C., Lin, C.-F., Li, C.-F., Sun, D.-P., Wang, L.-Y., & Hsing, C.-H. (2015). Anesthetic Propofol Overdose Causes Vascular Hyperpermeability by Reducing Endothelial Glycocalyx and ATP Production. International Journal of Molecular Sciences, 16(6), 12092-12107. https://doi.org/10.3390/ijms160612092







