Changes of Water Hydrogen Bond Network with Different Externalities
Abstract
:1. Introduction
2. Water Structure Changes with Temperature
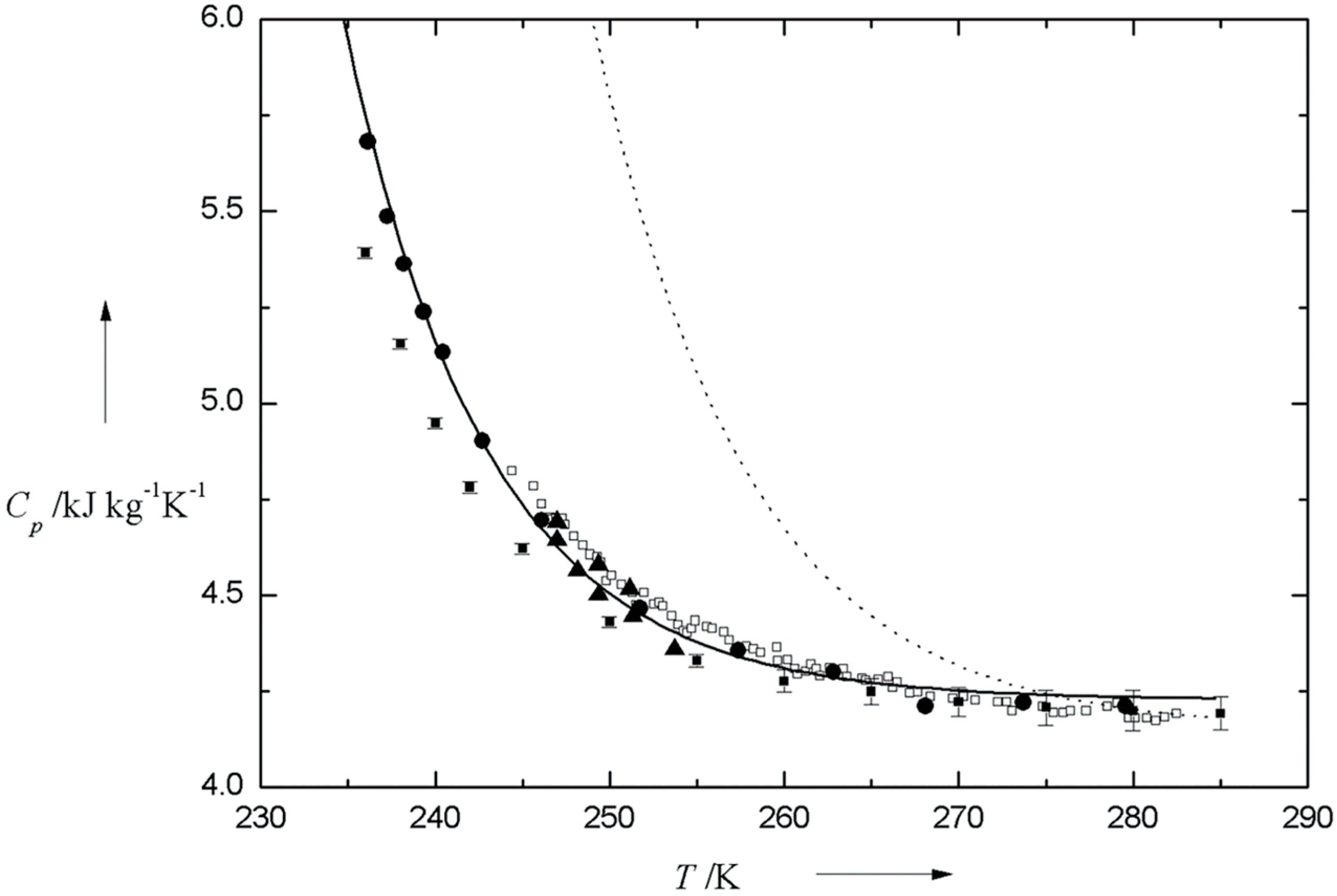
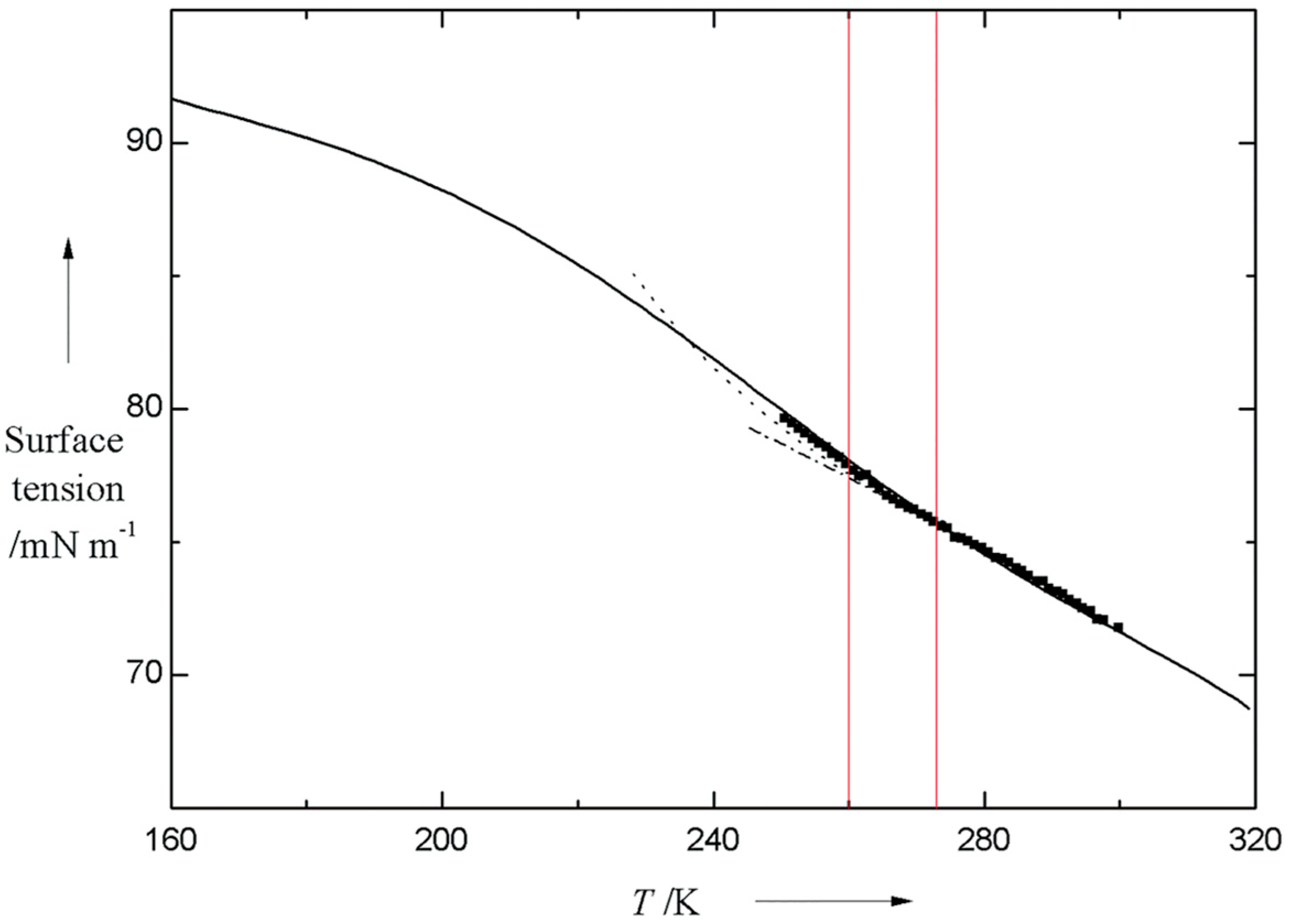
2.1. Supercooled Water
2.2. Ambient Water
| Kind of Point | Method | Interpretation of Point | Detailed Classification | Temperature Ranges (°C) | References |
|---|---|---|---|---|---|
| isosbestic point | near-IR | MCM a | HB-NHB b | 5–25 | Worley and Klotz [49] |
| isosbestic point | Raman spectra | MCM a | HB-NHB b | 3–85 | Walrafen et al. [14] |
| isosbestic point | Raman spectra | MCM a | free OH, DA, DDA, DAA, DDAA | −25–25 | Sun [13] |
| isosbestic point | Raman spectra and thermodynamic calculation | MCM a | CW-UCW c | 25–60 | Nino et al. [50] |
| isosbestic point | molecular simulations | CM d | none e | 0–100 | Geissler [11] |
| isosbestic point | Raman spectra and MC simulation | CM d | none e | 0–100 | Smith et al. [12] |
| isopermitive point | Dielectric spectra | MCM a | ions and dipoles | 26–40 | Angulo and Mercado [52] |
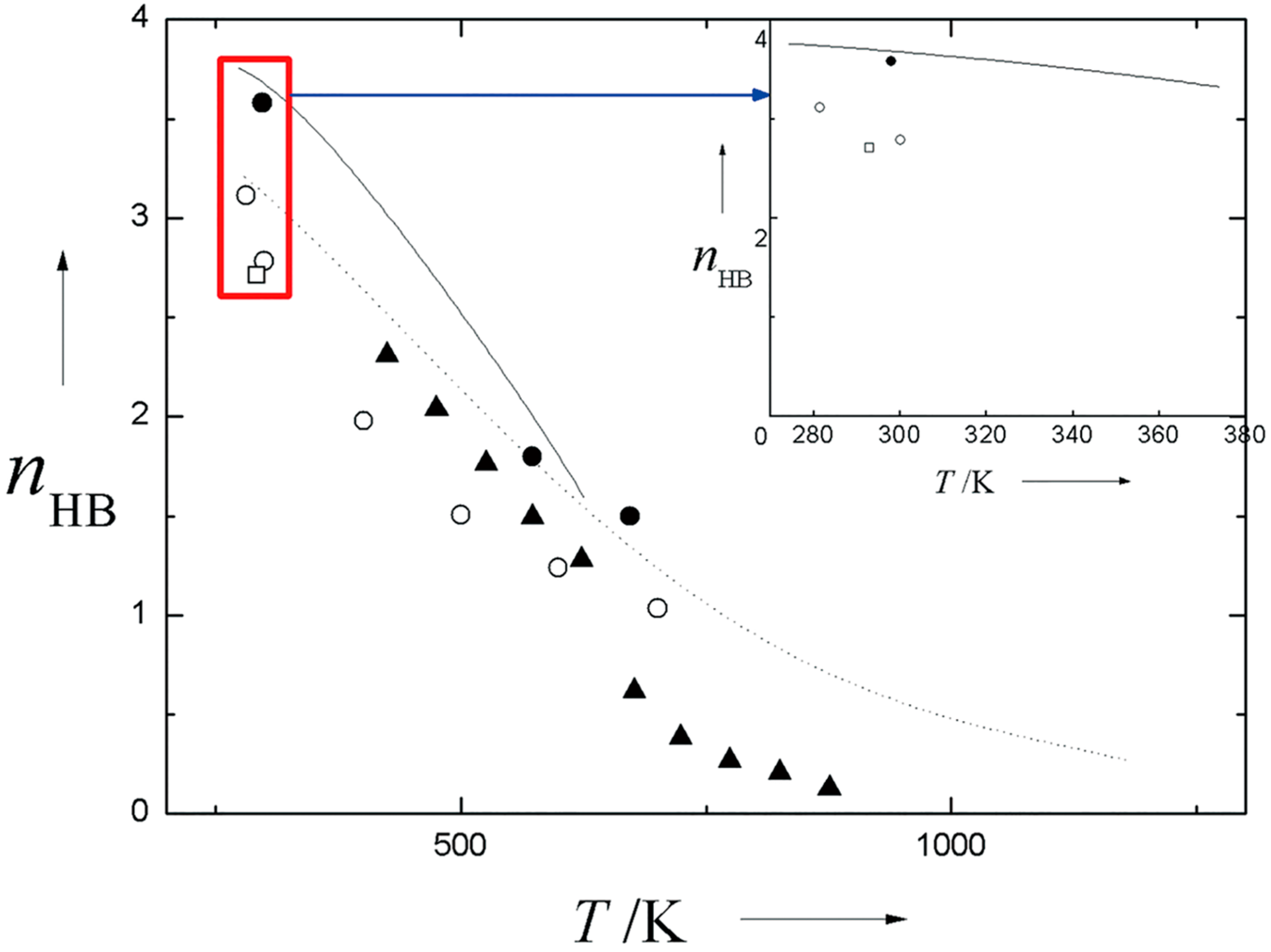
2.3. Gaseous Water
2.4. Sub- and Supercritical Water
| Parameters | Changing Trend with Temperature | Structural Features | References |
|---|---|---|---|
| dielectric constant | decreasing | Dipole moment decreasing and randomized orientation | Yoshii et al. [55] |
| electric conductivity | increasing | Transforming to ionic state | Chau et al. [84] |
| viscosity | decreasing | HB weakening a | Lamb and Jonas [85] |
| self-diffusion coefficient | increasing | HB weakening b | Lamb and Jonas [85] Brodholt and Wood [78] |
| HB vibration absorption | vanishment | HB breakage | Kalinichev [86] |
| OH stretching absorption | blue shift | HB breakage | Kohl et al. [80] |
3. Pressure-Induced Changes of Water
3.1. Different Forms of Ices
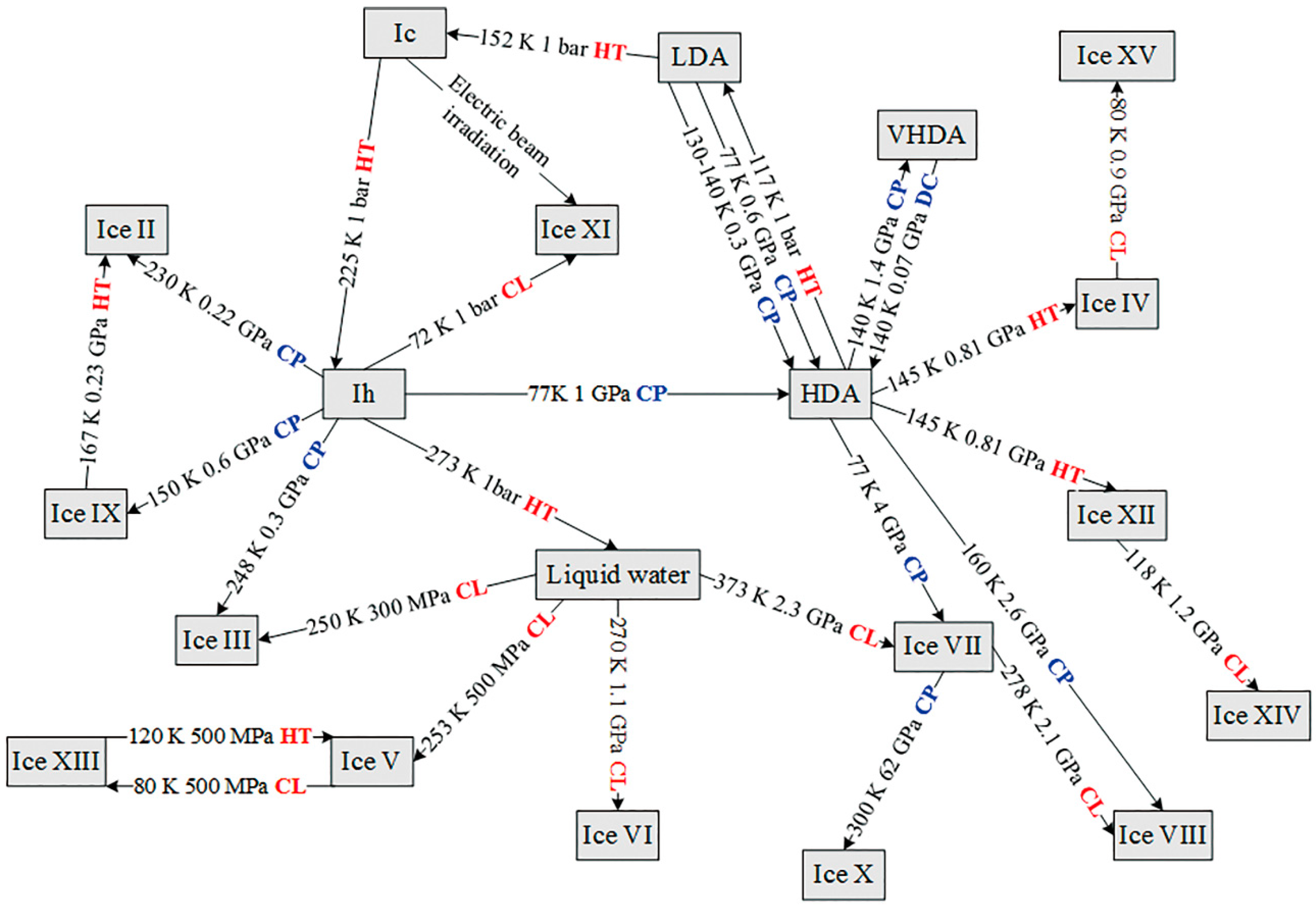
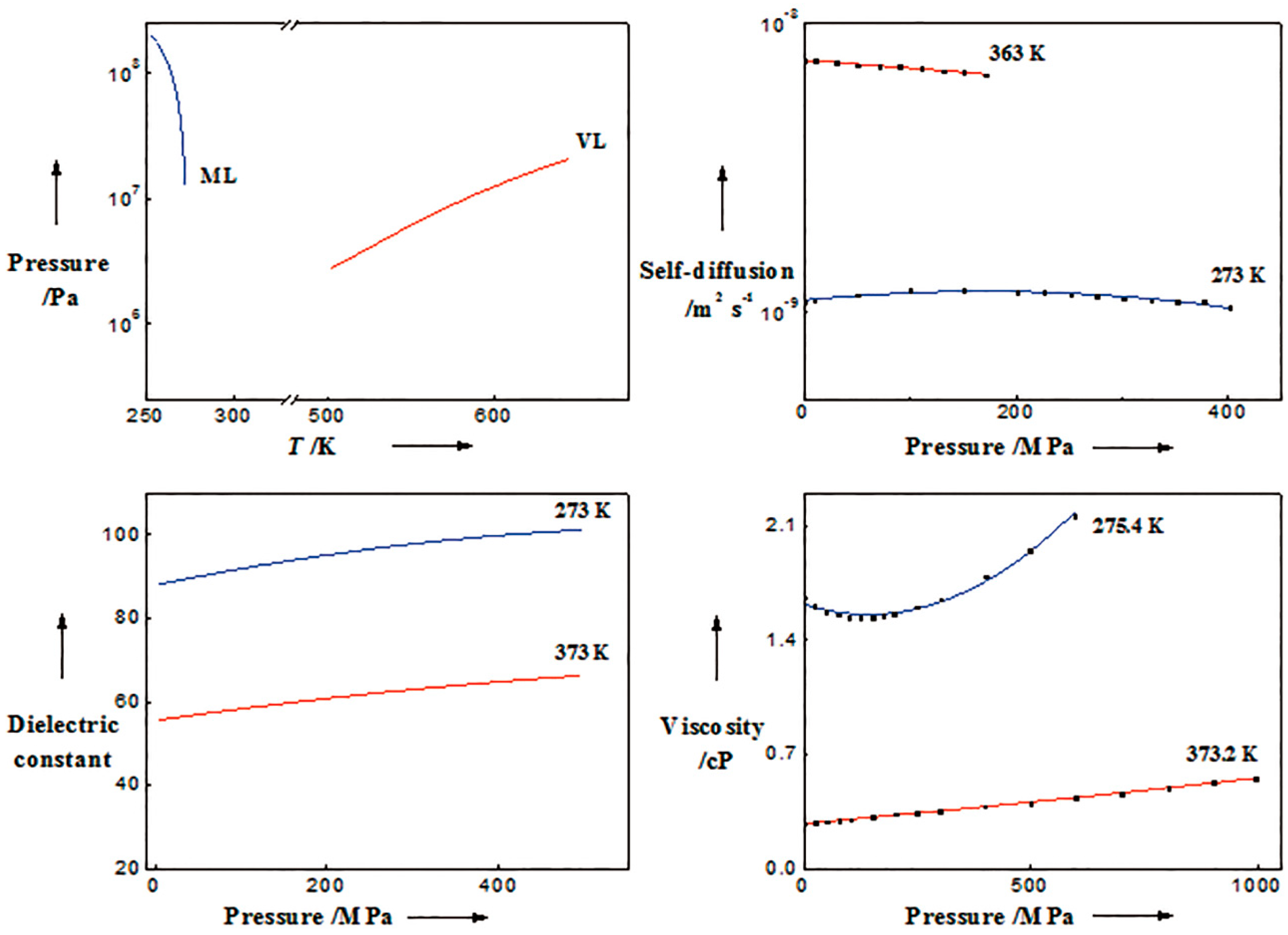
3.2. Liquid and Supercritical Water
4. Solutes-Dependent Effect on Water
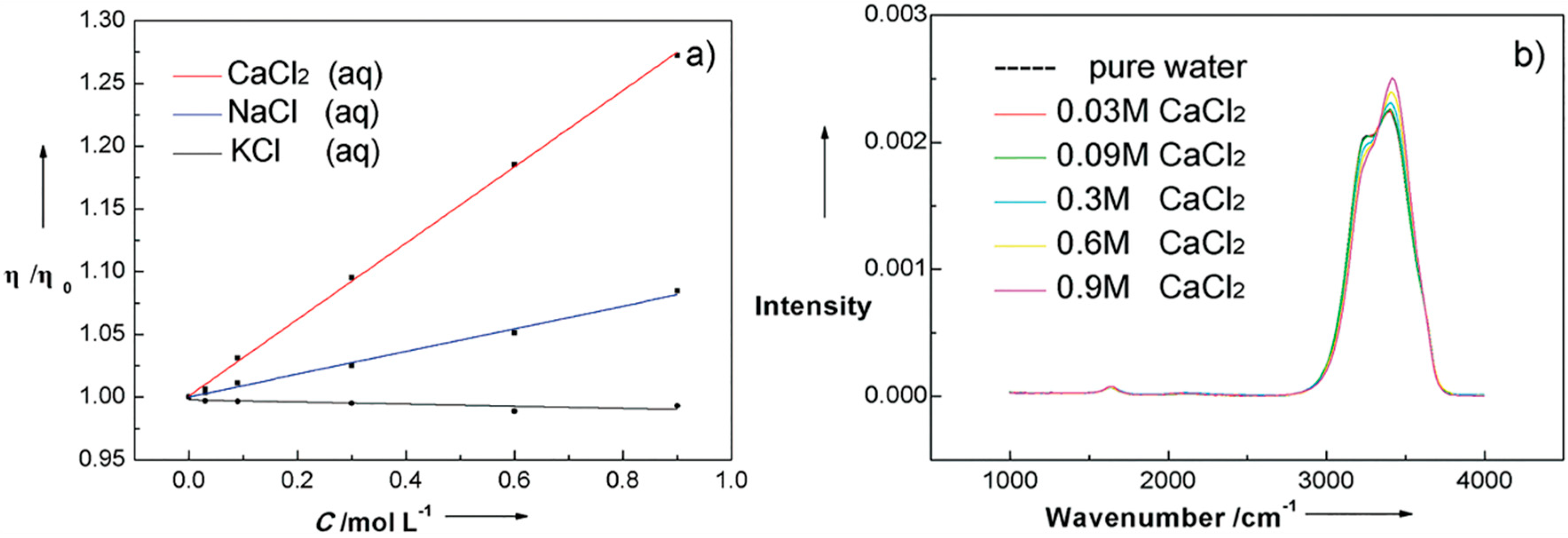
4.1. Effect of Inorganic Ions
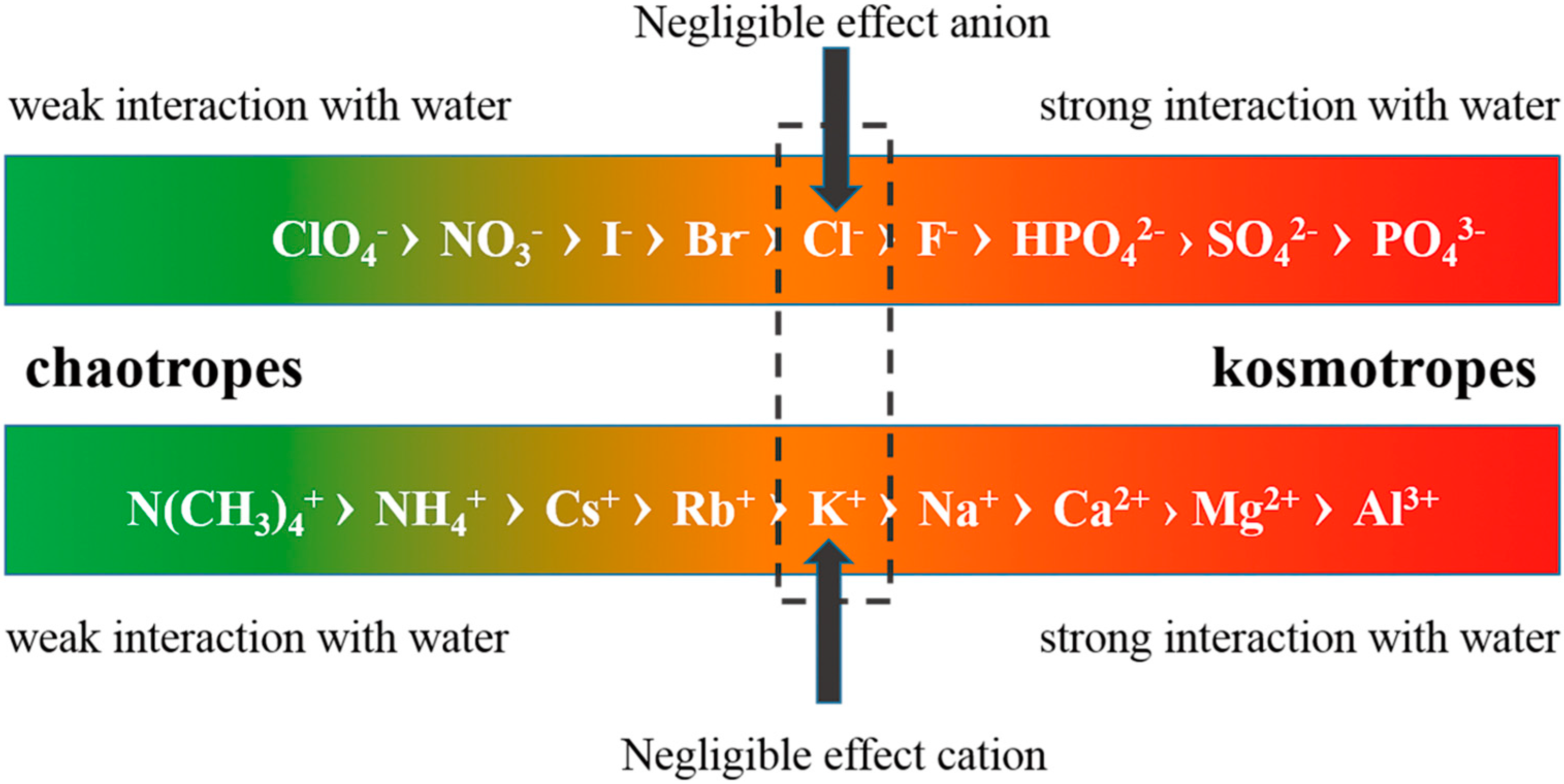
4.2. Effects of Proteins
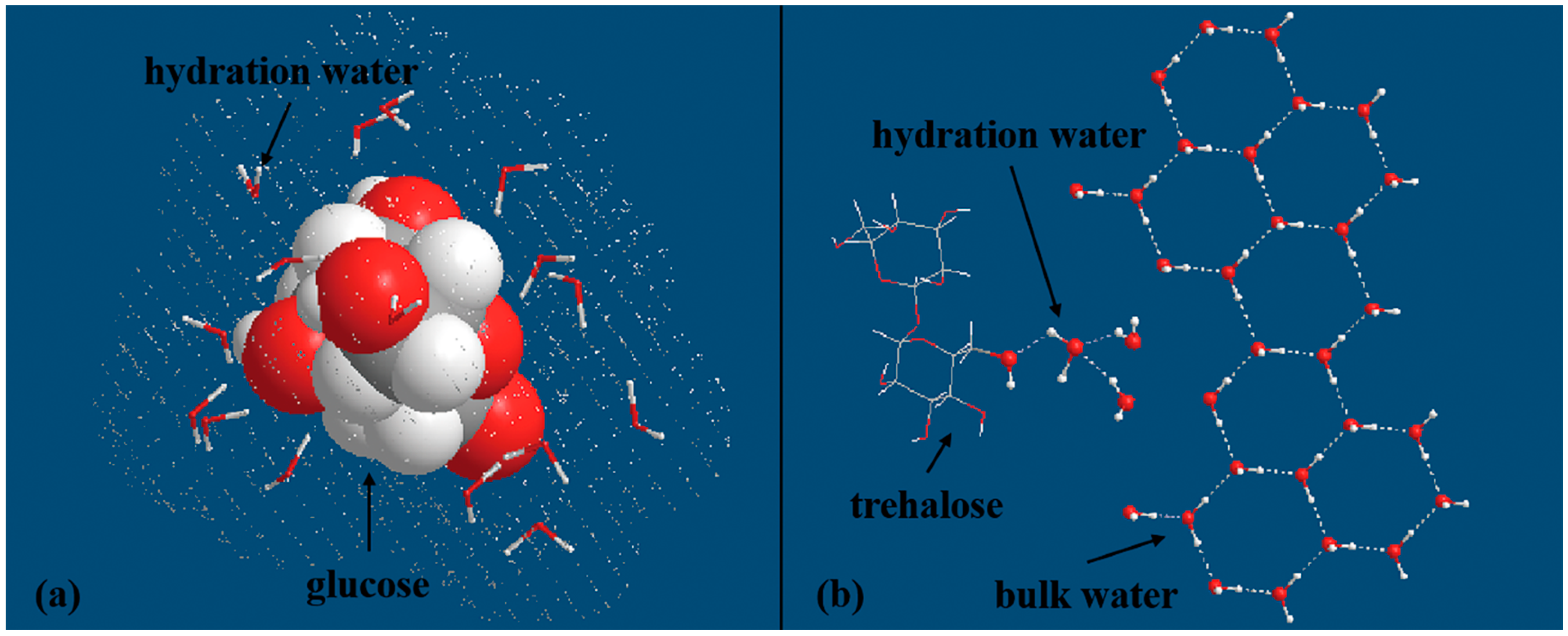
4.3. Effects of Carbohydrates
5. Mystery Effects Imposed by External Fields
5.1. Effect of Electric and Electromagnetic Field
5.2. Effect of Magnetic Field
6. Summary and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Perera, A.; Mazighi, R.; Kežić, B. Fluctuations and micro-heterogeneity in aqueous mixtures. J. Chem. Phys. 2012, 136. [Google Scholar] [CrossRef]
- Röntgen, W.C. Ueber die constitution des flüssigen wassers. Ann. Phys. Chem. 1892, 281, 91–97. [Google Scholar] [CrossRef]
- Bernal, J.D.; Fowler, R.H. A theory of water and ionic solution, with particular reference to hydrogen and hydroxyl ions. J. Chem. Phys. 1933, 1, 515–548. [Google Scholar] [CrossRef]
- Stanley, H.E.; Teixeira, J. Interpretation of the unusual behavior of H2O and D2O at low temperatures: Tests of a percolation model. J. Chem. Phys. 1980, 73, 3404–3422. [Google Scholar] [CrossRef]
- Laenen, R.; Roth, T.; Laubereau, A. Novel precursors of solvated electrons in water: Evidence for a charge transfer process. Phys. Rev. Lett. 2000, 85, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Woutersen, S.; Emmerichs, U.; Bakker, H.J. Femtosecond mid-IR pump-probe spectroscopy of liquid water: Evidence for a two-component structure. Science 1997, 278, 658–660. [Google Scholar] [CrossRef]
- Tokushima, T.; Harada, Y.; Takahashi, O.; Senba, Y.; Ohashi, H.; Pettersson, L.G.M.; Nilsson, A.; Shin, S. High resolution X-ray emission spectroscopy of liquid water: The observation of two structural motifs. Chem. Phys. Lett. 2008, 460, 387–400. [Google Scholar] [CrossRef]
- Lange, K.M.; Könnecke, R.; Soldatov, M.; Golnak, R.; Rubensson, J.-E.; Soldatov, A.; Aziz, E.F. On the origin of the hydrogen-bond-network nature of water: X-ray absorption and emission spectra of water–acetonitrile mixtures. Angew. Chem. Int. Ed. 2011, 50, 10621–10625. [Google Scholar] [CrossRef]
- Sellberg, J.A.; Kaya, S.; Segtnan, V.H.; Chen, C.; Tyliszczak, T.; Ogasawara, H.; Nordlund, D.; Pettersson, L.G.M.; Nilsson, A. Comparison of X-ray absorption spectra between water and ice: New ice data with low pre-edge absorption cross-section. J. Chem. Phys. 2014, 141. [Google Scholar] [CrossRef]
- Sahle, C.J.; Sternemann, C.; Schmidt, C.; Lehtola, S.; Jahn, S.; Simonelli, L.; Huotari, S.; Hakala, M.; Pylkkänen, T.; Nyrow, A. Microscopic structure of water at elevated pressures and temperatures. Proc. Natl. Acad. Sci. USA 2013, 110, 6301–6306. [Google Scholar] [CrossRef] [PubMed]
- Geissler, P.L. Temperature dependence of inhomogeneous broadening: On the meaning of isosbestic points. J. Am. Chem. Soc. 2005, 127, 14930–14935. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Cappa, C.D.; Wilson, K.R.; Cohen, R.C.; Geissler, P.L.; Saykally, R.J. Unified description of temperature-dependent hydrogen-bond rearrangements in liquid water. Proc. Natl. Acad. Sci. USA 2005, 102, 14171–14174. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q. Local statistical interpretation for water structure. Chem. Phys. Lett. 2013, 568–569, 90–94. [Google Scholar] [CrossRef]
- Walrafen, G.E.; Hokmabadi, M.H.; Yang, W.H. Raman isosbestic points from liquid water. J. Chem. Phys. 1986, 85, 6964–6969. [Google Scholar] [CrossRef]
- Hosoda, H.; Mori, H.; Sogoshi, N.; Nagasawa, A.; Nakabayashi, S. Refractive indices of water and aqueous electrolyte solutions under high magnetic fields. J. Phys. Chem. A 2004, 108, 1461–1464. [Google Scholar] [CrossRef]
- Marcus, Y. Effect of ions on the structure of water: Structure making and breaking. Chem. Rev. 2009, 109, 1346–1370. [Google Scholar] [CrossRef] [PubMed]
- Khan, A. A liquid water model: Density variation from supercooled to superheated states, prediction of H-bonds, and temperature limits. J. Phys. Chem. B 2000, 104, 11268–11274. [Google Scholar] [CrossRef]
- Cho, C.H.; Urquidi, J.; Robinson, G.W. Molecular-level description of temperature and pressure effects on the viscosity of water. J. Chem. Phys. 1999, 111, 10171–10176. [Google Scholar] [CrossRef]
- Kell, G.S. Density, thermal expansivity, and compressibility of liquid water from 0° to 150°. Correlations and tables for atmospheric pressure and saturation reviewed and expressed on 1968 temperature scale. J. Chem. Eng. Data 1975, 20, 97–105. [Google Scholar] [CrossRef]
- Angell, C.A.; Shuppert, J.; Tucker, J.C. Anomalous properties of supercooled water. Heat capacity, expansivity, and proton magnetic resonance chemical shift from 0 to −38%. J. Phys. Chem. 1973, 77, 3092–3099. [Google Scholar] [CrossRef]
- Angell, C.A.; Sichina, W.J.; Oguni, M. Heat capacity of water at extremes of supercooling and superheating. J. Phys. Chem. 1982, 86, 998–1002. [Google Scholar] [CrossRef]
- Yan, B.; Zhao, L.; Wang, W.H.; Tan, X. NMR and viscosity coefficients of CuCl2 and CuSO4 and their relationship to water structure. Acta Phys.-Chim. Sin. 2009, 25, 684–688. [Google Scholar]
- Wang, W.H.; Zhao, L.; Yan, B.; Tan, X.; He, B.; Pan, Z.Y. Effects of concentration and freeze-thaw treatment on the local structure of Cu2+ in CuCl2 aqueous solutions. Acta Phys. Chim. Sin. 2010, 26, 265–269. [Google Scholar]
- Floriano, M.A.; Angell, C.A. Surface tension and molar surface free energy and entropy of water to −27.2 °C. J. Phys. Chem. 1990, 94, 4199–4202. [Google Scholar] [CrossRef]
- Trinh, E.H.; Ohsaka, K. Measurement of density, sound velocity, surface tension, and viscosity of freely suspended supercooled liquids. Int. J. Thermophys. 1995, 16, 545–555. [Google Scholar] [CrossRef]
- Hacker, P.T. Experimental Values of the surface Tension Of Supercooled Water; Technical Note 2510; National Advisory Committee for Aeronautics: Washington, DC, USA, 1951. [Google Scholar]
- Hrubý, J.; Holten, V. A two-structure model of thermodynamic properties and surface tension of supercooled water. In Proceedings of the 14th International Conference on the Properties of Water and Steam, Kyoto, Japan, 29 August–3 September 2004; pp. 241–246.
- Bertolini, D.; Cassettari, M.; Salvetti, G. Anomalies in the “latent heat” of solidification of supercooled water. Chem. Phys. Lett. 1985, 119, 553–555. [Google Scholar] [CrossRef]
- Archer, D.G.; Carter, R.W. Thermodynamic properties of the NaCl + H2O system. 4: Heat capacities of H2O and NaCl (aq) in cold-stable and supercooled states. J. Phys. Chem. B 2000, 104, 8563–8584. [Google Scholar] [CrossRef]
- Tombari, E.; Ferrari, C.; Salvetti, G. Heat capacity anomaly in a large sample of supercooled water. Chem. Phys. Lett. 1999, 300, 749–751. [Google Scholar] [CrossRef]
- Wagner, W.; Pruß, A. The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific use. J. Phys. Chem. Ref. Data 2002, 31, 387–535. [Google Scholar] [CrossRef]
- IAPWS Release on Surface Tension of Ordinary Water Substance. Available online: http://www.iapws.org/relguide/surf.pdf (accessed on 8 December 2014).
- Kalova, J.; Mares, R. Second inflection point of the surface tension of water. Int. J. Thermophys. 2012, 33, 992–999. [Google Scholar] [CrossRef]
- Mishima, O.; Stanley, H.E. The relationship between liquid, supercooled and glassy water. Nature 1998, 396, 329–335. [Google Scholar] [CrossRef]
- Mishima, O.; Stanley, H.E. Decompression-induced melting of ice IV and the liquid–liquid transition in water. Nature 1998, 392, 164–168. [Google Scholar] [CrossRef]
- Poole, P.H.; Sciortino, F.; Essmann, U.; Stanley, H.E. Phase behaviour of metastable water. Nature 1992, 360, 324–328. [Google Scholar] [CrossRef]
- Yokoyama, H.; Kannami, M.; Kanno, H. Existence of clathrate-like structures in supercooled water: X-ray diffraction evidence. Chem. Phys. Lett. 2008, 463, 99–102. [Google Scholar] [CrossRef]
- Chaplin, M.F. A proposal for the structuring of water. Biophys. Chem. 2000, 83, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, S. Et tu, grotthuss! and other unfinished stories. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 876–885. [Google Scholar] [CrossRef]
- Gregory, J.K.; Clary, D.C.; Liu, K.; Brown, M.G.; Saykally, R.J. The water dipole moment in water clusters. Science 1997, 275, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Coulson, C.A.; Eisenberg, D. Interactions of H2O molecules in ice. II. Interaction energies of H2O molecules in ice. Proc. R. Soc. Lond. Ser. A 1966, 291, 454–459. [Google Scholar] [CrossRef]
- Suresh, S.J.; Naik, V.M. Hydrogen bond thermodynamic properties of water from dielectric constant data. J. Chem. Phys. 2000, 113, 9727–9732. [Google Scholar] [CrossRef]
- Sharma, M.; Resta, R.; Car, R. Dipolar correlations and the dielectric permittivity of water. Phys. Rev. Lett. 2007, 98. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, C.G.; Rice, S.A.; Bates, J.B. A Raman spectral study of amorphous solid water. J. Chem. Phys. 1975, 63, 1065–1071. [Google Scholar] [CrossRef]
- Saykally, R.J.; Blake, G.A. Molecular interactions and hydrogen bond tunneling dynamics: Some new perspectives. Science 1993, 259, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Nicodemus, R.A.; Ramasesha, K.; Roberts, S.T.; Tokmakoff, A. Hydrogen bond rearrangements in water probed with temperature-dependent 2D IR. J. Phys. Chem. Lett. 2010, 1, 1068–1072. [Google Scholar] [CrossRef]
- Bergmann, U.; Nordlund, D.; Wernet, P.; Odelius, M.; Pettersson, L.G.M.; Nilsson, A. Isotope effects in liquid water probed by X-ray raman spectroscopy. Phys. Rev. B 2007, 76. [Google Scholar] [CrossRef]
- Wernet, P.; Nordlund, D.; Bergmann, U.; Cavalleri, M.; Odelius, M.; Ogasawara, H.; Naslund, L.A.; Hirsch, T.K.; Ojamae, L.; Glatzel, P.; et al. The structure of the first coordination shell in liquid water. Science 2004, 304, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Worley, J.D.; Klotz, I.M. Near-infrared spectra of H2O-D2O solutions. J. Chem. Phys. 2004, 45, 2868–2871. [Google Scholar] [CrossRef]
- De Ninno, A.; Castellano, A.C.; del Giudice, E. The supramolecular structure of liquid water and quantum coherent processes in biology. J. Phys. Conf. Ser. 2013, 442. [Google Scholar] [CrossRef]
- Yada, H.; Nagai, M.; Tanaka, K. Origin of the fast relaxation component of water and heavy water revealed by terahertz time-domain attenuated total reflection spectroscopy. Chem. Phys. Lett. 2008, 464, 166–170. [Google Scholar] [CrossRef]
- Angulo-Sherman, A.; Mercado-Uribe, H. Dielectric spectroscopy of water at low frequencies: The existence of an isopermitive point. Chem. Phys. Lett. 2011, 503, 327–330. [Google Scholar] [CrossRef]
- Hoffmann, M.M.; Conradi, M.S. Are there hydrogen bonds in supercritical water? J. Am. Chem. Soc. 1997, 119, 3811–3817. [Google Scholar] [CrossRef]
- Soper, A.K.; Bruni, F.; Ricci, M.A. Site–site pair correlation functions of water from 25 to 400 °C: Revised analysis of new and old diffraction data. J. Chem. Phys. 1997, 106, 247–254. [Google Scholar] [CrossRef]
- Yoshii, N.; Miura, S.; Okazaki, S. A molecular dynamics study of dielectric constant of water from ambient to sub- and supercritical conditions using a fluctuating-charge potential model. Chem. Phys. Lett. 2001, 345, 195–200. [Google Scholar] [CrossRef]
- Kalinichev, A.G.; Bass, J.D. Hydrogen bonding in supercritical water. 2. Computer simulations. J. Phys. Chem. A 1997, 101, 9720–9727. [Google Scholar] [CrossRef]
- Huang, C.C.; Wikfeldt, K.T.; Nordlund, D.; Bergmann, U.; McQueen, T.; Sellberg, J.; Pettersson, L.G.M.; Nilsson, A. Wide-angle X-ray diffraction and molecular dynamics study of medium-range order in ambient and hot water. Phys. Chem. Chem. Phys. 2011, 13, 19997–20007. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, R.C. Temperature and pressure dependence of hydrogen bond strength: A perturbation molecular orbital approach. J. Chem. Phys. 1998, 109, 7372–7378. [Google Scholar] [CrossRef]
- Ramasesha, K.; Roberts, S.T.; Nicodemus, R.A.; Mandal, A.; Tokmakoff, A. Ultrafast 2D IR anisotropy of water reveals reorientation during hydrogen-bond switching. J. Chem. Phys. 2011, 135. [Google Scholar] [CrossRef]
- Wiggins, P.M. High and low density water in gels. Prog. Polym. Sci. 1995, 20, 1121–1163. [Google Scholar] [CrossRef]
- Ehre, D.; Lavert, E.; Lahav, M.; Lubomirsky, I. Water freezes differently on positively and negatively charged surfaces of pyroelectric materials. Science 2010, 327, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, P.M. Hydrophobic hydration, hydrophobic forces and protein folding. Phys. A 1997, 238, 113–128. [Google Scholar] [CrossRef]
- Zuo, G.H.; Hu, J.; Fang, H.P. Protein folding under mediation of ordering water: An off-lattice Gō-like model study. Chin. Phys. Lett. 2007, 24, 2426–2429. [Google Scholar] [CrossRef]
- Schrade, P.; Klein, H.; Egry, I.; Ademovic, Z.; Klee, D. Hydrophobic volume effects in albumin solutions. J. Colloid Interface Sci. 2001, 234, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Schenter, G.K.; Kathmann, S.M.; Garrett, B.C. Dynamical nucleation theory: A new molecular approach to vapor-liquid nucleation. Phys. Rev. Lett. 1999, 82, 3484–3487. [Google Scholar] [CrossRef]
- Keutsch, F.N.; Goldman, N.; Harker, H.A.; Leforestier, C.; Saykally, R.J. Complete characterization of the water dimer vibrational ground state and testing the VRT(ASP-W)III, SAPT-5ST, and VRT(MCY-5f) surfaces. Mol. Phys. 2003, 101, 3477–3492. [Google Scholar] [CrossRef]
- Kuyanov-Prozument, K.; Choi, M.Y.; Vilesov, A.F. Spectrum and infrared intensities of OH-stretching bands of water dimers. J. Chem. Phys. 2010, 132. [Google Scholar] [CrossRef]
- Tretyakov, M.Y.; Serov, E.A.; Koshelev, M.A.; Parshin, V.C.; Krupnov, A.F. Water dimer rotationally resolved millimeter-wave spectrum observation at room temperature. Phys. Rev. Lett. 2013, 110. [Google Scholar] [CrossRef] [PubMed]
- Lenz, A.; Ojamäe, L. A theoretical study of water equilibria: The cluster distribution versus temperature and pressure for (H2O)n, n = 1–60, and ice. J. Chem. Phys. 2009, 131. [Google Scholar] [CrossRef] [PubMed]
- Vaida, V. Perspective: Water cluster mediated atmospheric chemistry. J. Chem. Phys. 2011, 135. [Google Scholar] [CrossRef]
- Staikova, M.; Donaldson, D.J. Water complexes as catalysts in atmospheric reactions. Phys. Chem. Earth Part C 2001, 26, 473–478. [Google Scholar] [CrossRef]
- Miller, Y.; Chaban, G.M.; Finlayson-Pitts, B.J.; Gerber, R.B. Photochemical processes induced by vibrational overtone excitations: Dynamics simulations for cis-HONO, trans-HONO, HNO3, and HNO3-H2O. J. Phys. Chem. A 2006, 110, 5342–5354. [Google Scholar] [CrossRef] [PubMed]
- Kramer, Z.C.; Takahashi, K.; Skodje, R.T. Water catalysis and anticatalysis in photochemical reactions: Observation of a delayed threshold effect in the reaction quantum yield. J. Am. Chem. Soc. 2010, 132, 15154–15157. [Google Scholar] [CrossRef] [PubMed]
- Postorino, P.; Tromp, R.H.; Ricci, M.A.; Soper, A.K.; Neilson, G.W. The interatomic structure of water at supercritical temperatures. Nature 1993, 366, 668–670. [Google Scholar] [CrossRef]
- Kalinichev, A.G. Molecular simulations of liquid and supercritical water: Thermodynamics, structure, and hydrogen bonding. Rev. Mineral. Geochem. 2001, 42, 83–129. [Google Scholar] [CrossRef]
- Wernet, Ph.; Testemale, D.; Hazemann, J.-L.; Argoud, R.; Glatzel, P.; Pettersson, L.G.M.; Nilsson, A.; Bergmann, U. Spectroscopic characterization of microscopic hydrogen-bonding disparities in supercritical water. J. Chem. Phys. 2005, 123. [Google Scholar] [CrossRef]
- Bruges, E.A.; Gibson, M.R. Dynamic viscosity of compressed water to 10 kilobar and steam to 1500 °C. J. Mech. Eng. Sci. 1969, 11, 189–205. [Google Scholar] [CrossRef]
- Brodholt, J.; Wood, B. Molecular dynamics of water at high temperatures and pressures. Geochim. Cosmochim. Acta 1990, 54, 2611–2616. [Google Scholar] [CrossRef]
- Bernabei, M.; Botti, A.; Bruni, F.; Ricci, M.A.; Soper, A.K. Percolation and three-dimensional structure of supercritical water. Phys. Rev. E 2008, 78. [Google Scholar] [CrossRef]
- Kohl, W.; Lindner, H.A.; Franck, E.U. Raman spectra of water to 400 °C and 3000 bar. Ber. Bunsenges. Phys. Chem. 1991, 95, 1586–1593. [Google Scholar] [CrossRef]
- Weingärtner, H.; Franck, E.U. Supercritical water as a solvent. Angew. Chem. Int. Ed. 2005, 44, 2672–2692. [Google Scholar] [CrossRef]
- Jedlovszky, P. Voronoi polyhedra analysis of the local structure of water from ambient to supercritical conditions. J. Chem. Phys. 1999, 111, 5975–5985. [Google Scholar] [CrossRef]
- Chialvo, A.A.; Cummings, P.T. Molecular-based modeling of water and aqueous solutions at supercritical conditions. Adv. Chem. Phys. 1999, 109, 115–205. [Google Scholar]
- Chau, R.; Mitchell, A.C.; Minich, R.W.; Nellis, W.J. Electrical conductivity of water compressed dynamically to pressures of 70–180 GPa (0.7–1.8 Mbar). J. Chem. Phys. 2001, 114, 1361–1365. [Google Scholar] [CrossRef]
- Lamb, W.J.; Jonas, J. NMR study of compressed supercritical water. J. Chem. Phys. 1981, 74, 913–921. [Google Scholar] [CrossRef]
- Kalinichev, A.G. Molecular dynamics and self-diffusion in supercritical water. Ber. Bunsenges. Phys. Chem. 1993, 97, 872–876. [Google Scholar] [CrossRef]
- Dudziak, K.H.; Franck, E.U. Messungen der viskosität des wassers bis 560 °C und 3500 bar. Ber. Bunsenges. Phys. Chem. 1966, 70, 1120–1128. [Google Scholar]
- Chandler, D. Hydrophobicity: Two faces of water. Nature 2002, 417, 491–491. [Google Scholar] [CrossRef] [PubMed]
- Loerting, T.; Winkel, K.; Seidl, M.; Bauer, M.; Mitterdorfer, C.; Handle, P.H.; Salzmann, C.G.; Mayer, E.; Finney, J.L.; Bowron, D.T. How many amorphous ices are there? Phys. Chem. Chem. Phys. 2011, 13, 8783–8794. [Google Scholar] [CrossRef] [PubMed]
- Mishima, O.; Calvert, L.D.; Whalley, E. An apparently first-order transition between two amorphous phases of ice induced by pressure. Nature 1985, 314, 76–78. [Google Scholar] [CrossRef]
- Schober, H.; Koza, M.M.; Tölle, A.; Masciovecchio, C.; Sette, F.; Fujara, F. Crystal-like high frequency phonons in the amorphous phases of solid water. Phys. Rev. Lett. 2000, 85, 4100–4103. [Google Scholar] [CrossRef] [PubMed]
- Andersson, O. Dielectric relaxation of the amorphous ices. J. Phys. Condens. Matter 2008, 20. [Google Scholar] [CrossRef]
- Tulk, C.A.; Benmore, C.J.; Urquidi, J.; Klug, D.D.; Neuefeind, J.; Tomberli, B.; Egelstaff, P.A. Structural studies of several distinct metastable forms of amorphous ice. Science 2002, 297, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Winkel, K.; Mayer, E.; Loerting, T. Equilibrated high-density amorphous ice and its first-order transition to the low-density form. J. Phys. Chem. B 2011, 115, 14141–14148. [Google Scholar] [CrossRef] [PubMed]
- Makkonen, L. Surface melting of ice. J. Phys. Chem. B 1997, 101, 6196–6200. [Google Scholar] [CrossRef]
- Bett, K.E.; Cappi, J.B. Effect of pressure on the viscosity of water. Nature 1965, 207, 620–621. [Google Scholar] [CrossRef]
- Pfeiffer, H.; Heremans, K. The sound velocity in ideal liquid mixtures from thermal volume fluctuations. Chemphyschem 2005, 6, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Prielmeier, F.X.; Lang, E.W.; Speedy, R.J.; Lüdemann, H.-D. The pressure dependence of self-diffusion in supercooled light and heavy water. Ber. Bunsenges. Phys. Chem. 1988, 92, 1111–1117. [Google Scholar] [CrossRef]
- Uematsu, M.; Frank, E.U. Static dielectric constant of water and steam. J. Phys. Chem. Ref. Data 1980, 9, 1291–1306. [Google Scholar] [CrossRef]
- Sun, C.Q.; Zhang, X.; Zheng, W. The hidden force opposing ice compression. Chem. Sci. 2012, 3, 1455–1460. [Google Scholar] [CrossRef]
- Dunaeva, A.N.; Antsyshkin, D.V.; Kuskov, O.L. Phase diagram of H2O: Thermodynamic functions of the phase transitions of high-pressure ices. Sol. Syst. Res. 2010, 44, 202–222. [Google Scholar] [CrossRef]
- Water Structure and Science. Available online: http://www1.lsbu.ac.uk/water/index2.html (accessed on 14 February 2015).
- Franck, E.U.; Rosenzweig, S.; Christoforakos, M. Calculation of the dielectric constant of water to 1000 °C and very high pressures. Ber. Bunsenges. Phys. Chem. 1990, 94, 199–203. [Google Scholar] [CrossRef]
- Ohtaki, H. Effects of temperature and pressure on hydrogen bonds in water and in formamide. J. Mol. Liq. 2003, 103, 3–13. [Google Scholar] [CrossRef]
- Roche, J.; Caro, J.A.; Norberto, D.R.; Barthe, P.; Roumestand, C.; Schlessman, J.L.; Garcia, A.E.; Royer, C.A. Cavities determine the pressure unfolding of proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 6945–6950. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, M.D.; Cocero, M.J. Supercritical water oxidation: A technical review. AIChE J. 2006, 52, 3933–3951. [Google Scholar] [CrossRef]
- Koop, T.; Luo, B.; Tsias, A.; Peter, T. Water activity as the determinant for homogeneous ice nucleation in aqueous solutions. Nature 2000, 406, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Conde, M.R. Properties of aqueous solutions of lithium and calcium chlorides: Formulations for use in air conditioning equipment design. Int. J. Therm. Sci. 2004, 43, 367–382. [Google Scholar] [CrossRef]
- Loeffler, H.H.; Rode, B.M. The hydration structure of the lithium ion. J. Chem. Phys. 2002, 117, 110–117. [Google Scholar] [CrossRef]
- Harsányi, I.; Pusztai, L. Hydration of ions in aqueous RbCl solutions. J. Phys. Condens. Matter 2007, 19. [Google Scholar] [CrossRef]
- Collins, K.D.; Washabaugh, M.W. The Hofmeister effect and the behaviour of water at interfaces. Q. Rev. Biophys. 1985, 18, 323–422. [Google Scholar] [CrossRef] [PubMed]
- Zangi, R. Can salting-in/salting-out ions be classified as chaotropes/kosmotropes? J. Phys. Chem. B 2009, 114, 643–650. [Google Scholar] [CrossRef]
- Zavitsas, A.A. Properties of water solutions of electrolytes and nonelectrolytes. J. Phys. Chem. B 2001, 105, 7805–7817. [Google Scholar] [CrossRef]
- Fecko, C.J.; Eaves, J.D.; Loparo, J.J.; Tokmakoff, A.; Geissler, P.L. Ultrafast hydrogen-bond dynamics in the infrared spectroscopy of water. Science 2003, 301, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Lock, A.J.; Woutersen, S.; Bakker, H.J. Ultrafast energy equilibration in hydrogen-bonded liquids. J. Phys. Chem. A 2001, 105, 1238–1243. [Google Scholar] [CrossRef]
- Cowan, M.L.; Bruner, B.D.; Huse, N.; Dwyer, J.R.; Chugh, B.; Nibbering, E.T.J; Elsaesser, T.; Miller, R.J.D. Ultrafast memory loss and energy redistribution in the hydrogen bond network of liquid H2O. Nature 2005, 434, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Kropman, M.F.; Bakker, H.J. Femtosecond mid-infrared spectroscopy of aqueous solvation shells. J. Chem. Phys. 2001, 115, 8942–8948. [Google Scholar] [CrossRef]
- Fayer, M.D.; Moilanen, D.E.; Wong, D.; Rosenfeld, D.E.; Fenn, E.E.; Park, S. Water dynamics in salt solutions studied with ultrafast two-dimensional infrared (2D IR) vibrational echo spectroscopy. Acc. Chem. Res. 2009, 42, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Weissenborn, P.K.; Pugh, R.J. Surface tension of aqueous solutions of electrolytes: Relationship with ion hydration, oxygen solubility, and bubble coalescence. J. Colloid Interface Sci. 1996, 184, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Pestova, O.N.; Kostikov, Y.P.; Khripun, M.K. X-ray phase analysis of structure of water-salt systems: NaCl-H2O and KCl-H2O. Russ. J. Appl. Chem. 2004, 77, 1066–1069. [Google Scholar] [CrossRef]
- Suresh, S.J.; Kapoor, K.; Talwar, S.; Rastogi, A. Internal structure of water around cations. J. Mol. Liq. 2012, 174, 135–142. [Google Scholar] [CrossRef]
- O’Brien, J.T.; Williams, E.R. Effects of ions on hydrogen-bonding water networks in large aqueous nanodrops. J. Am. Chem. Soc. 2012, 134, 10228–10236. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, M.; Ohshima, Y.; Tsubouchi, M. Ion effects on the structure of water studied by terahertz time-domain spectroscopy. Chem. Phys. Lett. 2014, 591, 317–322. [Google Scholar] [CrossRef]
- Grijalba, M.T.; Cheron, M.; Borowski, E.; Bolard, J.; Schreier, S. Modulation of polyene antibiotics self-association by ions from the hofmeister series. Biochim. Biophys. Acta 2006, 1760, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Kameda, Y.; Sasaki, M.; Yaegashi, M.; Tsuji, K.; Oomori, S.; Hino, S.; Usuki, T. Hydration structure around the carboxyl group studied by neutron diffraction with 12C/13C and H/D isotopic substitution methods. J. Solut. Chem. 2004, 33, 733–745. [Google Scholar] [CrossRef]
- Fedotova, M.V.; Kruchinin, S.E. Hydration of acetic acid and acetate ion in water studied by 1d-RISM theory. J. Mol. Liq. 2011, 164, 201–206. [Google Scholar] [CrossRef]
- Despa, F.; Fernández, A.; Berry, R.S. Dielectric modulation of biological water. Phys. Rev. Lett. 2004, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Yang, Y.; Kao, Y.-T.; Wang, L.; Zhong, D.P. Protein hydration dynamics and molecular mechanism of coupled water-protein fluctuations. J. Am. Chem. Soc. 2009, 131, 10677–10691. [Google Scholar] [CrossRef] [PubMed]
- Stirnemann, G.; Rossky, P.J.; Hynes, J.T.; Laage, D. Water reorientation, hydrogen-bond dynamics and 2D-IR spectroscopy next to an extended hydrophobic surface. Faraday Discuss. 2010, 146, 263–281. [Google Scholar] [CrossRef] [PubMed]
- King, J.T.; Kubarych, K.J. Site-specific coupling of hydration water and protein flexibility studied in solution with ultrafast 2D-IR spectroscopy. J. Am. Chem. Soc. 2012, 134, 18705–18712. [Google Scholar] [CrossRef] [PubMed]
- Del Val, C.; Bondar, L.; Bondar, A.-N. Coupling between inter-helical hydrogen bonding and water dynamics in a proton transporter. J. Struct. Biol. 2014, 186, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Ebbinghaus, S.; Kim, S.J.; Heyden, M.; Yu, X.; Heugen, U.; Gruebele, M.; Leitner, D.M.; Havenith, M. An extended dynamical hydration shell around proteins. Proc. Natl. Acad. Sci. USA 2007, 104, 20749–20752. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Li, R.Y.; Zeitler, J.A.; Huber, T.L.; Gladden, L.F.; Middelberg, A.P.J.; Falconer, R.J. Terahertz and far infrared spectroscopy of alanine-rich peptides having variable ellipticity. Opt. Express 2010, 18, 27431–27444. [Google Scholar] [CrossRef] [PubMed]
- Bye, J.W.; Meliga, S.; Ferachou, D.; Cinque, G.; Zeitler, J.A.; Falconer, R.J. Analysis of the hydration water around bovine serum albumin using terahertz coherent synchrotron radiation. J. Phys. Chem. A 2014, 118, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, A.R.; Cannistraro, S. Molecular dynamics of water at the protein-solvent interface. J. Phys. Chem. B 2002, 106, 6617–6633. [Google Scholar] [CrossRef]
- Sirotkin, V.A.; Komissarov, I.A.; Khadiullina, A.V. Hydration of proteins: Excess partial volumes of water and proteins. J. Phys. Chem. B 2012, 116, 4098–4105. [Google Scholar] [CrossRef] [PubMed]
- Branca, C.; Magazu, S.; Maisano, G.; Migliardo, P. Anomalous cryoprotective effectiveness of trehalose: Raman scattering evidences. J. Chem. Phys. 1999, 111, 281–287. [Google Scholar] [CrossRef]
- Engelsen, S.B.; Perez, S. Unique similarity of the asymmetric trehalose solid-state hydration and the diluted aqueous-solution hydration. J. Phys. Chem. B 2000, 104, 9301–9311. [Google Scholar] [CrossRef]
- Lee, S.L.; Debenedetti, P.G.; Errington, J.R. A computational study of hydration, solution structure, and dynamics in dilute carbohydrate solutions. J. Chem. Phys. 2005, 122. [Google Scholar] [CrossRef]
- Lupi, L.; Comez, L.; Paolantoni, M.; Perticaroli, S.; Sassi, P.; Morresi, A.; Ladanyi, B.M.; Fioretto, D. Hydration and aggregation in mono- and disaccharide aqueous solutions by gigahertz-to-terahertz light scattering and molecular dynamics simulations. J. Phys. Chem. B 2012, 116, 14760–14767. [Google Scholar] [CrossRef] [PubMed]
- Heugen, U.; Schwaab, G.; Bründermann, E.; Heyden, M.; Yu, X.; Leitner, D.M.; Havenith, M. Solute-induced retardation of water dynamics probed directly by terahertz spectroscopy. Proc. Natl. Acad. Sci. USA 2006, 103, 12301–12306. [Google Scholar] [CrossRef] [PubMed]
- Heyden, M.; Bründermann, E.; Heugen, U.; Niehues, G.; Leitner, D.M.; Havenith, M. Long-range influence of carbohydrates on the solvation dynamics of water-answers from terahertz absorption measurements and molecular modeling simulations. J. Am. Chem. Soc. 2008, 130, 5773–5779. [Google Scholar] [CrossRef] [PubMed]
- Shiraga, K.; Suzuki, T.; Kondo, N.; de Baerdemaeker, J.; Ogawa, Y. Quantitative characterization of hydration state and destructuring effect of monosaccharides and disaccharides on water hydrogen bond network. Carbohydr. Res. 2015, 406, 46–54. [Google Scholar] [CrossRef]
- Sajadi, M.; Berndt, F.; Richter, C.; Gerecke, M.; Mahrwald, R.; Ernsting, N.P. Observing the hydration layer of trehalose with a linked molecular terahertz probe. J. Phys. Chem. Lett. 2014, 5, 1845–1849. [Google Scholar] [CrossRef]
- Marin, A.G.; Lohse, D. Building water bridges in air: Electrohydrodynamics of the floating water bridge. Phys. Fluids 2010, 22. [Google Scholar] [CrossRef]
- Min, L.Y.; Yao, T.; Chao, L.J. The affection to water clusters on temperatures and electric fields by molecular dynamics simulation. J. Southwest Univ. Natl. Nat. Sci. Ed. 2009, 35, 151–156. [Google Scholar]
- Hayashi, H. Microwater, the Natural Solution; Water Institute: Tokyo, Japan, 1996. [Google Scholar]
- Danielewicz-Ferchmin, I.; Ferchmin, A.R. Water at ions, biomolecules and charged surfaces. Phys. Chem. Liq. 2004, 42, 1–36. [Google Scholar] [CrossRef]
- Shevkunov, S.V.; Vegiri, A. Electric field induced transitions in water clusters. J. Mol. Struct. THEOCHEM 2002, 593, 19–32. [Google Scholar] [CrossRef]
- Sun, W.; Xu, X.B.; Zhang, H.; Xu, C.X. Effects of dipole polarization of water molecules on ice formation under an electrostatic field. Cryobiology 2008, 56, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Kimiko, E.; Fumiko, N.; Kumiko, H.; Takahiko, H.; Katsumi, S.; Kousaku, M.; Shigeo, A. Effects of high-voltage electric field treatment on the water activity of bread. Biosci. Biotechnol. Biochem. 1996, 60, 1444–1449. [Google Scholar] [CrossRef]
- Bateni, A.; Susnar, S.S.; Amirfazli, A.; Neumann, A.W. Development of a new methodology to study drop shape and surface tension in electric fields. Langmuir 2004, 20, 7589–7597. [Google Scholar] [CrossRef] [PubMed]
- Han, J.U.; Zhou, X.Y.; Liu, H.T. Ab initio simulation on the mechanism of proton transport in water. J. Power Sources 2006, 161, 1420–1427. [Google Scholar] [CrossRef]
- Vegiri, A. Reorientational relaxation and rotational-translational coupling in water clusters in a d.c. External electric field. J. Mol. Liq. 2004, 110, 155–168. [Google Scholar] [CrossRef]
- English, N.J.; MacElroy, J. Molecular dynamics simulations of microwave heating of water. J. Chem. Phys. 2003, 118, 1589–1592. [Google Scholar] [CrossRef]
- De Ninno, A.; Castellano, A.C. On the effect of weak magnetic field on solutions of glutamic acid: The function of water. J. Phys. Conf. Ser. 2011. [Google Scholar] [CrossRef]
- Shen, X. Increased dielectric constant in the water treated by extremely low frequency electromagnetic field and its possible biological implication. J. Phys. Conf. Ser. 2011, 329. [Google Scholar] [CrossRef]
- Goldsworthy, A.; Whitney, H.; Morris, E. Biological effects of physically conditioned water. Water Res. 1999, 33, 1618–1626. [Google Scholar] [CrossRef]
- Zhou, K.X.; Lu, G.W.; Zhou, Q.C.; Song, J.H.; Jiang, S.T.; Xia, H.R. Monte Carlo simulation of liquid water in a magnetic field. J. Appl. Phys. 2000, 88, 1802–1805. [Google Scholar] [CrossRef]
- Pang, X.F.; Deng, B. Investigation of changes in properties of water under the action of a magnetic field. Sci. China Ser. G Phys. Mech. Astron. 2008, 51, 1621–1632. [Google Scholar] [CrossRef]
- Iwasaka, M.; Ueno, S. Structure of water molecules under 14 T magnetic field. J. Appl. Phys. 1998, 83, 6459–6461. [Google Scholar] [CrossRef]
- Cai, R.; Yang, H.W.; He, J.S.; Zhu, W.P. The effects of magnetic fields on water molecular hydrogen bonds. J. Mol. Struct. 2009, 938, 15–19. [Google Scholar] [CrossRef]
- Krems, R.V. Breaking van der waals molecules with magnetic fields. Phys. Rev. Lett. 2004, 93. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.f.; Deng, B. Infrared absorption spectra of pure and magnetized water at elevated temperatures. EPL 2010, 92. [Google Scholar] [CrossRef]
- Coey, J.M.D. Magnetic water treatment-how might it work? Philos. Mag. 2012, 92, 3857–3865. [Google Scholar] [CrossRef]
- Szkatula, A.; Balanda, M.; Kopec, M. Magnetic treatment of industrial water. Silica activation. Eur. Phys. J. Appl. Phys. 2002, 18, 41–49. [Google Scholar] [CrossRef]
- Su, N.; Wu, C.-F. Effect of magnetic field treated water on mortar and concrete containing fly ash. Cem. Concr. Compos. 2003, 25, 681–688. [Google Scholar] [CrossRef]
- Ozeki, S.; Wakai, C.; Ono, S. Is a magnetic effect on water adsorption possible? J. Phys. Chem. 1991, 95, 10557–10559. [Google Scholar] [CrossRef]
- Tao, R.; Huang, K. Reducing blood viscosity with magnetic fields. Phys. Rev. E 2011, 84. [Google Scholar] [CrossRef]
- Guo, J.; Meng, X.Z.; Chen, J.; Peng, J.B.; Sheng, J.M.; Li, X.-Z.; Xu, L.M.; Shi, J.-R.; Wang, E.; Jiang, Y.; et al. Real-space imaging of interfacial water with submolecular resolution. Nat. Mater. 2014, 13, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Tanugi, D.; Grossman, J.C. Water desalination across nanoporous graphene. Nano Lett. 2012, 12, 3602–3608. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Ma, K.; Yang, Z. Changes of Water Hydrogen Bond Network with Different Externalities. Int. J. Mol. Sci. 2015, 16, 8454-8489. https://doi.org/10.3390/ijms16048454
Zhao L, Ma K, Yang Z. Changes of Water Hydrogen Bond Network with Different Externalities. International Journal of Molecular Sciences. 2015; 16(4):8454-8489. https://doi.org/10.3390/ijms16048454
Chicago/Turabian StyleZhao, Lin, Kai Ma, and Zi Yang. 2015. "Changes of Water Hydrogen Bond Network with Different Externalities" International Journal of Molecular Sciences 16, no. 4: 8454-8489. https://doi.org/10.3390/ijms16048454
APA StyleZhao, L., Ma, K., & Yang, Z. (2015). Changes of Water Hydrogen Bond Network with Different Externalities. International Journal of Molecular Sciences, 16(4), 8454-8489. https://doi.org/10.3390/ijms16048454