Abstract
Aims: To determine the effects of resistance training (RT) on the expression of microRNA (miRNA)-214 and its target in sarcoplasmic reticulum Ca2+-ATPase (SERCA2a), and on the morphological and mechanical properties of isolated left ventricular myocytes. Main methods: Male Wistar rats were divided into two groups (n = 7/group): Control (CO) or trained (TR). The exercise-training protocol consisted of: 4 × 12 bouts, 5×/week during 8 weeks, with 80% of one repetition maximum. Key findings: RT increased the left ventricular myocyte width by 15% and volume by 12%, compared with control animals (p < 0.05). The time to half relaxation and time to peak were 8.4% and 4.4% lower, respectively, in cells from TR group as compared to CO group (p < 0.05). RT decreased miRNA-214 level by 18.5% while its target SERCA2a expression were 18.5% higher (p < 0.05). Significance: Our findings showed that RT increases single left ventricular myocyte dimensions and also leads to faster cell contraction and relaxation. These mechanical adaptations may be related to the augmented expression of SERCA2a which, in turn, may be associated with the epigenetic modification of decreased miRNA-214 expression.
1. Introduction
Several studies suggest that resistance training (RT) has beneficial effects on the cardiovascular system and can potentially be an effective treatment for various clinical conditions as such heart disease [1,2,3,4,5]. However, the molecular cardiovascular adaptations induced by RT are not as well described as those induced by aerobic training. We and others have previously demonstrated that RT may lead to a physiological type of concentric cardiac hypertrophy, and some molecular and cellular adaptations have been described [4,5,6,7].
Aerobic exercise is characterized by the use of large muscle groups in dynamic physical activities, such as running and swimming. It increases cardiac preload and is known to induce the eccentric type of cardiac hypertrophy, which manifests as increased left ventricular (LV) cavity dimensions and proportionally augmented LV wall thickness to normalize myocardial strain [8,9,10,11]. At the ultrastructural level, new sarcomeres added in series predominate resulting in increased cardiomyocyte length [12,13,14,15,16].
On the other hand, the strength or resistance type of exercise, which increases cardiac after load due to increased peripheral vascular resistance, leads to concentric cardiac hypertrophy that differs from that induced by aerobic exercise. This physiological concentric LV hypertrophy is characterized by increased LV wall thickness and no changes in the LV cavity dimensions [7,17]. This type of hypertrophy induces the addition of new sarcomeres in parallel, which results in increased cardiomyocyte width [7,18,19]. However, this hypothesis has not yet been investigated in isolated cardiomyocytes after RT.
The LV remodeling after aerobic exercise training is well known to improve ventricular function in both healthy and disease conditions [8,10] which has been demonstrated both in isolated papillary muscle as well as in isolated cardiomyocytes from animal models [15,20,21]. The improvement in cell function by aerobic exercise training is accompanied by an increase in Ca2+ uptake by the sarcoplasmic reticulum Ca2+-ATPase (SERCA2a) that actively transports Ca2+ into the cardiac sarcoplasmic reticulum, regulates cytosolic Ca2+ concentration and plays a pivotal role in myocardial contractility [15,22]. This intrinsic contractility adaptation of cardiomyocytes is the key mechanism to explain the improved myocardial contractile function induced by exercise.
MicroRNA (miRNAs) are small (approximately 22 nucleotides) single-stranded non-coding RNAs that are transcribed into the nucleus, processed by specific enzymes and incorporated in RNA-induced silencing complexes (RISC), which inhibit the transcription of the target mRNA and its translational processing into the mature protein. We have recently described and reviewed some of the miRNAs involved in the physiological cardiac hypertrophy induced by exercise training [23,24,25,26].
Thus, since SERCA2a is an interesting target involved on cardiomyocyte function and is modulated by miRNA-214, we sought to determine the effects of RT on miRNA-214 expression and its target protein SERCA2a, and on the morphological and mechanical properties of isolated LV myocytes.
2. Results
2.1. Body Mass and Left Ventricular Mass
No difference was observed between groups for body weight (BW) during the study period. Cardiac hypertrophy analyzed by LV weight/BW ratio was 21.7% greater in the trained (TR) group compared with the control (CO) group (Table 1).

Table 1.
Body weight (BW), left ventricular (LV), left ventricular weight/body weight ratio of 7 animals per control (CO) and trained (TR) groups. Results are presented as mean ± standard deviation. * p < 0.05 when compared with the control group. One repetition maximum (1RM).
| Parameters | CO | TR |
|---|---|---|
| BW (g) | 348.9 ± 6.7 | 341.7 ± 8.5 |
| LVW (mg) | 632.1 ± 30.9 | 753.5 ± 35.4 |
| LV/BW (mg/g) | 1.8 ± 0.06 | 2.2 ± 0.04 * |
| 1RM Initial (g) | 639.2 ± 35.2 | 643.7 ± 41.4 |
| 1RM Final (g) | 1070.4 ± 41.7 | 2168.2 ± 37.8 * |
2.2. Maximal Strength
Analysis of the 1 repetition maximum (1RM) test showed an increase in the absolute weight lifted by the TR group obtained during the 1RM test. Both the CO and the TR group had similar values for 1RM at the beginning (day 0) of the protocol. After the 8-weeks of RT protocol, the load lifted by the TR group was higher (2168 ± 37 g) than that of the control (1070 ± 41 g) group, which represented a 2-fold increase for the TR group (Table 1).
2.3. Effects of Training on Cardiomyocyte Dimensions
2.4. Cell Contractility
To measure cardiomyocyte contractility, isolated LV cells were used. The time to half relaxation (Figure 2A) and time to peak (Figure 2B) were 8.4% and 4.4% lower, respectively, in cells from TR group (n = 7 rats and 108 cells) compared to those from CO group (n = 7 rats and 96 cells). These data demonstrate that resistance trained animals exhibit faster cell contraction and relaxation.
2.5. Protein and miRNA Expression
To further understand the molecular mechanisms that could explain the functional data on isolated LV myocytes, SERCA2a protein expression, a key molecule involved in Ca2+ sequestration into the sarcoplasmic reticulum, was analyzed. SERCA2a expression levels were 18.5% higher in the TR group than in CO group (Figure 3B), which may be explained by the 18.5% decrease in the miRNA-214 level (Figure 3A).
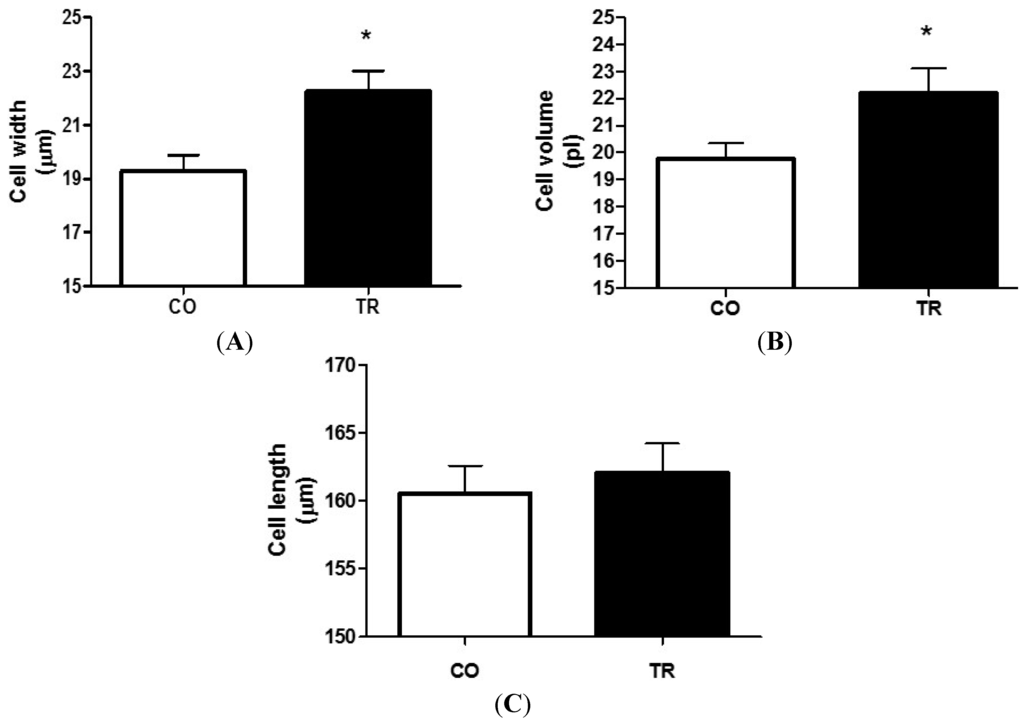
Figure 1.
Cardiomyocyte dimensions of isolated LV myocytes. (A) Cell width; (B) Cell volume; and (C) Cell length. Results are presented as mean ± standard deviation of 70 cells per group and 7 animals each group. At least 10 cells were analyzed from each animal. * p < 0.05 when compared to control group.
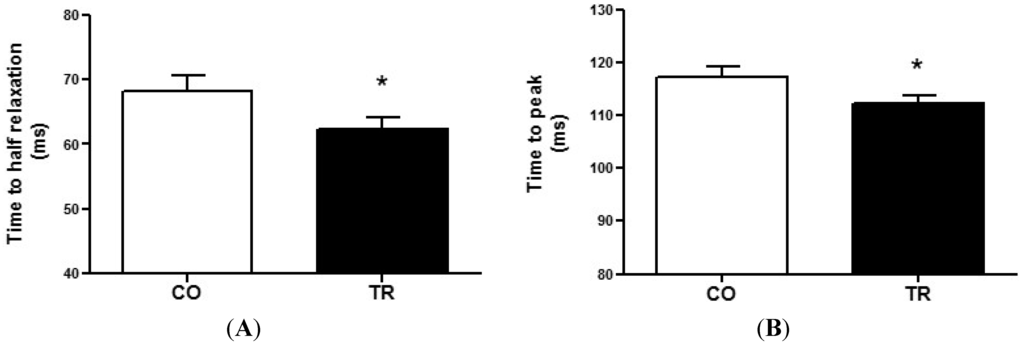
Figure 2.
Cell contractility (A) Time to half relaxation; and (B) Time to peak contraction of cardiomyocytes from trained (n = 7 rats and 108 cells) and control (n = 7 rats and 96 cells) groups. Results are presented as mean ± standard deviation. * p < 0.05 when compared to control group.
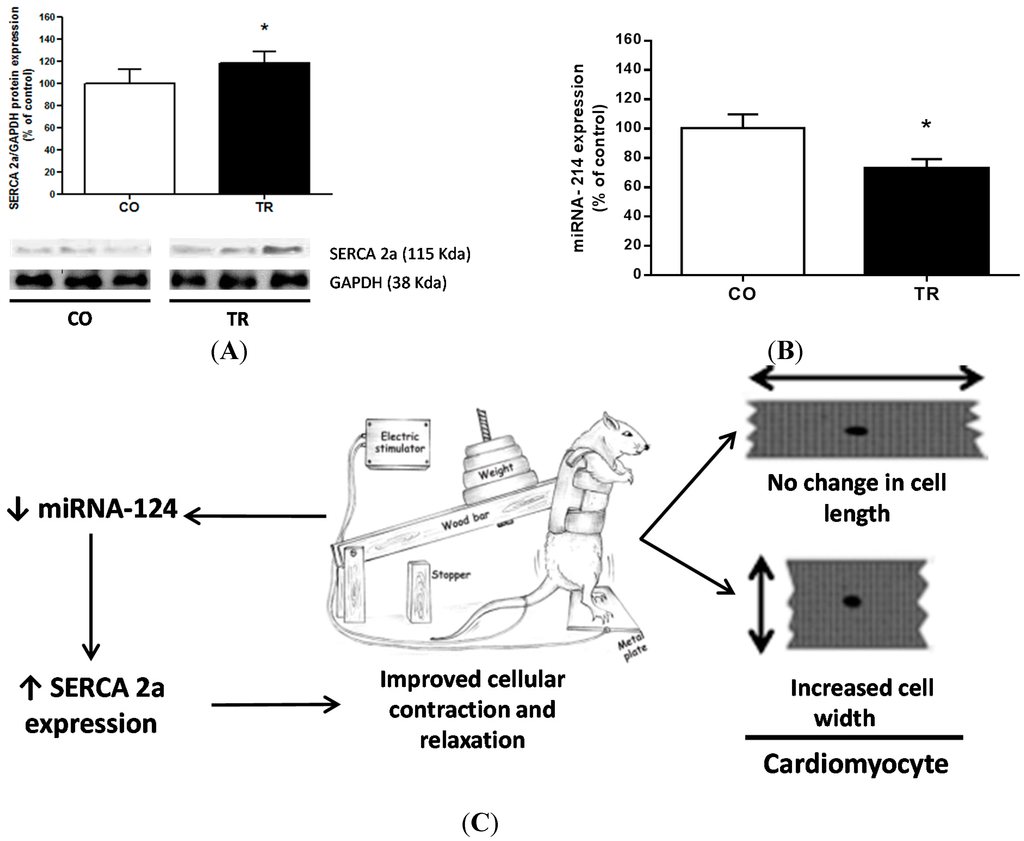
Figure 3.
Protein expression level and miRNA. (A) Representative western blot image and SERCA2a protein expression; (B) miRNA expression level; and (C) Schematic summary of the results showing both the morphological and functional adaptations induced by the resistance training. Results are presented as mean ± standard deviation. * p < 0.05 when compared to control group.
3. Discussion
The present study provides the first observations regarding the effects of RT on morphological and mechanical properties of single LV myocytes. RT increased width and volume of isolated LV myocytes. Also, exercised animals exhibited faster cell contraction and relaxation, which can be explained by the decreased miRNA-214 expression and increased SERCA2a protein levels.
In study involving humans, Fagard (1996) [11] observed that athletes engaged in static training had LV dimension increased by 2.5% and LV wall thickness increased by 12% compared with non-athletes. In addition, Dickerman et al. (1998) [27] demonstrated that a LV wall thickness greater than 13 mm can routinely be found in elite resistance-trained athletes. Previous data from our group has confirmed this data in the animal model used here showing that RT induces concentric cardiac hypertrophy. Echocardiography analysis in this animal model of RT showed a similar increase in both septum and free posterior wall mass, but no reduction in the end-diastolic LV internal diameter during 3 months of exercise training [28].
Cardiomyocytes isolated from aerobic exercise-trained rats showed increased cell length [20,29,30], with no change in thickness [13,31,32]. At the same time, in other studies, it was observed that the intermittent type of aerobic exercise training increased the thickness of LV myocytes in rats with no change in their length. It was also observed that this adaptation was more evident in cardiomyocytes from the region near to the endocardium [14,21]. Here we are showing that RT, differently from the aerobic exercise training, resulted in increased width and volume of LV myocytes when compared with sedentary control animals, while cell length was unaltered. Thus, the results show that due to the increased cardiac after load during the RT exercise sessions, cardiac cell width and volume were also increased which are in agreement with previous echocardiographic data from our group [28].
In addition to these morphological adaptations, we also observed that single cardiomyocytes had improved contraction and relaxation function. Pinter et al. (2008) [5], showed increased myosin ATPase activity associated with increased papillary muscle contractility after 8 weeks of RT. The papillary muscles of exercised rats showed increased isometric force, indicating that the RT might improve cardiac performance. In humans, data are controversial. Echocardiography results have demonstrated that cardiac function is not altered in RT athletes [18,33], although other studies have shown enhanced systolic function [9,34] and one study indicated enhanced diastolic function [35]. However, with respect to the results observed by echocardiography, although it is a measure of the cardiac function in the whole organ, it is necessary to consider that this involves a large number of mathematical calculations while the direct cellular measurements in isolated myocytes are more reliable.
Aerobic exercise training improves cardiomyocyte contractility and Ca2+ handling [31,32], which optimizes cardiac performance [36,37]. Cardiac myocyte shortening and relaxation kinetics are regulated by Ca2+ regulatory proteins, contractile protein isoform expression patterns, and different action potential waveform and duration [22,38]. Although we did not analyze all these possible mechanisms, the increase in SERCA2a protein expression, which is responsible for 92% of Ca2+ reuptake in rat ventricular cells [37] may partly explain our findings. The time to peak and time to half relaxation were lower in cells of the TR group. Our findings might also be explained by recent studies suggesting that mice with overexpression of the SERCA2a pump exhibit increased sarcoplasmic reticulum Ca2+ transport, which in turn increases the rates of cardiac contraction and relaxation without developing cardiac pathology [39,40,41,42]. Furthermore, a possible role for other important modulators of SERCA2a, such as its ATPase activity and the phospholamban phosphorylation, which regulates SERCA2a function, must be considered as well [43].
miRNAs have been implicated in regulating the expression of genes that are involved in multiple biological processes of cardiovascular disease and also as potential drug targets[44]. Recently, Gurha et al. (2012) [45], showed that genetic ablation of miRNA-22 regulates target proteins that function as transcription factors for SERCA2a expression, and Wahlquist et al. (2014) [46], showed that miRNA-25 regulates SERCA2a and contributes to declining cardiac function during heart failure. On the other hand, Aurora et al. (2012) [47], reported that miRNA-214 targets both sodium/calcium exchanger 1 (NCX) and proapoptotic effectors of Ca2+-signaling pathways like CaMKII and cyclophilin D. Through in silico analysis of predicted targets for miRNA, we verified a possible relationship between SERCA2a and miRNA-214. Here, our results showed that decreased miRNA-214 levels in the trained group may explain the increased expression of SERCA2a. This relationship becomes of great interest because our results show that the regulation of SERCA2a by miRNA-214 occurs by exercise training. Such regulation is crucial in as much as SERCA2a represents 90% of the total membrane proteins in the sarcoplasmic reticulum of the rat myocardium and has a massive impact on cardiac contractile function.
The SERCA2a isoform is also found in skeletal muscle and is modulated by aerobic exercise training. In this context, SERCA2a expression has been shown to be modulated by the adenosine monophosphate-activated protein kinase (AMPK)-α2 in type I skeletal muscle fibers [48] and by adiponectin [49], two other possible pathways that were not investigated here.
4. Methods
4.1. Animals
All procedures were in accordance with the Brazilian Society for Laboratory Animal Science (COBEA) and were approved by the Ethics Committee of the School of Physical Education and Sport of the University of São Paulo (Process Number: 2009/34, Date of Approval: 9 April 2009). Fourteen male Wistar rats (250–300 g and 10-weeks-old) were randomly divided into two groups (n = 7): Control (CO) and trained (TR). Animals were housed in standard cages, with food and water ad libitum. The environmental temperature was kept at 23 ± 1 °C, and a 12:12-h dark-light cycle was maintained throughout the experiment.
4.2. Exercise Training Protocol
Animals were trained following previous studies by our group [3,4] (Figure 4) and adapted from Tamaki et al. (1992) [50]. Briefly, rats were wearing canvas jackets to be able to regulate the twisting and flexion of their torsos and were suspended in a standard position on their hindlimbs. Electrical stimulation (20 V, 0.3-s duration, at 3-s intervals) was applied to the tail through a surface electrode. Stimulated rats flexed their legs repeatedly, which lifted the weight arm of the training apparatus. The rats were trained by 4 × 12 repetitions, with a 90-s rest period between each set for eight weeks. The animals were adapted for one week and on the last day of adaptation, the maximum weight lifted [one repetition maximum (1RM)] was measured with the squat-training apparatus, and the training load was set at 80% of this value. The 1RM value was defined as the minimum load in which the rats were unable to jump after electrical stimulation. Exercise training sessions were performed in a dark room.
4.3. Cardiomyocytes Isolation
Twenty-four hours after the last 1RM test, the rats were killed by decapitation under resting conditions, and their hearts were quickly removed and weighed. Ventricular cardiomyocytes were enzymatically isolated as previously described [14,16]. Briefly, the hearts were mounted on a home-made Langendorff system and perfused for 5 min with a modified Hepes–Tyrode’s solution of the following composition (in mM): 130 NaCl, 1.43 MgCl2, 5.4 KCl, 0.75 CaCl2, 5.0 Hepes [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], 10.0 glucose, 20.0 taurine, and 10.0 creatine, pH 7.4 at 37 °C. The perfusion solution was changed for the calcium-free solution containing 0.1 mM EGTA (Ethylene glycol-bis(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid) for 6 min. Afterwards, the hearts were perfused for 15–20 min with a solution containing 1 mg/mL collagenase type II (Worthington, Cooper Biomedical Co., Freehold, NJ, USA). The digested heart was then removed from the cannula, the LV was dissected and cut into small pieces. The left ventricle tissues were placed into small conical flasks with collagenase-containing solution supplemented with 1% bovine serum albumin (Sigma Chemicals, St. Louis, MO, USA). The cells were dispersed by shaking the flasks at 37 °C for periods of 5 min. Then, single cells were separated from the non-dispersed tissue by filtration. The resulting cell suspension was centrifuged and resuspended in Hepes–Tyrode’s solution. Non-dispersed tissue was subjected to further enzyme treatment. The isolated cells were stored at 5 °C until use. Only Ca2+ tolerant, quiescent, rod-shaped myocytes showing clear cross striations were studied. The isolated cardiomyocytes were used within 2–3 h after isolation.
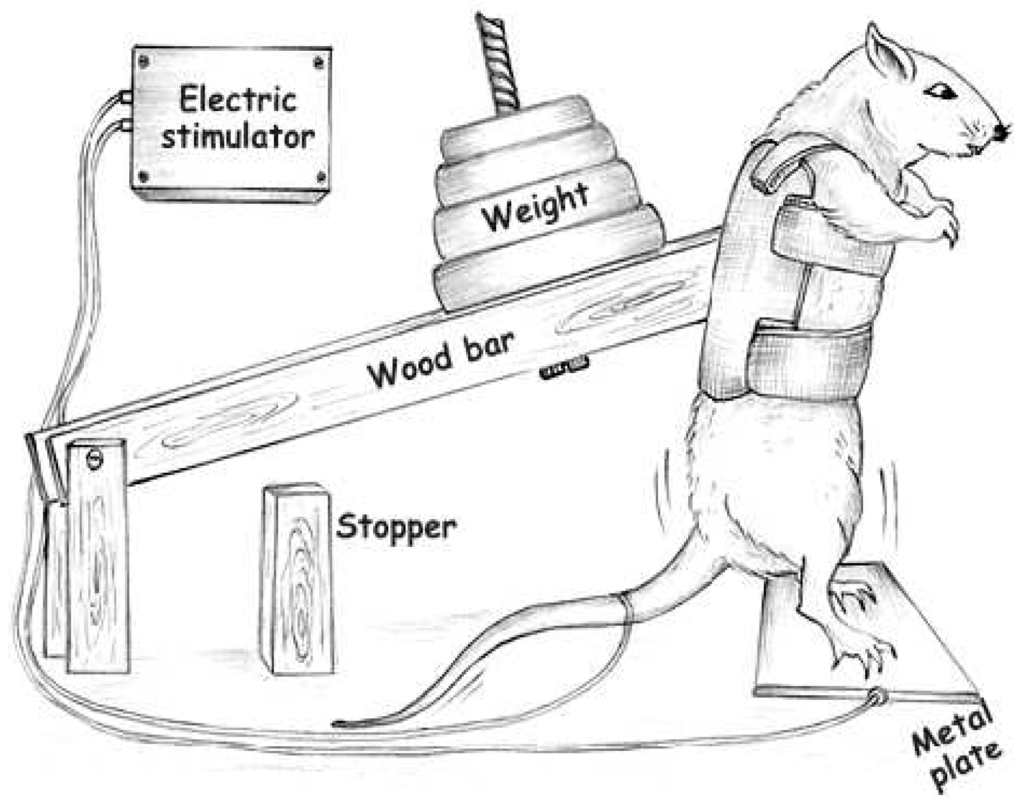
Figure 4.
Apparatus adapted from Tamaki et al. (1992) [50], used to perform resistance training in the rats [3,4].
4.4. Measurements of Cell Contractility and Morphology
Cell contractility was evaluated as previously described [16]. Briefly, isolated cells were placed in a chamber with a glass coverslip base mounted on the stage of an inverted microscope (Nikon Eclipse TS100, Nikon, Kawasaki, Japan). The chamber was perfused with Hepes-Tyrode’s solution at room temperature [16]. Steady-state 1-Hz contractions were elicited via platinum bath electrodes (MyoPacer field stimulator; IonOptix, Milton, MA, USA) with 5-ms-duration voltage pulses and an intensity of 20 V. Cells were visualized on a personal computer monitor with a NTSC camera (MyoCam, IonOptix, Milton, MA, USA) in partial scanning mode. This image was used to measure cell shortening (our index of contractility) in response to electrical stimulation using a video motion edge detector (IonWizard, IonOptix). The cell image was sampled at 240 Hz. Cell shortening was calculated from the output of the edge detector using an IonWizard analog-to-digital converter (IonOptix). Time to peak of contraction and time to half-relaxation was calculated as previously described [16]. The cell image was also used to determine cell lengths and widths, which were used to calculate the cell volume as previously described [21]. Measurements were performed in at least 10 cells from each animal, and in 7 animals from each group. The total numbers of cells analyzed are described in the legend of each figure.
4.5. Western Blot Analysis
Frozen ventricles were thawed and minced into small pieces and homogenized in cell lysis buffer containing 100 mM Tris, 50 mM NaCl, 10 mM EDTA, 1% Triton X-100, and a mixture of protease inhibitors [phenylmethanesulfonyl fluoride (1 mM) o-phenanthroline (30 mM), pepstatin A (1 mM) and 4-(chloromercuribenzoic acid) (1 mM)]. The heart debris tissues were removed by centrifugation at 3000× g, 4 °C, for 10 min. A total of 20 µg of protein per sample was loaded and subjected to SDS-PAGE gels. After electrophoresis, proteins were electro transferred to nitrocellulose membrane (Amersham Biosciences; Piscataway, NJ, USA). The blot membrane was incubated in a blocking buffer (5% nonfat dry milk, 10 mM Tris-HCl, pH 7.6, 150 mM NaCl, and 0.1% Tween 20) for 2 h at room temperature and probed with a polyclonal antibody directed against SERCA2a (1:2500; Abcam, Cambridge, UK) and a polyclonal anti-GAPDH antibody (1:2000; Abcam). The level of expression of GAPDH was used to normalize the results. Binding of the primary antibody was detected with the use of peroxidase-conjugated secondary antibodies, and enhanced chemiluminescence reagents (Amersham Biosciences; Piscataway, NJ, USA) were used to visualize the autoradiogram, which was later exposed to film. The film was developed, and the bands were analyzed using Scion Image software (Scion based on National Institutes of Health image). Total GAPDH protein expression was used to normalize the results. Protein content was determined using the protein assay by Bradford method (Bio-Rad, Richmond, CA, USA) and BSA (0.1–1 mg/mL) as standard.
4.6. miRNA Quantification Using Real-Time PCR
The relative expression of miRNA-214 and U6 were analyzed by polymerase chain reaction (PCR), described as follows. cDNA for miRNA analysis was synthesized from total RNA using gene-specific primers according to the TaqMan MicroRNA Assay protocol (Applied Biosystems, Foster City, CA, USA). In order to accurately detect the expression of miRNAs, real-time PCR quantification was performed using TaqMan MicroRNA Assay protocol (Applied Biosystems). TaqMan MicroRNA Assay protocol for miRNA-214 (ID000517). Samples were normalized by evaluating U6 (ID4373381) expression. Relative quantities of target gene expressions were compared after normalization to the values of reference gene (ΔCt). Fold changes in miRNA expression were calculated using the differences in ΔCt values between the two samples (ΔΔCt) and equation 2−ΔΔCt. The results were expressed as % of control.
4.7. Statistical Analysis
Differences between groups were assessed using unpaired t-tests. The initial and final 1RM was assessed using paired t-test. Results are presented as mean ± standard deviation (SD). Data were significant when p < 0.05 compared with the control group.
5. Conclusions
We have shown that RT increased the width and volume of LV myocytes. In addition, we have observed that trained animals exhibited faster cardiomyocyte contraction and relaxation, and that this adaptation is at least partly explained by improved SERCA2a expression and decrease of miRNA-214 with RT. The morphological and mechanical cellular adaptations reported here may contribute to our understanding of mechanisms involved in cardiac hypertrophy and contractile activity in the heart as a result of RT. In summary our results suggest that the RT induces cardiac hypertrophy with improved contractile function of isolated cardiomyocytes.
Acknowledgments
Melo SFS is the recipient of a FAPESP Fellowship (No. 2010/09438-0). Oliveira EM was the recipient of a CNPq-PDE Fellowship (No. 308267/2013-3) and Barauna VG CNPq-Universal (No. 485873/2012-5). Natali AJ is a CNPq fellow. This work was supported by FAPESP (Project No. 2009/18370-3, 2010/50048-1) and USP/PRP-NAPmiR (No. 476515/2012-2).
Author Contributions
All authors contributed equally to this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alves, J.P.; Nunes, R.B.; Stefani, G.P.; dal Lago, P. Resistance training improves hemodynamic function, collagen deposition and inflammatory profiles: Experimental model of heart failure. PLoS ONE 2014, 9, e110317. [Google Scholar] [CrossRef] [PubMed]
- Braith, R.W.; Beck, D.T. Resistance exercise: Training adaptations and developing a safe exercise prescription. Heart Fail. Rev. 2008, 13, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Drummond, L.R.; Carlo, R.J.; Melo, S.F.S.; Carneiro-Junior, M.A.; Silva, K.A.; Rodrigues, A.C.; Soares, P.N.P.; Gomes, T.N.P.; Lousada, M.J.Q.; Oliveira, E.M.; et al. Enhanced femoral neck strength in response to weightlifting exercise training in maturing male rats. Int. Sport Med. J. 2013, 14, 155–167. [Google Scholar]
- Barauna, V.G.; Magalhaes, F.C.; Krieger, J.E.; Oliveira, E.M. AT1 receptor participates in the cardiac hypertrophy induced by resistance training in rats. Am. J. Physiol. 2008, 295, R381–R387. [Google Scholar]
- Pinter, R.D.E.; Padilha, A.S.; Oliveira, E.M.; Vassallo, D.V.; Fucio, L.J.H. Cardiovascular adaptive responses in rats submitted to moderate resistance training. Eur. J. Appl. Physiol. 2008, 103, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Naylor, L.H.; George, K.; O’Driscoll, G.; Green, D.J. The athlete’s heart: A contemporary appraisal of the “Morganroth hypothesis”. Sports Med. 2008, 38, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, B.C.; Weeks, K.L.; Pretorius, L.; McMullen, J.R. Molecular distinction between physiological and pathological cardiac hypertrophy: Experimental findings and therapeutic strategies. Pharmacol. Ther. 2010, 128, 191–227. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.F.S.; Lunz, W.; Fontes, E.P.B.; Dias, C.M.G.C.; Júnior, M.A.C.; de Moura, A.G.; del Carlo, R.J.; Natali, A.J. Different levels of Hsp72 in female rat myocardium in response to voluntary exercise and forced exercise. Arq. Bras. Cardiol. 2009, 93, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Colan, S.D.; Sanders, S.P.; Borow, K.M. Physiologic hypertrophy: Effects on left ventricular systolic mechanics in athletes. J. Am. Coll. Cardiol. 1987, 9, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Hossack, K.F. Cardiovascular responses to dynamic exercise. Cardiol. Clin. 1987, 5, 147–156. [Google Scholar] [PubMed]
- Fagard, R.H. Athlete’s heart: A meta-analysis of the echocardiographic experience. Int. J. Sports Med. 1996, 17, 140–144. [Google Scholar] [CrossRef] [PubMed]
- White, F.C.; Witzel, G.; Breisch, E.A.; Bloor, C.M.; Nimmo, L.E. Regional capillary and myocyte distribution in normal and exercise trained male and female rat hearts. Am. J. Cardiovasc. Pathol. 1988, 2, 247–253. [Google Scholar] [PubMed]
- Laughlin, M.H.; Schaefer, M.E.; Sturek, M. Effect of exercise training on intracellular free Ca2+ transients in ventricular myocytes of rats. J. Appl. Physiol. 1992, 73, 1441–1448. [Google Scholar] [PubMed]
- Natali, A.J.; Turner, D.L.; Harrison, S.M.; White, E. Regional effects of voluntary exercise on cell size and contraction-frequency responses in rat cardiac myocytes. J. Exp. Biol. 2001, 204, 1191–1199. [Google Scholar] [PubMed]
- Wisløff, U.; Loennechen, J.P.; Falck, G.; Beisvag, V.; Currie, S.; Smith, G.; Ellingsen, O. Increased contractility and calcium sensitivity in cardiac myocytes isolated from endurance trained rats. Cardiovasc. Res. 2001, 50, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Primola-Gomes, T.N.; Campos, L.A.; Lauton-Santos, S.; Balthazar, C.H.; Guatimosim, S.; Capettini, L.S.; Lemos, V.S.; Coimbra, C.C.; Soares, D.D.; Carneiro-Júnior, M.A.; et al. Exercise capacity is related to calcium transients in ventricular cardiomyocytes. J. Appl. Physiol. 2009, 107, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Grossman, W.; Jones, D.; McLaurin, L.P. Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Investig. 1975, 56, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Longhurst, J.C.; Kelly, A.R.; Gonyea, W.J.; Mitchell, J.H. Echocardiographic left ventricular masses in distance runners and weight lifters. J. Appl. Physiol. 1980, 48, 154–162. [Google Scholar] [PubMed]
- Barauna, V.G.; Batista, M.L., Jr.; Costa Rosa, L.F.; Casarini, D.E.; Krieger, J.E.; Oliveira, E.M. Cardiovascular adaptations in rats submitted to a resistance-training model. Clin. Exp. Pharmacol. Physiol. 2005, 32, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.L.; Korzick, D.H. Cellular adaptations of the myocardium to chronic exercise. Prog. Cardiovasc. Dis. 1995, 37, 371–396. [Google Scholar] [CrossRef] [PubMed]
- Natali, A.J.; Wilson, L.A.; Peckham, M.; Turner, D.L.; Harrison, S.M.; White, E. Different regional effects of voluntary exercise on the mechanical and electrical properties of rat ventricular myocytes. J. Physiol. 2002, 541, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Kemi, O.J.; MacQuaide, N.; Hoydal, M.A.; Ellingsen, O.; Smith, G.L.; Wisloff, U. Exercise training corrects control of spontaneous calcium waves in hearts from myocardial infarction heart failure rats. J. Cell. Physiol. 2012, 227, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Soci, U.P.; Fernandes, T.; Hashimoto, N.Y.; Mota, G.F.; Amadeu, M.A.; Rosa, K.T.; Irigoyen, M.C.; Phillips, M.I.; Oliveira, E.M. MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rats. Physiol. Genomics 2011, 43, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Das Neves, V.J.; Fernandes, T.; Redondo, F.R.R.; Soci, U.P.R.; Melo, S.F.S.; de Oliveira, E.M. Exercise training in hypertension: Role of microRNAs. World J. Cardiol. 2014, 6, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Magalhães, F.C.; Roque, F.R.; Phillips, M.I.; Oliveira, E.M. Exercise training prevents the microvascular rarefaction in hypertension balancing angiogenic and apoptotic factors: Role of microRNAs-16, -21, and -126. Hypertension 2012, 59, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.T.F.; Fernandes, T.; Baraúna, V.G.; Matos, K.C.; Santos, A.A.; Tucci, P.J.F.; Oliveira, E.M. Expression of microRNA-29 and collagen in cardiac muscle after swimming training in myocardial-infarcted rats. Cell. Physiol. Biochem. 2014, 33, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Dickerman, R.D.; Schaller, F.; McConathy, W.J. Left ventricular wall thickening does occur in elite power athletes with or without anabolic steroid use. Cardiology 1998, 90, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Barauna, V.G.; Rosa, K.T.; Irigoyen, M.C.; Oliveira, E.M. Effects of resistance training on ventricular function and hypertrophy in a rat model. Clin. Med. Res. 2007, 5, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Mokelke, E.A.; Palmer, B.M.; Cheung, J.Y.; Moore, R.L. Endurance training does not affect intrinsic calcium current characteristics in rat myocardium. Am. J. Physiol. 1997, 273, H1193–H1197. [Google Scholar] [PubMed]
- Palmer, B.M.; Thayer, A.M.; Snyder, S.M.; Moore, R.L. Shortening and [Ca2+] dynamics of left ventricular myocytes isolated from exercise-trained rats. J. Appl. Physiol. 1998, 85, 2159–2168. [Google Scholar] [PubMed]
- Diffee, G.M.; Nagle, D.F. Exercise training alters length dependence of contractile properties in rat myocardium. J. Appl. Physiol. 2003, 94, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Diffee, G.M.; Seversen, E.A.; Titus, M.M. Exercise training increases the Ca2+ sensitivity of tension in rat cardiac myocytes. J. Appl. Physiol. 2001, 91, 309–315. [Google Scholar] [PubMed]
- Pluim, B.M.; Zwinderman, A.H.; van der Laarse, A.; van der Wall, E.E. The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation 2000, 101, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Luo, X.; Murohara, T.; Yang, B.; Dobrev, D.; Nattel, S. MicroRNA regulation and cardiac calcium signaling: Role in cardiac disease and therapeutic potential. Circ. Res. 2014, 114, 689–705. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, J.D.; Tuxen, D.; Sale, D.G.; Moroz, J.R.; Sutton, J.R. Arterial blood pressure response to heavy resistance exercise. J. Appl. Physiol. 1985, 58, 785–790. [Google Scholar] [PubMed]
- Tibbits, G.F.; Barnard, R.J.; Baldwin, K.M.; Cugalj, N.; Roberts, N.K. Influence of exercise on excitation-contraction coupling in rat myocardium. Am. J. Physiol. 1981, 240, 472–480. [Google Scholar]
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.; Ramirez, R.J.; Oudit, G.Y.; Gidrewicz, D.; Trivieri, M.G.; Zobel, C.; Backx, P.H. Regulation of cardiac excitation-contraction coupling by action potential repolarization: Role of the transient outward potassium current (Ito). J. Physiol. 2003, 546, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.L.; Hashimoto, K.; Grupp, I.L.; Ji, Y.; Reed, T.; Loukianov, E.; Grupp, G.; Bhagwhat, A.; Hoit, B.; Walsh, R.; et al. Targeted overexpression of the sarcoplasmic reticulum Ca2+-ATPase increases cardiac contractility in transgenic mouse hearts. Circ. Res. 1998, 83, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, M.; Bhupathy, P.; Babu, G.J. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc. Res. 2008, 77, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, M.; Huke, S. SERCA pump level is a critical determinant of Ca2+ homeostasis and cardiac contractility. J. Mol. Cell. Cardiol. 2001, 33, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Vetter, R.; Rehfeld, U.; Reissfelder, C.; Weiss, W.; Wagner, K.D.; Günther, J.; Hammes, A.; Tschöpe, C.; Dillmann, W.; Paul, M. Transgenic overexpression of the sarcoplasmic reticulum Ca2+ATPase improves reticular Ca2+ handling in normal and diabetic rat hearts. FASEB J. 2002, 16, 1657–1659. [Google Scholar] [PubMed]
- Kemi, O.J.; Ellingsen, Ø.; Ceci, M.; Grimaldi, S.; Smith, G.L.; Condorelli, G.; Wisløff, U. Aerobic interval training enhances cardiomyocyte contractility and Ca2+ cycling by phosphorylation of CaMKII and Thr-17 of phospholamban. J. Mol. Cell. Cardiol. 2007, 43, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, E.; Olson, E.N. MicroRNA therapeutics for cardiovascular disease: Opportunities and obstacles. Nat. Rev. Drug. Discov. 2012, 11, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Gurha, P.; Abreu-Goodger, C.; Wang, T.; Ramirez, M.O.; Drumond, A.L.; van Dongen, S.; Chen, Y.; Bartonicek, N.; Enright, A.J.; Lee, B.; et al. Targeted deletion of microRNA-22 promotes stress-induced cardiac dilation and contractile dysfunction. Circulation 2012, 125, 2751–2761. [Google Scholar] [CrossRef] [PubMed]
- Wahlquist, C.; Jeong, D.; Rojas-Muñoz, A.; Kho, C.; Lee, A.; Mitsuyama, S.; van Mil, A.; Park, W.J.; Sluijter, J.P.; Doevendans, P.A.; et al. Inhibition of miR-25 improves cardiac contractility in the failing heart. Nature 2014, 508, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Aurora, A.B; Mahmoud, A.I.; Luo, X.; Johnson, B.A.; van Rooij, E.; Matsuzaki, S.; Humphries, K.M.; Hill, J.A.; Bassel-Duby, R.; Sadek, H.A.; et al. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca2+ overload and cell death. J. Clin. Investig. 2012, 122, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Morissette, M.P.; Susser, S.E.; Stammers, A.N.; O’Hara, K.A.; Gardiner, P.F.; Sheppard, P.; Moffatt, T.L.; Duhamel, T.A. Differential regulation of the fibert ype-specific gene expression of the sarcoplasmic reticulum calcium-ATPase isoforms induced by exercise training. J. Appl. Physiol. 2014, 117, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Safwat, Y.; Yassin, N.; Gamal El Din, M.; Kassem, L. Modulation of skeletal muscle performance and SERCA by exercise and adiponectin gene therapy in insulin-resistant rat. DNA Cell Biol. 2013, 32, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Uchiyama, S.; Nakano, S. A weight-lifting exercise model for inducing hypertrophy in the hindlimb muscles of rats. Med. Sci. Sports Exerc. 1992, 24, 881–886. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).