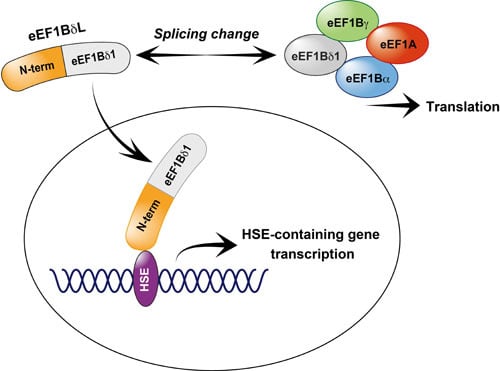
Regulation of Translation Factor EEF1D Gene Function by Alternative Splicing
Abstract
1. Introduction
2. EEF1D Gene Structure and EEF1D Homologs
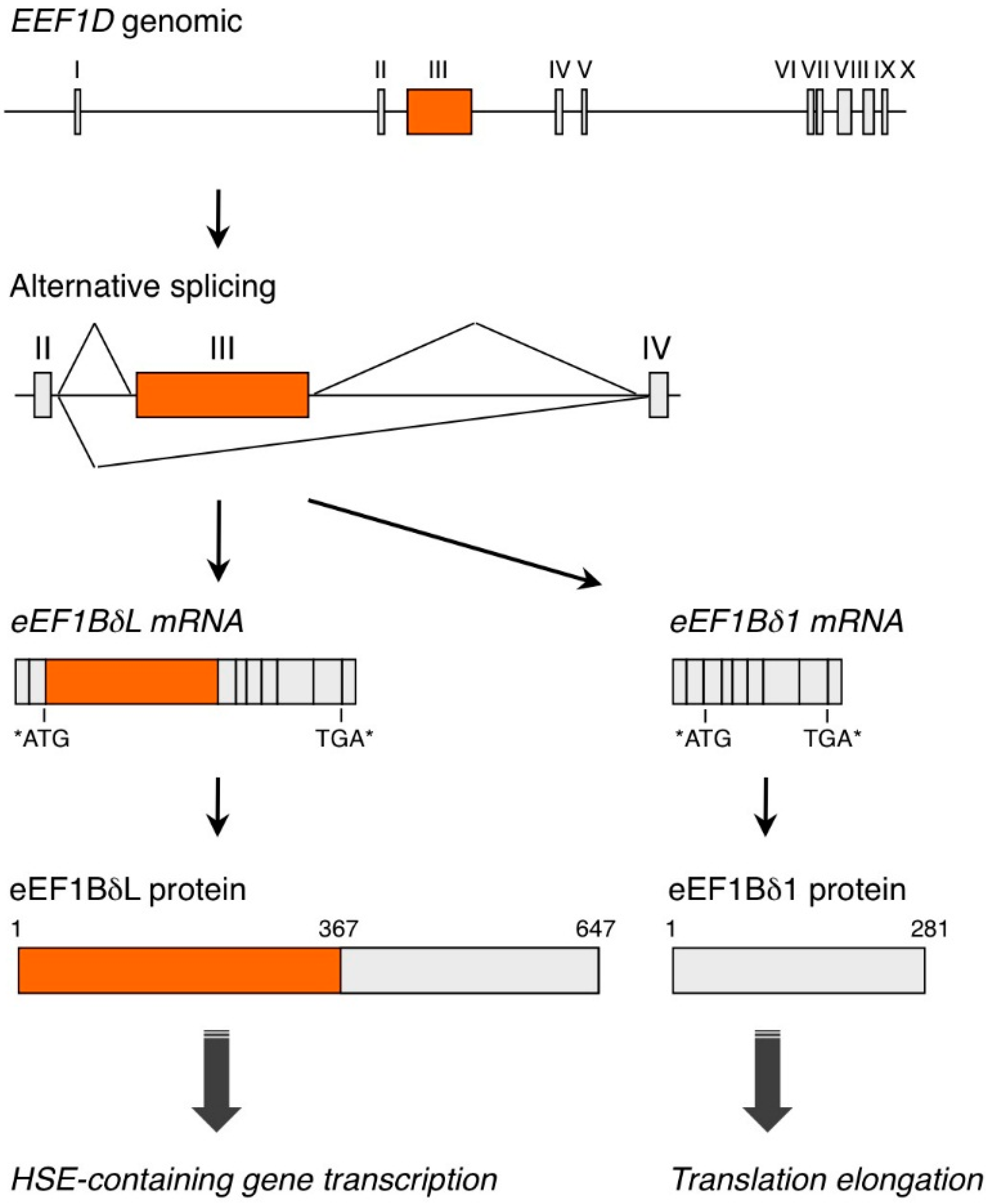
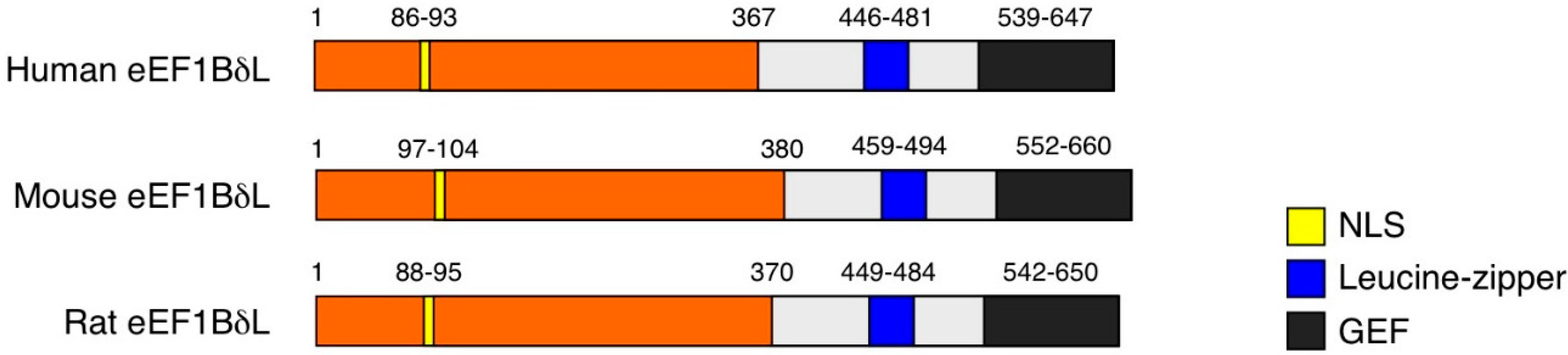
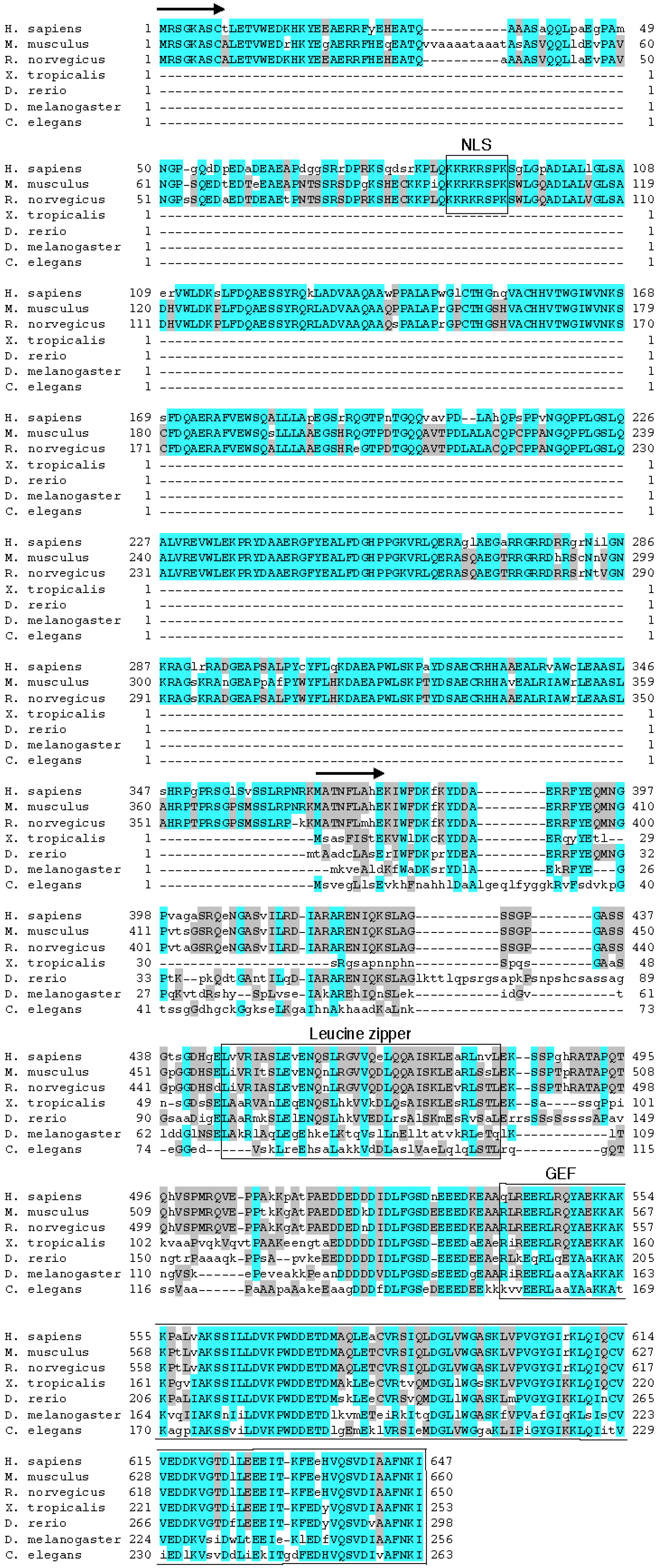
3. eEF1BδL Target Genes
4. The Role of eEF1Bδ and eEF1BδL in Stress Response
5. A Putative Role for eEF1BδL in Vivo
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kalsotra, A.; Cooper, T.A. Functional consequences of developmentally regulated alternative splicing. Nat. Rev. Genet. 2011, 12, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Manley, J.L. Mechanisms of alternative splicing regulation: Insights from molecular and genomics approaches. Nat. Rev. Mol. Cell Biol. 2009, 10, 741–754. [Google Scholar] [PubMed]
- Wang, E.T.; Sandberg, R.; Luo, S.; Khrebtukova, I.; Zhang, L.; Mayr, C.; Kingsmore, S.F.; Schroth, G.P.; Burge, C.B. Alternative isoform regulation in human tissue transcriptomes. Nature 2008, 456, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Licatalosi, D.D.; Darnell, R.B. RNA processing and its regulation: Global insights into biological networks. Nat. Rev. Genet. 2010, 11, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Kaitsuka, T.; Tomizawa, K.; Matsushita, M. Transformation of eEF1Bδ into heat-shock response transcription factor by alternative splicing. EMBO Rep. 2011, 12, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Le Sourd, F.; Boulben, S.; le Bouffant, R.; Cormier, P.; Morales, J.; Belle, R.; Mulner-Lorillon, O. eEF1B: At the dawn of the 21st century. Biochim. Biophys. Acta 2006, 1759, 13–31. [Google Scholar]
- Morales, J.; Cormier, P.; Mulner-Lorillon, O.; Poulhe, R.; Bellé, R. Molecular cloning of a new guanine nucleotide-exchange protein, EF1δ. Nucleic Acids Res. 1992, 20, 4091. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.; Raggiaschi, R.; Morales, J.; Möller, W. The human leucine zipper-containing guanine-nucleotide exchange protein elongation factor-1δ. Biochim. Biophys. Acta 1993, 1174, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Andersen, G.R.; Nissen, P.; Nyborg, J. Elongation factors in protein biosynthesis. Trends Biochem. Sci. 2003, 28, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Browne, G.J.; Proud, C.G. Regulation of peptide-chain elongation in mammalian cells. Eur. J. Biochem. 2002, 269, 5360–5368. [Google Scholar] [CrossRef] [PubMed]
- Merkin, J.; Russell, C.; Chen, P.; Burge, C.B. Evolutionary dynamics of gene and isoform regulation in Mammalian tissues. Science 2012, 338, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Ule, J.; Darnell, R.B. RNA binding proteins and the regulation of neuronal synaptic plasticity. Curr. Opin. Neurobiol. 2006, 16, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lee, J.A.; Black, D.L. Neuronal regulation of alternative pre-mRNA splicing. Nat. Rev. Neurosci. 2007, 8, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Hattori, D.; Millard, S.S.; Wojtowicz, W.M.; Zipursky, S.L. Dscam-mediated cell recognition regulates neural circuit formation. Annu. Rev. Cell Dev. Biol. 2008, 24, 597–620. [Google Scholar] [CrossRef] [PubMed]
- Treutlein, B.; Gokce, O.; Quake, S.R.; Südhof, T.C. Cartography of neurexin alternative splicing mapped by single-molecule long-read mRNA sequencing. Proc. Natl. Acad. Sci. USA 2014, 111, E1291–E1299. [Google Scholar] [CrossRef] [PubMed]
- Simms, B.A.; Zamponi, G.W. Neuronal voltage-gated calcium channels: Structure, function, and dysfunction. Neuron 2014, 82, 24–45. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.K.; Rajendran, M.Y.; Monfries, C.; Hall, C.; Lim, L. The human heat-shock protein family. Expression of a novel heat-inducible HSP70 (HSP70B) and isolation of its cDNA and genomic DNA. Biochem. J. 1990, 267, 125–132. [Google Scholar] [PubMed]
- Parsian, A.J.; Sheren, J.E.; Tao, T.Y.; Goswami, P.C.; Malyapa, R.; van Rheeden, R.; Watson, M.S.; Hunt, C.R. The human hsp70B gene at the HSPA7 locus of chromosome 1 is transcribed but nonfunctional. Biochim. Biophys. Acta 2000, 1494, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Noonan, E.J.; Place, R.F.; Giardina, C.; Hightower, L.E. Hsp70B' regulation and function. Cell Stress Chaperones 2007, 12, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Noonan, E.; Giardina, C.; Hightower, L. Hsp70B' and Hsp72 form a complex in stressed human colon cells and each contributes to cytoprotection. Exp. Cell Res. 2008, 314, 2468–2476. [Google Scholar] [CrossRef] [PubMed]
- Sax, C.M.; Piatigorsky, J. Expression of the α-crystallin/small heat shock protein/molecular chaperone genes in the lens and other tissues. Adv. Enzymol. Relat. Areas Mol. Biol. 1994, 69, 155–201. [Google Scholar] [PubMed]
- Kelley, W.L. The J-domain family and the recruitment of chaperone power. Trends Biochem. Sci. 1998, 23, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Esfarjani, P.; Benzer, S. Genetic suppression of polyglutamine toxicity in Drosophila. Science 2000, 287, 1837–1840. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Cook, J.L. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am. J. Respir. Cell Mol. Biol. 2007, 36, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Holcik, M.; Sonenberg, N. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 2005, 6, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Tettweiler, G.; Miron, M.; Jenkins, M.; Sonenberg, N.; Lasko, P.F. Starvation and oxidative stress resistance in Drosophila are mediated through the eIF4E-binding protein, d4E-BP. Genes Dev. 2005, 19, 1840–1843. [Google Scholar] [CrossRef] [PubMed]
- Scheper, G.C.; van der Knaap, M.S.; Proud, C.G. Translation matters: Protein synthesis defects in inherited disease. Nat. Rev. Genet. 2007, 8, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.L.; Mao, X.; Scott, B.A.; Crowder, C.M. Survival from hypoxia in C. elegans by inactivation of aminoacyl-tRNA synthetases. Science 2009, 323, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, A.G. Mechanism and regulation of methionyl-tRNA binding to ribosomes. In Translational Control of Gene Expression; Sonenberg, N., Hershey, J.W.B., Mathews, M.B., Eds.; Cold Spring Harbor Laboratory Press, Cold Spring Harbor Laboratory: New York, NY, USA, 2000; pp. 185–243. [Google Scholar]
- Proud, C.G. eIF2 and the control of cell physiology. Semin. Cell Dev. Biol. 2005, 16, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cash, T.P.; Jones, R.G.; Keith, B.; Thompson, C.B.; Simon, M.C. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol. Cell 2006, 21, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Browne, G.J.; Finn, S.G.; Proud, C.G. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J. Biol. Chem. 2004, 279, 12220–12231. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; McLeod, L.E.; Vries, R.G.; Flynn, A.; Wang, X.; Proud, C.G. Cellular stresses profoundly inhibit protein synthesis and modulate the states of phosphorylation of multiple translation factors. Eur. J. Biochem. 2002, 269, 3076–3085. [Google Scholar] [CrossRef] [PubMed]
- Proud, C.G. Regulation of mammalian translation factors by nutrients. Eur. J. Biochem. 2002, 269, 5338–5349. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, D.F.; Brown, G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997, 77, 731–758. [Google Scholar] [PubMed]
- Buttgereit, F.; Brand, M.D. A hierarchy of ATP-consuming processes in mammalian cells. Biochem. J. 1995, 312, 163–167. [Google Scholar] [PubMed]
- Olarewaju, O.; Ortiz, P.A.; Chowdhury, W.Q.; Chatterjee, I.; Kinzy, T.G. The translation elongation factor eEF1B plays a role in the oxidative stress response pathway. RNA Biol. 2004, 1, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.I. Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998, 12, 3788–3796. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. HIF-1 and human disease: One highly involved factor. Genes Dev. 2000, 14, 1983–1991. [Google Scholar] [PubMed]
- Jacquier-Sarlin, M.R.; Polla, B.S. Dual regulation of heat-shock transcription factor (HSF) activation and DNA-binding activity by H2O2: Role of thioredoxin. Biochem. J. 1996, 318, 187–193. [Google Scholar] [PubMed]
- Morimoto, R.I. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008, 22, 1427–1438. [Google Scholar] [CrossRef] [PubMed]
- Bukau, B.; Weissman, J.; Horwich, A. Molecular chaperones and protein quality control. Cell 2006, 125, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Wu, C. Heat shock transcription factors: Structure and regulation. Annu. Rev. Cell Dev. Biol. 1995, 11, 441–469. [Google Scholar] [CrossRef] [PubMed]
- Morano, K.A.; Thiele, D.J. Heat shock factor function and regulation in response to cellular stress, growth, and differentiation signals. Gene Expr. 1999, 7, 271–282. [Google Scholar] [PubMed]
- Pirkkala, L.; Nykänen, P.; Sistonen, L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001, 15, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Vihervaara, A.; Sistonen, L. HSF1 at a glance. J. Cell Sci. 2014, 127, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Akerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Christians, E.; Davis, A.A.; Thomas, S.D.; Benjamin, I.J. Embryonic development: Maternal effect of hsf1 on reproductive success. Nature 2000, 407, 693–694. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zuo, X.; Davis, A.A.; McMillan, D.R.; Curry, B.B.; Richardson, J.A.; Benjamin, I.J. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999, 18, 5943–5952. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Whitesell, L.; Rogers, A.B.; Lindquist, S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell 2007, 130, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Neef, D.W.; Jaeger, A.M.; Thiele, D.J. Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases. Nat. Rev. Drug Discov. 2011, 10, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Motohashi, H.; Yamamoto, M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol. Sci. 2013, 34, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Schulte, E.C.; Ellwanger, D.C.; Dihanich, S.; Manzoni, C.; Stangl, K.; Schormair, B.; Graf, E.; Eck, S.; Mollenhauer, B.; Haubenberger, D.; et al. Rare variants in LRRK1 and Parkinson’s disease. Neurogenetics 2014, 15, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kallio, M.; Chang, Y.; Manuel, M.; Alastalo, T.P.; Rallu, M.; Gitton, Y.; Pirkkala, L.; Loones, M.T.; Paslaru, L.; Larney, S.; et al. Brain abnormalities, defective meiotic chromosome synapsis and female subfertility in HSF2 null mice. EMBO J. 2002, 21, 2591–2601. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, J.; Moskophidis, D.; Mivechi, N.F. Targeted disruption of the heat shock transcription factor (hsf)-2 gene results in increased embryonic lethality, neuronal defects, and reduced spermatogenesis. Genesis 2003, 36, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ying, Z.; Jin, X.; Tu, N.; Zhang, Y.; Phillips, M.; Moskophidis, D.; Mivechi, N.F. Essential requirement for both hsf1 and hsf2 transcriptional activity in spermatogenesis and male fertility. Genesis 2004, 38, 66–80. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaitsuka, T.; Matsushita, M. Regulation of Translation Factor EEF1D Gene Function by Alternative Splicing. Int. J. Mol. Sci. 2015, 16, 3970-3979. https://doi.org/10.3390/ijms16023970
Kaitsuka T, Matsushita M. Regulation of Translation Factor EEF1D Gene Function by Alternative Splicing. International Journal of Molecular Sciences. 2015; 16(2):3970-3979. https://doi.org/10.3390/ijms16023970
Chicago/Turabian StyleKaitsuka, Taku, and Masayuki Matsushita. 2015. "Regulation of Translation Factor EEF1D Gene Function by Alternative Splicing" International Journal of Molecular Sciences 16, no. 2: 3970-3979. https://doi.org/10.3390/ijms16023970
APA StyleKaitsuka, T., & Matsushita, M. (2015). Regulation of Translation Factor EEF1D Gene Function by Alternative Splicing. International Journal of Molecular Sciences, 16(2), 3970-3979. https://doi.org/10.3390/ijms16023970