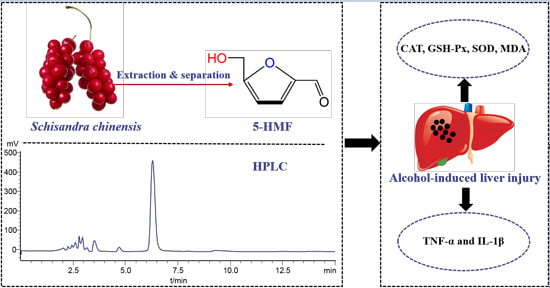
Ameliorative Effects of 5-Hydroxymethyl-2-furfural (5-HMF) from Schisandra chinensis on Alcoholic Liver Oxidative Injury in Mice
Abstract
:1. Introduction
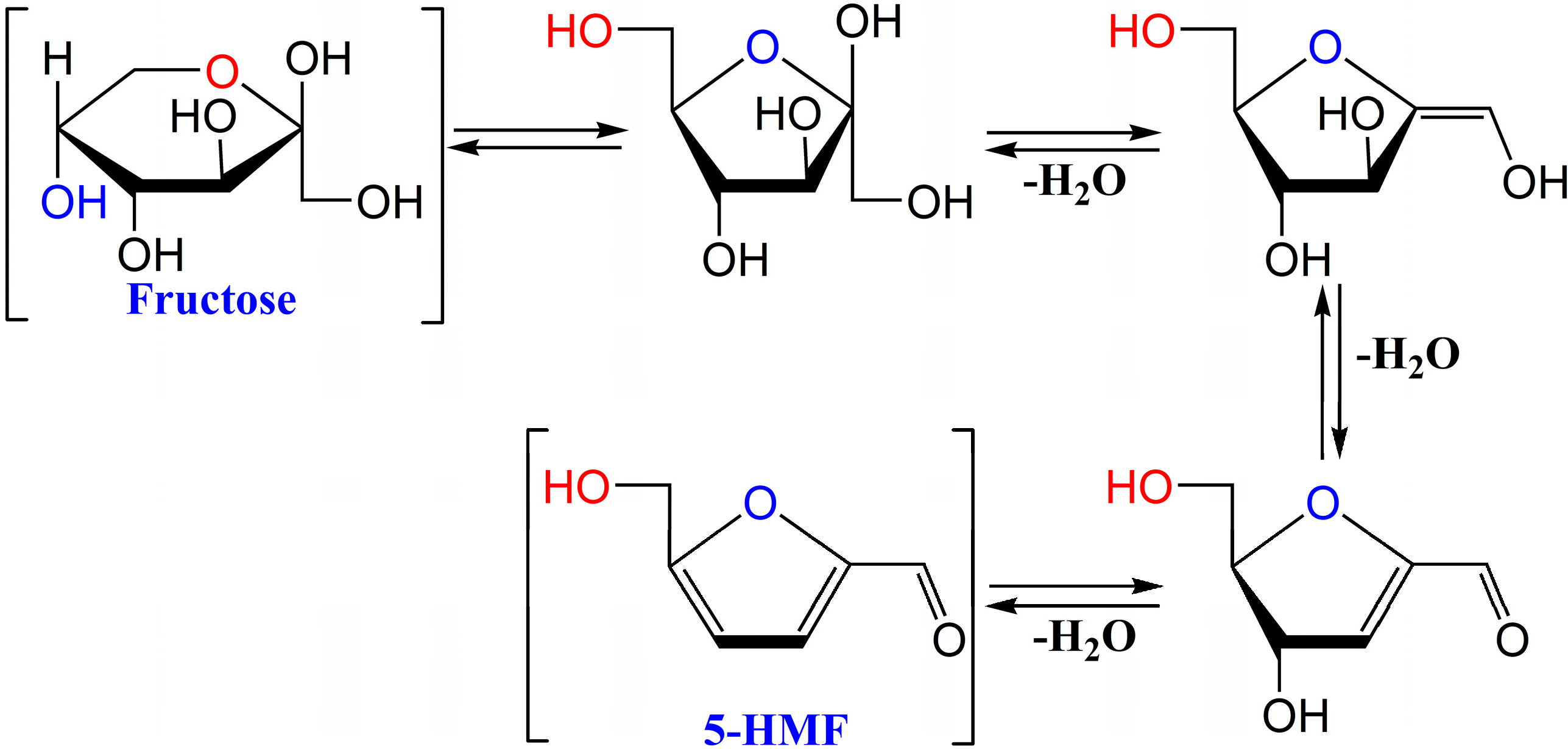
2. Results and Discussion
2.1. Analysis and Isolation of 5-HMF from Schisandra chinensis

2.2. Effects of 5-HMF on Body Weight and Organ Index
| Group | Dosage (mg/kg) | Initial Wts (g) | Final Wts (g) | Liver Index (×100, mg·g−1) | Spleen Index (×100, mg·g−1) |
|---|---|---|---|---|---|
| Control | — | 22.3 ± 3.1 | 27.5 ± 2.9 | 4.31 ± 0.14 | 0.32 ± 0.05 |
| Alcohol | — | 23.3 ± 2.6 | 28.2 ± 3.1 | 4.74 ± 0.14 * | 0.42 ± 0.03 * |
| Huganpian | 350 | 22.6 ± 2.1 | 29.3 ± 2.7 | 4.38 ± 0.14 # | 0.36 ± 0.04 # |
| 5-HMF | 7.5 | 23.1 ± 2.9 | 27.8 ± 3.3 | 4.31 ± 0.54 | 0.40 ± 0.06 |
| 15 | 22.8 ± 2.6 | 28.6 ± 3.4 | 4.48 ± 0.19 # | 0.37 ± 0.04 # | |
| 30 | 23.2 ± 2.7 | 29.1 ± 3.2 | 4.51 ± 0.14 # | 0.35 ± 0.03 # |
2.3. Effect of 5-HMF on Serum Biochemical Markers
| Group | Dosage (mg/kg) | ALT (U/L) | AST (U/L) | TC (mM) | TG (mM) | L-DLC (mM) |
|---|---|---|---|---|---|---|
| Control | — | 3.91 ± 0.63 | 12.62 ± 0.78 | 2.24 ± 0.23 | 0.38 ± 0.10 | 0.75 ± 0.15 |
| Alcohol | — | 5.32 ± 0.75 * | 28.22 ± 0.71 * | 3.77 ± 0.32 * | 2.15 ± 0.28 * | 1.77 ± 0.31 * |
| Huganpian | 350 | 4.02 ± 0.44 # | 21.25 ± 0.72 # | 3.54 ± 0.28 # | 0.51 ± 0.19 # | 1.36 ± 0.21 # |
| 5-HMF | 12.5 | 4.25 ± 0.24 # | 24.23 ± 0.55 # | 3.32 ± 0.26 # | 1.29 ± 0.15 # | 1.55 ± 0.32 # |
| 25 | 4.21 ± 0.22 # | 23.59 ± 0.64 # | 3.12 ± 0.22 # | 1.12 ± 0.23 # | 1.52 ± 0.26 # | |
| 50 | 4.01 ± 1.02 # | 22.45 ± 0.73 # | 2.98 ± 0.33 # | 0.80 ± 0.11 # | 1.43 ± 0.21 # |
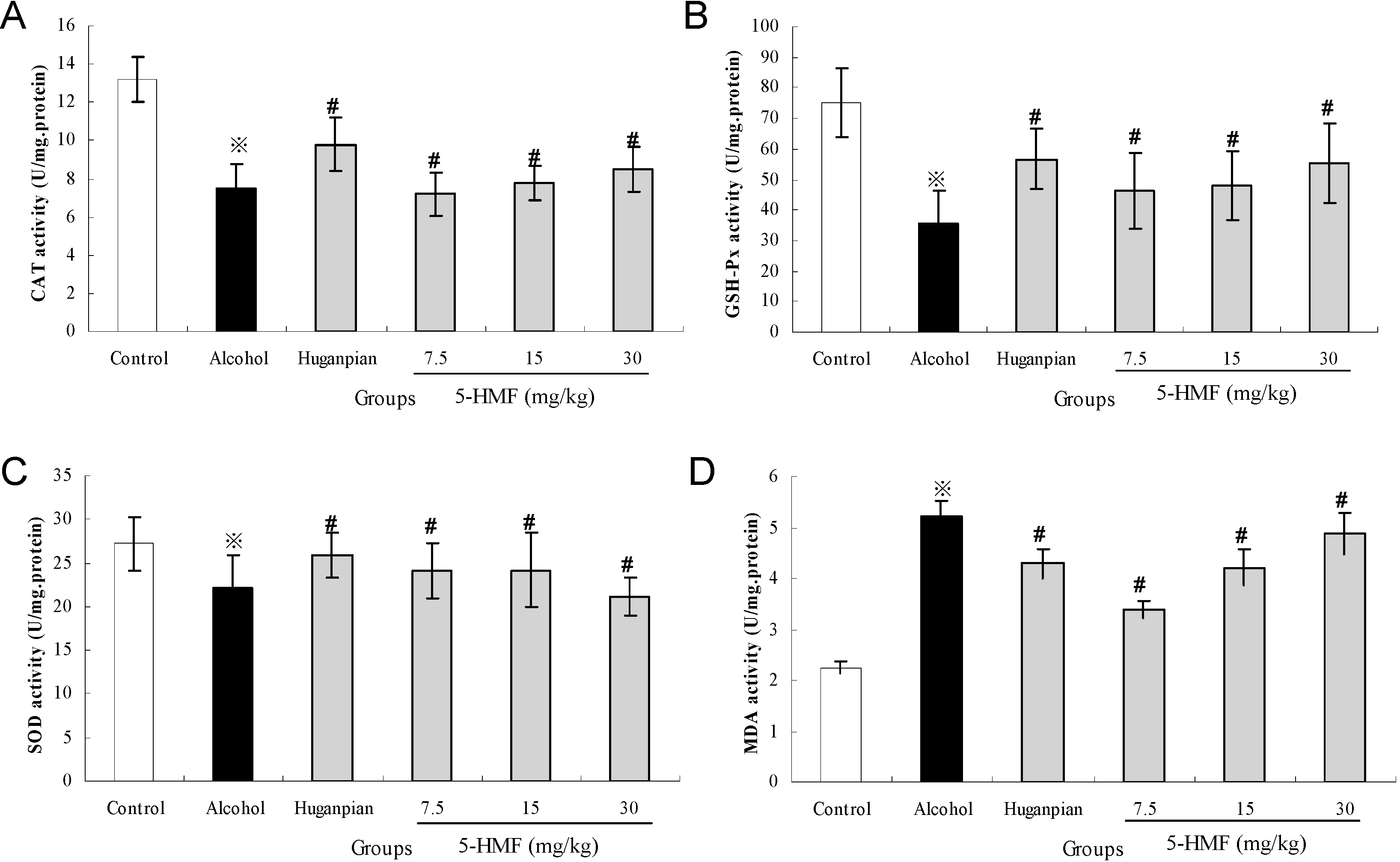
2.4. Effect of 5-HMF on Hepatic Biochemical Markers Activities
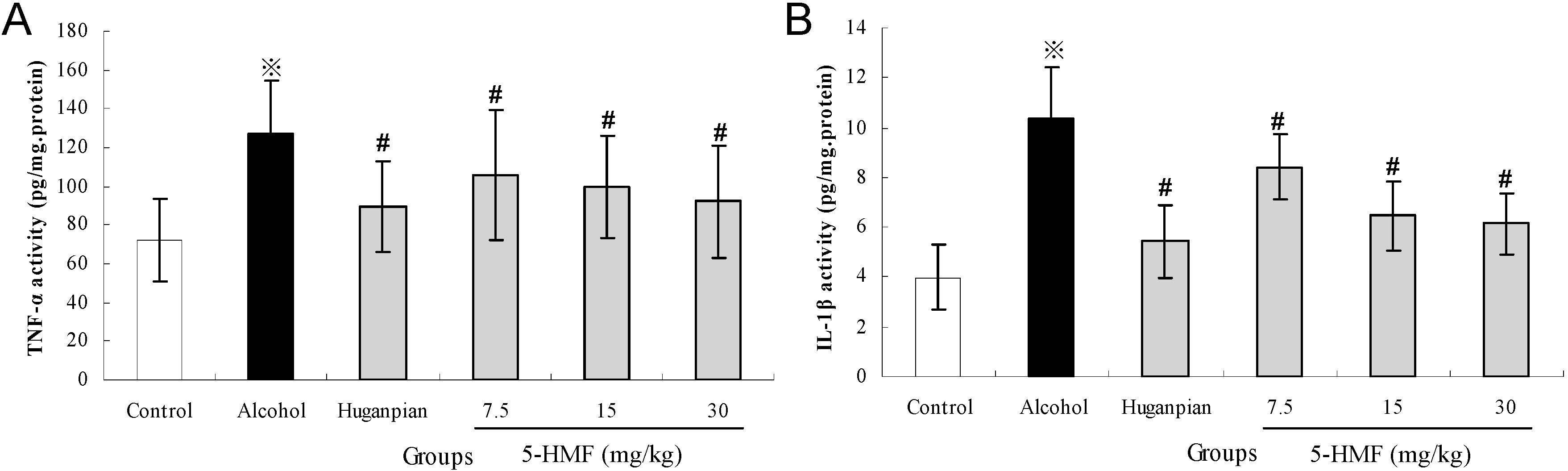
2.5. Effect of 5-HMF on Hepatic Inflammatory Markers
2.6. Pathological Observations
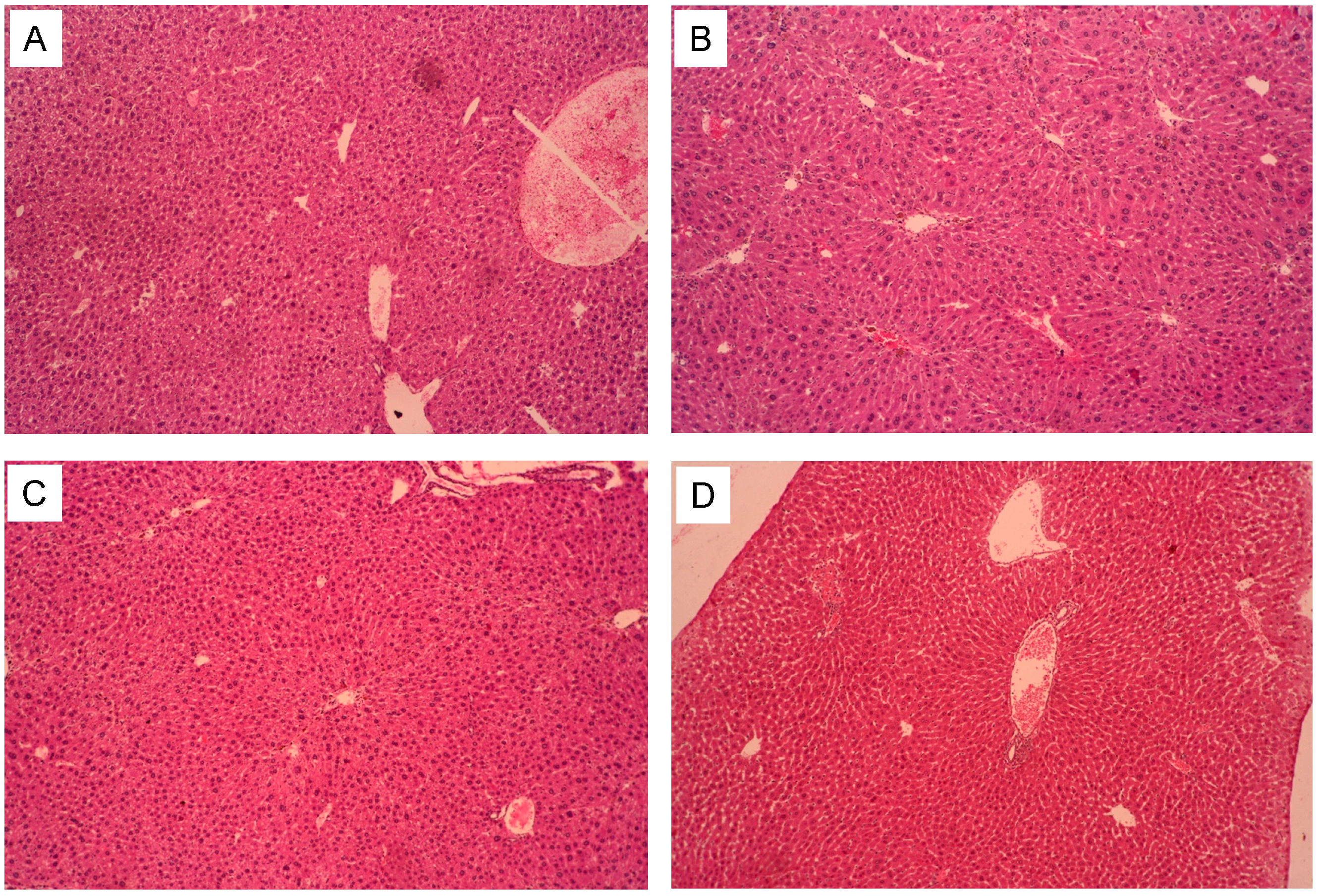
2.7. The Liver Pathological Classification and Grading
| Groups | Dosage (mg/kg) | n | Steatosis Grade | Ridit Analysis | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||
| Control | — | 8 | 8 | 0 | 0 | 0 | 0 | 0.23 |
| Alcohol | — | 8 | 0 | 3 | 4 | 1 | 0 | 0.78 * |
| Huganpian | 350 | 8 | 5 | 2 | 1 | 0 | 0 | 0.41 |
| 5-HMF | 7.5 | 8 | 2 | 4 | 1 | 1 | 0 | 0.59 # |
| 15 | 8 | 3 | 4 | 1 | 0 | 0 | 0.51 # | |
| 30 | 8 | 4 | 2 | 1 | 1 | 0 | 0.49 # | |
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Extraction, Isolation and Analysis of 5-HMF
3.3. Animals
3.4. Experimental Groups and Treatment
3.5. Assay for Serum Biochemical Markers
3.6. Assay for Hepatic Antioxidant Activities and Oxidative Stress Marker
3.7. Assay for Inflammatory Markers Activities
3.8. Histopathological Examination
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gramenzi, A.; Caputo, F.; Biselli, M.; Kuria, F.; Loggi, E.; Andreone, P.; Bernardi, M. Review article: Alcoholic liver disease—pathophysiological aspects and risk factors. Aliment. Pharmacol. Ther. 2006, 24, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.C.; Huang, H.P.; Lee, Y.J.; Tang, Y.H.; Wang, C.J. Hepatoprotective effect of mulberry water extracts on ethanol-induced liver injury via anti-inflammation and inhibition of lipogenesis in c57bl/6j mice. Food Chem. Toxicol. 2013, 62, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; French, S.W.; Casey, C.A.; Kharbanda, K.K.; Nanau, R.M.; Rasineni, K.; McVicker, B.L.; Kong, V.; Donohue, T.M., Jr. Changes in the pathogenesis of alcohol-induced liver disease—preclinical studies. Exp. Mol. Pathol. 2013, 95, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Guo, F.F.; Zhang, C.L.; Zhao, S.; Dou, D.D.; Gao, X.C.; Xie, K.Q. The anti-fatty liver effects of garlic oil on acute ethanol-exposed mice. Chem.-Biol. Interact. 2008, 176, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Mesias-Garcia, M.; Guerra-Hernandez, E.; Garcia-Villanova, B. Determination of furan precursors and some thermal damage markers in baby foods: Ascorbic acid, dehydroascorbic acid, hydroxymethylfurfural and furfural. J. Agric. Food Chem. 2010, 58, 6027–6032. [Google Scholar] [CrossRef] [PubMed]
- Zirbes, L.; Nguyen, B.K.; de Graaf, D.C.; de Meulenaer, B.; Reybroeck, W.; Haubruge, E.; Saegerman, C. Hydroxymethylfurfural: A possible emergent cause of honey bee mortality? J. Agric. Food Chem. 2013, 61, 11865–11870. [Google Scholar] [CrossRef] [PubMed]
- Yamada, P.; Nemoto, M.; Shigemori, H.; Yokota, S.; Isoda, H. Isolation of 5-(hydroxymethyl)furfural from lycium chinense and its inhibitory effect on the chemical mediator release by basophilic cells. Planta Med. 2011, 77, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Cai, H.; Cai, B.; Tu, S. Effect of 5-hydroxymethylfurfural derived from processed cornus officinalis on the prevention of high glucose-induced oxidative stress in human umbilical vein endothelial cells and its mechanism. Food Chem. 2013, 140, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Won, T.H.; Liao, L.; Kang, S.S.; Shin, J. Simultaneous analysis of furfural metabolites from rehmanniae radix preparata by hplc-dad-esi-ms. Food Chem. 2014, 142, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chao, Z.; Liu, Y.; Song, Z.; Lu, A. Maillard reaction involved in the steaming process of the root of polygonum multiflorum. Planta Med. 2009, 75, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, Y.H.; Lu, X.Y. Investigation on influencing factors of 5-hmf content in schisandra. J. Zhejiang Univ. Sci. B 2007, 8, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Abraham, K.; Gurtler, R.; Berg, K.; Heinemeyer, G.; Lampen, A.; Appel, K.E. Toxicology and risk assessment of 5-hydroxymethylfurfural in food. Mol. Nutr. Food Res. 2011, 55, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (hmf): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Truzzi, C.; Annibaldi, A.; Illuminati, S.; Finale, C.; Rossetti, M.; Scarponi, G. Determination of very low levels of 5-(hydroxymethyl)-2-furaldehyde (hmf) in natural honey: Comparison between the hplc technique and the spectrophotometric white method. J. Food Sci. 2012, 77, C784–C790. [Google Scholar] [CrossRef] [PubMed]
- Gidamis, A.B.; Chove, B.E.; Shayo, N.B.; Nnko, S.A.; Bangu, N.T. Quality evaluation of honey harvested from selected areas in tanzania with special emphasis on hydroxymethyl furfural (hmf) levels. Plant Foods Hum. Nutr. 2004, 59, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Monakhova, Y.B.; Lachenmeier, D.W. The margin of exposure of 5-hydroxymethylfurfural (hmf) in alcoholic beverages. Environ. Health Toxicol. 2012, 27, e2012016. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, G.; Berregi, I.; Caracena, R.; Zuriarrain, J. Quantitative determination of caffeine, formic acid, trigonelline and 5-(hydroxymethyl)furfural in soluble coffees by 1 h nmr spectrometry. Talanta 2010, 81, 367–371. [Google Scholar]
- Zhao, L.; Chen, J.; Su, J.; Li, L.; Hu, S.; Li, B.; Zhang, X.; Xu, Z.; Chen, T. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J. Agric. Food Chem. 2013, 61, 10604–10611. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Wu, L.Y.; Zhao, T.; Xiong, L.; Huang, X.; Liu, Z.H.; Fan, X.L.; Xiao, C.R.; Gao, Y.; Ma, Y.B.; et al. The protective role of 5-hmf against hypoxic injury. Cell Stress Chaperones 2011, 16, 267–273. [Google Scholar] [CrossRef]
- Janzowski, C.; Glaab, V.; Samimi, E.; Schlatter, J.; Eisenbrand, G. 5-hydroxymethylfurfural: Assessment of mutagenicity, DNA-damaging potential and reactivity towards cellular glutathione. Food Chem. Toxicol. 2000, 38, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Powell, R.D.; Swet, J.H.; Kennedy, K.L.; Huynh, T.T.; McKillop, I.H.; Evans, S.L. Resveratrol attenuates hypoxic injury in a primary hepatocyte model of hemorrhagic shock and resuscitation. J. Trauma Acute Care Surg. 2014, 76, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Wang, M.Y.; Yao, Y.X.; Li, G.Y.; Cai, B.C. Protective effect of 5-hydroxymethylfurfural derived from processed fructus corni on human hepatocyte lo2 injured by hydrogen peroxide and its mechanism. J. Ethnopharmacol. 2010, 128, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.S.; Kim, J.E.; Lee, Y.J.; Kwak, M.H.; Choi, Y.H.; Kang, B.C.; Hong, J.T.; Hwang, D.Y. Protective effects of gomisin a isolated from schisandra chinensis against ccl(4)-induced hepatic and renal injury. Int. J. Mol. Med. 2013, 31, 888–898. [Google Scholar] [PubMed]
- Zeng, T.; Zhang, C.L.; Song, F.Y.; Zhao, X.L.; Yu, L.H.; Zhu, Z.P.; Xie, K.Q. The activation of ho-1/nrf-2 contributes to the protective effects of diallyl disulfide (dads) against ethanol-induced oxidative stress. Biochim. Biophys. Acta 2013, 1830, 4848–4859. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Hassan, W.; Meinerz, D.F.; Leite Gde, O.; Nogueira, C.W.; Rocha, J.B. Ethanol-induced oxidative stress: The role of binaphthyl diselenide as a potent antioxidant. Biol. Trace Elem. Res. 2012, 147, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qi, X.; Cao, S.; Li, P. Protective effect of flavonoid extract from Chinese bayberry (myrica rubra sieb. Et zucc.) fruit on alcoholic liver oxidative injury in mice. J. Nat. Med. 2014, 68, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Mantena, S.K.; King, A.L.; Andringa, K.K.; Landar, A.; Darley-Usmar, V.; Bailey, S.M. Novel interactions of mitochondria and reactive oxygen/nitrogen species in alcohol mediated liver disease. World J. Gastroenterol. 2007, 13, 4967–4973. [Google Scholar] [PubMed]
- Parlesak, A.; Schafer, C.; Paulus, S.B.; Hammes, S.; Diedrich, J.P.; Bode, C. Phagocytosis and production of reactive oxygen species by peripheral blood phagocytes in patients with different stages of alcohol-induced liver disease: Effect of acute exposure to low ethanol concentrations. Alcohol. Clin. Exp. Res. 2003, 27, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Kawaratani, H.; Tsujimoto, T.; Douhara, A.; Takaya, H.; Moriya, K.; Namisaki, T.; Noguchi, R.; Yoshiji, H.; Fujimoto, M.; Fukui, H. The effect of inflammatory cytokines in alcoholic liver disease. Mediat. Inflamm. 2013, 2013, 495156. [Google Scholar] [CrossRef]
- Orman, E.S.; Odena, G.; Bataller, R. Alcoholic liver disease: Pathogenesis, management, and novel targets for therapy. J. Gastroenterol. Hepatol. 2013, 28, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Choi, Y.W.; Lee, E.N.; Park, J.K.; Kim, S.G.; Park, D.J.; Kim, B.S.; Lim, Y.T.; Yoon, S. 5-hydroxymethylfurfural from black garlic extract prevents tnfalpha-induced monocytic cell adhesion to huvecs by suppression of vascular cell adhesion molecule-1 expression, reactive oxygen species generation and nf-kappab activation. Phytother. Res. 2011, 25, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M. Grading and staging the histopathological lesions of chronic hepatitis: The knodell histology activity index and beyond. Hepatology 2000, 31, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.L. Handbook helps students improve writing skills. J. Am. Med. Rec. Assoc. 1985, 56, 42–45. [Google Scholar] [PubMed]
- Mohandas, J.; Marshall, J.J.; Duggin, G.G.; Horvath, J.S.; Tiller, D.J. Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer Res. 1984, 44, 5086–5091. [Google Scholar] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Qu, X.-N.; Han, Y.; Zheng, S.-W.; Wang, J.; Wang, Y.-P. Ameliorative Effects of 5-Hydroxymethyl-2-furfural (5-HMF) from Schisandra chinensis on Alcoholic Liver Oxidative Injury in Mice. Int. J. Mol. Sci. 2015, 16, 2446-2457. https://doi.org/10.3390/ijms16022446
Li W, Qu X-N, Han Y, Zheng S-W, Wang J, Wang Y-P. Ameliorative Effects of 5-Hydroxymethyl-2-furfural (5-HMF) from Schisandra chinensis on Alcoholic Liver Oxidative Injury in Mice. International Journal of Molecular Sciences. 2015; 16(2):2446-2457. https://doi.org/10.3390/ijms16022446
Chicago/Turabian StyleLi, Wei, Xin-Nan Qu, Ye Han, Si-Wen Zheng, Jia Wang, and Ying-Ping Wang. 2015. "Ameliorative Effects of 5-Hydroxymethyl-2-furfural (5-HMF) from Schisandra chinensis on Alcoholic Liver Oxidative Injury in Mice" International Journal of Molecular Sciences 16, no. 2: 2446-2457. https://doi.org/10.3390/ijms16022446
APA StyleLi, W., Qu, X.-N., Han, Y., Zheng, S.-W., Wang, J., & Wang, Y.-P. (2015). Ameliorative Effects of 5-Hydroxymethyl-2-furfural (5-HMF) from Schisandra chinensis on Alcoholic Liver Oxidative Injury in Mice. International Journal of Molecular Sciences, 16(2), 2446-2457. https://doi.org/10.3390/ijms16022446